Abstract
Background
Cardioplegic arrest (CP) followed by reperfusion after cardiopulmonary bypass induces coronary microvascular dysfunction. We investigated the role of calcium-activated potassium (KCa) channels in this dysfunction in the human coronary microvasculature.
Methods and Results
Human atrial tissue was harvested before CP from a nonischemic segment and after CP from an atrial segment exposed to hyperkalemic cold blood CP (mean CP time, 58 minutes) followed by 10-minute reperfusion. In vitro relaxation responses of precontracted arterioles (80 to 180 μm in diameter) in a pressurized no-flow state were examined in the presence of KCa channel activators/blockers and several other vasodilators. We also examined expression and localization of KCa channel gene products in the coronary microvasculature using reverse transcriptase-polymerase chain reaction, immunoblot, and immunofluorescence photomicroscopy. Post-CP reperfusion relaxation responses to the activator of intermediate and small conductance KCa channels (IKCa/SKCa), NS309 (10-5 M), and to the endothelium-dependent vasodilators, substance P (10-8 M) and adenosine 5′diphosphate (10-5 M), were significantly reduced compared with pre-CP responses (P<0.05, n=8/group). In contrast, relaxation responses to the activator of large conductance KCa channels (BKCa), NS1619 (10-5 M), and to the endothelium-independent vasodilator, sodium nitroprusside (10-4 M), were unchanged pre- and post-CP reperfusion (n=8/group). Endothelial denudation significantly diminished NS309-induced vasodilatation and abolished substance P- or adenosine 5′ diphosphate-induced relaxation (P<0.05), but had no effect on relaxation induced by either NS1619 or sodium nitroprusside. The total polypeptide levels of BKCa, IKCa, and SKCa and the expression of IKCa mRNA were not altered post-CP reperfusion.
Conclusion
Cardioplegic arrest followed by reperfusion after cardiopulmonary bypass causes microvascular dysfunction associated with and likely in part due to impaired function of SKCa and IKCa channels in the coronary microcirculation. These results suggest novel mechanisms of endothelial and smooth muscle microvascular dysfunction after cardiac surgery.
Keywords: calcium-activated potassium channels, cardioplegia, ischemia and reperfusion, microcirculation
Microvascular endothelial dysfunction and altered vascular reactivity often occur after ischemic arrest and cardiopulmonary bypass, although cardioplegia has been routinely used for the protection of the myocardium against ischemic injury during cardiac surgery.1-3 Endothelium has been found to release 3 major endothelium-dependent relaxing factors: nitric oxide, prostacyclin, and endothelium-dependent hyperpolarization factor (EDHF).4 Impaired endothelial synthesis and release of nitric oxide, prostacyclin, and EDHF associated with cardioplegic ischemia and reperfusion may contribute to microvascular endothelial dysfunction.1-3 These observations have prompted addition to cardioplegic solutions of nitric oxide donors, exogenous prostacyclin, and EDHF agonists in attempts to attenuate ischemia/reperfusion-mediated endothelial dysfunction.5-7 The vasodilatory influence of EDHF is believed predominant in smaller arterioles.
EDHF has been proposed to dilate the coronary arteries through opening of calcium-activated potassium channels (KCa) in endothelial and smooth muscle cells.4 Recently, we found that bradykinin preconditioning of coronary microvasculature in isolated rabbit hearts undergoing cardioplegic arrest was mediated in part by the activation of KCa channels.8 However, the roles of KCa channel activation in cardioplegic arrest (CP) and reperfusion-related microvascular dysfunction in human coronary resistance arterioles and the regulatory properties of these KCa channels in this vascular bed have little been investigated.
This study was designed to examine the effect of blood CP and brief reperfusion (Rep) on vascular responses of human atrial microvessels to KCa channel openers and other vasoactive substances and to correlate these responses to possible alterations in expression of KCa channel mRNAs and polypeptides in human atrial tissue.
Materials and Methods
Human Subjects and Tissue Harvesting
Samples of right atrial appendage were harvested from clinically similar patients undergoing coronary artery bypass graft surgery before and after exposure of the heart to blood CP and short-term reperfusion under conditions of cardiopulmonary bypass. Samples were harvested with cold sharp dissection and handled in a nontraumatic fashion. Double 3-0 polypropylene pursestring sutures (Ethicon, Somerville, NJ) were placed in the atrial appendage. During placement of the venous cannula, the first sample of atrial appendage was harvested before cardioplegia and reperfusion (pre-CP Rep). The superior suture was tightened to secure the venous cannula. The inferior suture remained loose to allow this portion of the atrium to be perfused with blood, exposed to blood CP, and reperfused (post-CP Rep) after removal of the aortic crossclamp. An initial 800 to 1000 mL of cold-blood (0°C to 4°C) hyperkalemic (15 mmol/L K+) cardioplegic solution was delivered antegrade into the aortic root. This was followed at 8- to 15-minute intervals with 250 to 300 mL of cold CP solution (15 mmol/L K+). The CP solutions consisted of a mixture of oxygenated blood with crystalloid solution of the following final composition (in mmol/L): 15 KCl, 3.5 MgSO4, 135NaCl, 1.0 CaCl2, 11 glucose, 11 mannitol, and 4 tromethamine.
The second sample of atrial appendage was harvested during removal of the venous cannula. Tissue samples for immunoblot analysis assay were immediately frozen in liquid nitrogen. Tissue for immunofluorescent staining was fixed in 10% formalin-buffered solution for 24 hours followed by paraffin mounting and sectioning into 5-μm slices. Tissue for microvascular studies was placed in cold (5° to 10°C) Krebs buffer solution. All procedures were approved by the Institutional Review Board of Beth Israel Deaconess Medical Center, Harvard Medical School, and informed consent was obtained from all enrolled patients required by the Institutional Review Board.
Coronary Microvessel Relaxation Studies
Coronary arterioles (80- to 180-μm internal diameters) were dissected from the right atria appendage pre- and post-CP Rep. Microvessel studies were performed by in vitro organ bath video-microscopy as described previously.3,6 After a 60-minute stabilization period in the organ chamber, the microvessels were preconstricted with thromboxane A2 analog U46619×30% to 40% of the baseline diameter. After achievement of this constricted steady state, dose-dependent relaxation was measured in response to (extraluminal) application of the following vasodilators: the activator of intermediate and small conductance KCa channels (IKCa/SKCa), NS309, the activator of large conductance KCa channels (BKCa), NS1619 (both from 10-9 to 10-5 M), sodium nitroprusside (SNP), adenosine 5′-diphosphate (both from 10-9 to 10-4 M), and substance P (10-12 to 10-7 M). After exposure to substance P, the vessel was discarded to avoid tachyphylaxis. One or 3 interventions were performed on each vessel. The order of drug administration was random. Six vessels harvested before CP Rep were pretreated with a mixture of the IKCa blocker, TRAM34 (10-7 M), and the SKCa blocker, apamin (10-6 M), before perfusion with NS309. Six additional vessels were pretreated with the BKCa blocker, iberiotoxin (10-7 M) before perfusion with NS1619. In some cases, endothelial denudation was carried out by advancing a human hair into the lumen and gently abrading the luminal surface.
Immunoblot
Small coronary arteries were dissected and cleaned of connective tissues and solubilized in SDS-PAGE buffer. Total protein (40 μg) was fractionated on an 8% to 16% SDS-PAGE and then transferred to a polyvinylidene diflouride membrane (Immobilon-P; Millipore Corporation, Bedford, Mass) as previously described.3 Membranes were incubated for 1 hour at room temperature with 1:200 dilutions of individual rabbit polyclonal primary antibodies to BKCa-α, BKCa-β1 (Santa Cruz Biotechnology, Santa Cruz, Calif), IKCa,or SK3Ca-α (Alomone Labs Ltd, Jerusalem, Israel). The membranes were then incubated for 1 hour with horseradish peroxidase-conjugated secondary anti-Ig, washed 3× in Tris saline buffer, and processed for chemiluminescent detection (Pierce, Rockford, Ill) on x-ray film (Kodak, Rochester, NY). Band intensity was measured by densitometric analysis of autoradiograph films using NIH Image J1.33. Specificities of the anti-BKCa-α, anti-BKCa-β1, anti-IKCa, and anti-SK3Ca antibodies were demonstrated in the previous studies, respectively.8-10
Reverse Transcriptase-Polymerase Chain Reaction of IKCa mRNA
Total RNA was prepared with the RNeasy kit (Qiagen, Valencia, Calif) from flash-frozen pre- and post-CP Rep atrial tissue samples. RNA integrity was verified by formaldehyde-agarose gel electrophoresis, and RNA concentration was estimated by absorbance at 260 nm. Reverse transcription was performed with the First Strand cDNA Synthesis Kit (Ambion, Austin, Texas) using equal amounts of total RNA. Five percent of the cDNA mixture was used for polymerase chain reaction with HotStart DNA Polymerase (Qiagen; total reaction volume 20 μL in supplier’s recommended buffer). Two primer pairs tested yielded nearly identical results: forward primer IK1.F6 5′-GGAAGCTCCGGGAACAAGTG-3′ coupled with reverse primer IKCa.R3 5′-CTACTTKGACTGCTGGCTKGGTTC-3′ (209 bp polymerase chain reaction product) or forward primer IK1.F7 5′-GGTGGACATCTCCAAGATGCAC-3′ coupled with the same reverse primer (181 bp polymerase chain reaction product). Complete reaction mixes were incubated 15 minutes at 95°C, then cycled through 45 seconds denaturation at 94°C, 2 minutes annealing at 60°C, and 2 minutes elongation at 72°C. A 7-minute final extension at 72°C was terminated by rapid cooling to room temperature. Polymerase chain reaction products were separated in 1% agarose gels, visualized with ethidium bromide, and imaged (GelDoc; Biorad, Hercules, Calif). Polymerase chain reaction cycle number was adjusted for low abundance transcript detection within the range of log linear amplification. Thirty-six cycles was chosen for amplification of IKCa transcript and 23 cycles for βactin controls.
Immunofluorescence Photomicroscopy
Atrial tissue sections from 5 patients were deparaffinized in xylene, rehydrated in graded ethanol and phosphate-buffered saline solution, and antigen-unmasked with sodium citrate (10 mmol/L, pH=6.0) followed by phosphate-buffered saline wash and blocking with 2% bovine serum albumin in phosphate-buffered saline at room temperature for 2 hours. After phosphate-buffered saline wash, overnight incubation with anti-BKCa-α and BKCa-β1 (each used 1:200; Santa Cruz Biotechnology) was performed at 4°C. Antimouse, α-smooth muscle actin (1:1000; Sigma, St Louis, Mo) was used to detect microvascular smooth muscle. Sections were then washed in phosphate-buffered saline and incubated with the appropriate Alexa fluor secondary antibody and mounted using fluorescent mounting medium (Vector Labs, Burlingame, Calif). Tissue was visualized using a Zeiss LSM510 confocal microscope system (Carl Zeiss MicroImaging, Inc, Thornwood, NY). Tissue labeling with secondary antibody alone or with normal rabbit IgG or serum in place of primary antibody served as a negative control.
Chemicals
NS309, NS1619, U46619, SNP, adenosine 5′ diphosphate (ADP), and substance P were obtained from Sigma. SNP, ADP, and substance P were dissolved in ultrapure distilled water and prepared on the day of the study. U46619 was dissolved in ethanol to make a stock solution. NS309 and NS1619 were dissolved in dimethylsulfoxide to make a stock solution. All stock solutions were stored at -20°C. All dilutions were prepared daily.
Data Analysis
Data are presented as the mean and SEM. The relaxation responses were expressed as the percentage of relaxation of the U46619-preconstricted diameter of the microvessels. Repeated-measures analysis of variance and Student t test were used to compare variables between or among vessels. The treatment effects were statistically examined by paired or independent 2-tailed Student t test. Statistical significance was taken at a probability value of <0.05.
Statement of Responsibility
The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Patient Characteristics
Tissue samples from 27 patients were studied. Twenty-seven patients (mean age 67±6 years) underwent coronary artery bypass graft with duration of cardioplegic arrest of 58±4 minutes and cardiopulmonary bypass time of 71.0±18 minutes. Twenty-one patients were male and 6 were female. Twenty-two of the 27 patients carried a preoperative diagnosis of hypertension. All patients with preoperative hypertension were on medication (β-blocker, calcium channel blocker, or angiotensin-converting enzyme inhibitor) and received perioperative β-blockade. Diabetes mellitus (type I or type II) was present in 5 of 27 patients.
Vessel Characteristics
Coronary microvessels ranged from 80 to 180 μm in internal diameter. Diameters in the NS309 groups were 107±4 μmin the pre-CP Rep group, 110±8 μm in the post-CP Rep group, 103±7 μm in the endothelium denudation group, and 137+9 μm in the TRAM34/apamin+NS309 group. Diameters in the NS1619 groups were 125±10 μm in the pre-CP Rep group, 114±9 μm in the post-CP Rep group, 146±11 μm in the endothelium denudation group, and 126±8 μm in the iberiotoxin+NS1619 group. In the substance P groups, diameters were 134±6 μm in the pre-CP Rep group, 112±7 μm in the post-CP Rep group, and 119±7 μm in the endothelium denudation group. In the ADP groups, diameters were 118±10 μm in the pre-CP Rep group, 130±12 μm in the post-CP Rep group, and 135±7 μm in the endothelium denudation group. In the SNP groups, diameters were 116±6 μm in the pre-CP Rep group, 108±7 μm in the pre-CP Rep group, and 122±11 μm in the endothelium denudation group. Most intergroup differences of the vessel diameters were statistically insignificant. The degree of precontraction by the thromboxane A2 analog U46619 was 29±2% in the pre-CP Rep group and 31±2% in the post-CP Rep group. Similar U46619 concentrations were required to produce adequate precontraction in both groups.
Responses to NS309 and NS1619
Both NS309 (Figure 1A) and NS1619 (Figure 2A) induced dose-dependent relaxation of human coronary arterioles. Cardioplegic arrest and reperfusion significantly impaired the relaxation response to NS 309 compared with pre-CP Rep responses (P<0.05; Figure 1A). In contrast, the pre-CP Rep responses to NS1619 were unchanged post-CP Rep (Figure 2A). Pretreatment with TRAM34/apamin abolished vessel relaxation induced by NS309 (P<0.001; Figure 1B), and NS1619-induced relaxation was inhibited by pretreatment with iberiotoxin (P<0.001; Figure 2B). Furthermore, NS309-mediated vasorelaxation of the coronary arterioles was significantly diminished by removal of the endothelium (P<0.001; Figure 1C), whereas responses to NS1619 were unaffected (Figure 2C).
Figure 1.
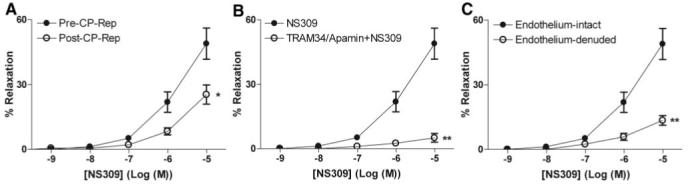
Vasoreactivity of human coronary arterioles to the IK/SK channel activator NS309. A, Dose-dependent relaxation of coronary arterioles (n=8) in response to NS309 before CP and Rep and afterward (post-CP Rep); *P<0.05. B, Dose-dependent NS309-mediated relaxation of pre-CP Rep human coronary arterioles pretreated in the absence or presence of TRAM34 and apamin (n=6); **P<0.001. C, Dose-dependent relaxation of pre-CP Rep human coronary arterioles from pre-CP Rep tissues (n=6) with intact or denuded endothelium in response to NS309; **P<0.001. Data are presented as percent relaxation after preconstriction with U46619.
Figure 2.
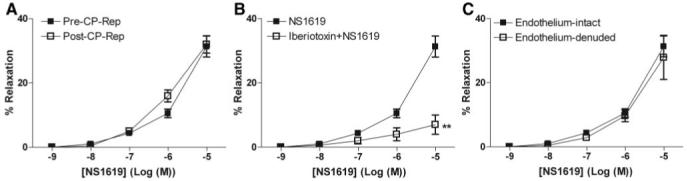
Vasoreactivity of human coronary arterioles to the BK channel activator NS1619. A, Dose-dependent relaxation of coronary arterioles in response to NS1619 either pre-CP Rep or post-CP Rep (n=8/group). B, Dose-dependent NS1619-mediated relaxation of human coronary arterioles from pre-CP Rep atrial tissues pretreated in the absence or presence of iberiotoxin (n=6; **P<0.001). C, Dose-dependent NS1619-induced relaxation of pre-CP Rep human coronary arterioles with intact or denuded endothelium (n=6/group). Data are presented as percent relaxation after preconstriction with U46619.
Responses to Adenosine 5′ Diphosphate, Substance P, and Sodium Nitroprusside
ADP, substance P, and SNP induced dose-dependent relaxation of coronary arterioles (Figure 3A-C). Cardioplegic arrest and reperfusion remarkably reduced the relaxation responses both to ADP and to substance P compared with pre-CP Rep responses (P<0.05; Figure 3A-B). In contrast, pre-CP Rep responses to SNP were unchanged post-CP Rep (Figure 3C). Endothelium denudation abolished responses to both ADP and substance P (P<0.001; Figure 3A-B), but left intact SNP-induced relaxation.
Figure 3.
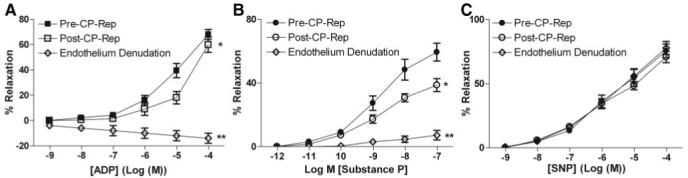
In vitro response of preconstricted human atrial arterioles to endogenous vasodilators. Arterioles were compared pre-CP Rep and post-CP Rep (n=8/group). Pre-CP Rep arterioles were compared with intact endothelium and after endothelial denudation (n=6/group) as indicated. A, Response to the endothelium-dependent vasodilator ADP. *P<0.05 versus pre-CP Rep; **P<0.001 versus intact pre-CP Rep. B, Response to substance P. *P<0.05 versus pre-CP Rep; *P<0.001 versus pre-CP Rep. C, Response to the endothelium-independent vasodilator, SNP.
Effect of Cardioplegic Arrest Reperfusion on levels of KCa Polypeptides and IKCa mRNA
Pre-CP Rep coronary artery levels of the KCa channel polypeptides BKCa-α and BKCa BKCa-β1, IKCa-α, and SK3Ca-α were unchanged in the post-CP Rep period as detected by immunoblot (Figure 4A-B). Levels of IKCa mRNA were similarly unaltered after cardioplegic ischemia and reperfusion (Figure 4C).
Figure 4.
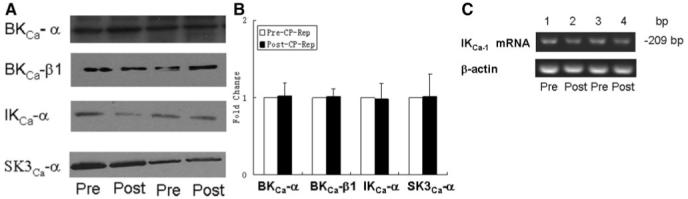
A, top left, Representative immunoblot of human coronary microvessels. Lanes 1 to 4 loaded with 40 μg protein were developed for BK-α, BK-β1, IKCa-α, and SK3Ca-α polypeptides B, top right, Densitometric evaluation of immunoblot band intensity shows unaltered levels of KCa polypeptides after CP Rep (n=5/group). C, bottom, Semiquantitative reverse transcriptase-polymerase chain reaction showing steady-state levels of mRNA for IKCa and β-actin control in human atrial tissue pre- and CP and Rep.
Immunolocalization of Microvessel KCa Polypeptides Pre- and Postcardioplegic Arrest Reperfusion
Immunofluorescent staining of coronary microvessels displayed a strong signal for BKCa-α (Figure 5A) and BKCa-β1 (Figure 5B) subunits localized to the microvascular smooth muscle and less abundantly to endothelium. Negative controls documented low-level background fluorescence.
Figure 5.
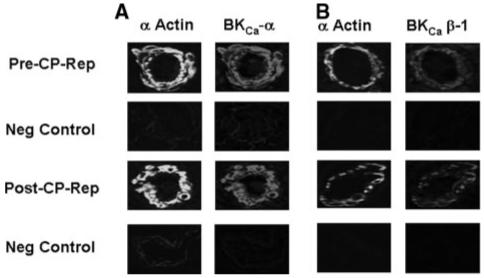
Immunolocalization of KCa channel polypeptides in human coronary microvessels. Vessels were costained for smooth muscle α-actin and either (A) BKCa-α or (B) BKCa-β1. Matched negative controls are displayed below each row of primary antibody staining as indicated.
Discussion
This study presents several novel findings. First, both the IKCa/SKCa activator NS309 and the BKCa-specific opener NS1619 induced dose-dependent vasodilatation of human coronary arterioles. Second, the relaxation response to NS309 was significantly impaired after CP and Rep, whereas the response to NS1619 was unaltered. Third, the response to NS309 was significantly diminished after endothelium denudation, whereas the response to NS1619 was unaffected. Fourth, the presence of BKCa, IKCa, and SKCa polypeptides in human coronary microvasculature was documented by immunoblot and by immunofluorescence microscopy. BKCa immunostaining was present predominantly in smooth muscle cells and less apparent in endothelium. However, SKCa immunostaining was detected predominantly in endothelium and less in smooth muscle cells. IKCa mRNA was also present in human atrial tissue. Finally, cardioplegic ischemia and Rep change neither total polypeptide levels of BKCa, IKCa,orSKCa polypeptides nor the level of IKCa mRNA.
We and several others have previously shown that cardioplegic ischemia and Rep resulted in microvascular endothelial dysfunction and decreased hyperpolarization in animals and humans.1-3,5 The present study confirmed previous findings indicating that responses to several endothelial-dependent vasodilators such as ADP and substance P were significantly reduced after cardioplegic ischemia and Rep. Recent studies have demonstrated that KCa channels contribute to the vasodilatation and hyperpolarization induced by the EDHF stimulators, substance P and bradykinin.4,10 Three types of KCa channels, BKCa, IKCa, and SKCa, are present in the vasculature.4,11,12 In the present study, relaxation responses to NS309 were completely blocked in the combined presence of the IKCa blocker TRAM 34 and the SKCa inhibitor apamin, supporting specificity of NS309 as an activator of both IKCa and SKCa channels. Relaxation responses to NS1619 were abolished in the presence of the BKCa blocker iberiotoxin, consistent with the specificity of NS1619 as a BKCa activator. Endothelium denudation significantly diminished NS309-induced relaxation but failed to affect the response to NS1619. These results show that whereas NS309-induced relaxation is endothelium-dependent, relaxation induced by NS1619 is endothelium-independent. Another important finding of the present study is that cardioplegic ischemia and Rep remarkably impaired IKCa/SKCa channel activator NS309-induced dose-dependent vasodilatation, but not the BKCa opener NS1619-induced relaxation, suggesting that the inactivation of IKCa/SKCa may be involved in endothelial dysfunction of human coronary microvasculature. All the results presented were obtained in vessels precontracted with a thromboxane agonist. It is thus possible that the proportional vasodilatory contributions of IKCa/SKCa and BKCa channels may vary in coronary microvessels precontracted by other agonists such as endothelin, norepinephrine, or elevated extracellular calcium.
To detect IKCa/SKCa and BKCa gene products in the human coronary microvasculature, we performed immunoblot and immunohistochemistry to localize channel polypeptides. We found that BKCa was predominantly present in smooth muscle cells. This finding is consistent with the microvessel relaxation data, indicating that BKCa activator-mediated relaxation is endothelium-independent. BKCa, IKCa, and SK3Ca polypeptides were detected by immunoblot of extracts from human coronary microvessels. IKCa mRNA was detected in human atrial appendage. However, cardioplegic ischemia and Rep did not alter expression of BKCa, IKCa, and SK3Ca polypeptides or IKCa mRNA levels, suggesting that short periods of intraoperative hypothermic ischemia and reperfusion may modify the functional state of channel protein activation or intracellular distribution rather than the steady-state levels of protein and (in the case of IKCa) mRNA.
The molecular mechanisms responsible for the CP Reprelated IKCa/SKCa channel dysfunction remain unclear. Ischemia and Rep may induce activation and phosphorylation of several protein and tyrosine kinases, which in turn may phosphorylate IKCa/SKCa channels or their regulatory proteins, resulting in channel inactivation13 either by decreasing open probability or by reduction of channel number at the cell surface. Generation of free radicals and cytokine release during ischemia and Rep may also inhibit KCa channel activity.14
In conclusion, CP followed by Rep after cardiopulmonary bypass causes microvascular dysfunction, likely due in part to impaired function of IKCa/SKCa channels in human coronary microcirculation. These results may provide novel mechanisms by which to explain the presently unavoidable endothelial and smooth muscle microvascular dysfunction after cardiac surgery. Pharmacological administration of KCa openers may represent a novel strategy to improve microvascular protection during cardiac surgery and minimally invasive revascularization.
Acknowledgments
We thank Dr Boris E. Shmukler for discussion and advice.
Sources of Funding
This research project was supported in part by the National Heart, Lung, and Blood Institute HL-69024-05 and HL-46716-15 (F.W.S.) and HL-077765 (S.L.A.). Y.L., R.T.C., and N.R.S. were supported by a cardiovascular research training grant (T32) from the National Institutes of Health (HL076130-02 [F.W.S.]).
Footnotes
Presented at the American Heart Association Scientific Sessions, November 4-7, 2007, Orlando, Fla.
Disclosures
F.W.S. has received grant support from Ikaria and Orthologic and is on the steering committee for Novo Nordisk and on the DSMB for Dyax.
References
- 1.Sellke FW, Boyle EM, Jr, Verrier ED. Endothelial cell injury in cardiovascular surgery the pathophysiology of vasomotor dysfunction. Ann Thorac Surg. 1996;62:1222–1228. doi: 10.1016/0003-4975(96)00538-3. [DOI] [PubMed] [Google Scholar]
- 2.Metias C, Li J, Simons M, Sellke FW. Serotonin-induced coronary contraction increases after blood cardioplegia-reperfusion: role of COX-2 expression. Circulation. 1999;100(suppl II):II328–334. doi: 10.1161/01.cir.100.suppl_2.ii-328. [DOI] [PubMed] [Google Scholar]
- 3.Feng J, Bianchi C, Sandmeyer JL, Li JY, Sellke FW. Molecular indices of apoptosis after intermittent blood and crystalloid cardioplegic ischemia. Circulation. 2005;112(suppl I):I-184–189. doi: 10.1161/CIRCULATIONAHA.104.526160. [DOI] [PubMed] [Google Scholar]
- 4.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology. 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 5.Feng J, Li HL, Rosenkranz ER. Bradykinin protects the rabbit heart after cardioplegia via NO-dependent pathways. Ann Thorac Surg. 2000;70:2119–2124. doi: 10.1016/s0003-4975(00)02148-2. [DOI] [PubMed] [Google Scholar]
- 6.Feng J, Liu RY, Wu GZ, Tang SB. PGE1 reduces cardiac-derived TXA2 in ischemic arrest in isolated working rat heart. Int J Cardiol. 1996;55:265–270. doi: 10.1016/0167-5273(96)02715-5. [DOI] [PubMed] [Google Scholar]
- 7.Feng J, Sellke ME, Ramlawi B, Boodhwani M, Clements R, Li JY, Bianchi C, Sellke FW. Bradykinin induces microvascular preconditioning via opening of calcium-activated potassium channels. Surgery. 2006;140:192–197. doi: 10.1016/j.surg.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Si H, Heyken WT, Wolfle SE, Tysiac M, Schubert R, Grgic I, Vilianovich L, Giebing G, Maier T, Gross V, Bader M, de Wit C, Hoyer J, Kohler R. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- 9.Ambroisine ML, Favre J, Oliviero P, Rodriguez C, Gao J, Thuillez C, Samuel J, Richard LV, Delcayre C. Aldosterone-induced coronary dysfunction in transgenic mice involves the calcium-activated potassium (BKCa) channels of vascular smooth muscle cells. Circulation. 2007;116:2435–2443. doi: 10.1161/CIRCULATIONAHA.107.722009. [DOI] [PubMed] [Google Scholar]
- 10.Weston AH, Absi M, Ward DT, Ohanian J, Dodd RH, Dauban P, Petrel C, Ruat M, Edwards G. Evidence in favor of a calcium-sensing receptor in arterial endothelial cells: studies with calindol and Calhex 231. Circ Res. 2005;19(97):391–398. doi: 10.1161/01.RES.0000178787.59594.a0. [DOI] [PubMed] [Google Scholar]
- 11.Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev. 2005;5:463–472. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- 12.Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper SL, Melvin JE. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J Biol Chem. 2004;279:47681–47687. doi: 10.1074/jbc.M409627200. [DOI] [PubMed] [Google Scholar]
- 13.Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22:505–512. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Terata K, Chai Q, Li H, Kleinman LH, Gutterman DD. Peroxynitrite inhibits Ca2+-activated K+ channel activity in smooth muscle of human coronary arterioles. Circ Res. 2002;9:1070–1076. doi: 10.1161/01.res.0000046003.14031.98. [DOI] [PubMed] [Google Scholar]


