Abstract
In and evaginations of 2D cell sheets are major shape generating processes in animal development. They result from directed movement and intercalation of polarized cells associated with cell shape changes. Work on several bilaterian model organisms has emphasized the role of noncanonical Wnt signaling in cell polarization and movement. However, the molecular processes responsible for generating tissue and body shape in ancestral, prebilaterian animals are unknown. We show that noncanonical Wnt signaling acts in mass tissue movements during bud and tentacle evagination and regeneration in the cnidarian polyp Hydra. The wnt5, wnt8, frizzled2 (fz2), and dishevelled-expressing cell clusters define the positions, where bud and tentacle evaginations are initiated; wnt8, fz2, and dishevelled remain up-regulated in those epithelial cells, undergoing cell shape changes during the entire evagination process. Downstream of wnt and dsh expression, JNK activity is required for the evagination process. Multiple ectopic wnt5, wnt8, fz2, and dishevelled-expressing centers and the subsequent evagination of ectopic tentacles are induced throughout the body column by activation of Wnt/β-Catenin signaling. Our results indicate that integration of axial patterning and tissue morphogenesis by the coordinated action of canonical and noncanonical Wnt pathways was crucial for the evolution of eumetazoan body plans.
Keywords: cnidaria, convergent extension, morphogenesis, actin, JNK
Animal form is mainly elaborated by curvature of initially flat sheets of cells (1, 2). Such processes of in and evagination can be mediated by an interplay of movement, intercalation, and division of cells as well as by changes in cell polarization and shape (3, 4). The molecular events underlying these cellular behaviors have been studied in a number of bilaterian model systems to some detail (5–8). However, it is unknown how curvature is generated at the cellular and molecular level in more ancestral, prebilaterian animals. In consequence, we know little about the establishment of body plans of ancestral eumetazoans.
Hydra belongs to the basal eumetazoan phylum Cnidaria, the sister group to the bilaterian clade, whose members arose >550 million years ago and have since remained relatively unchanged. Hydra polyps are composed of 2 monolayers of epithelial muscle cells, ectoderm and endoderm, separated by a basement membrane called mesoglea. Ectodermal and endodermal epithelial cells, which constitute 2 distinct cell lineages, exhibit planar polarization by 2 contractile muscle processes extending from the basal end of each cell above the mesoglea. These muscle processes are oriented along (ectoderm) or perpendicular (endoderm) to the major oral-aboral body axis. Epithelial cells in the gastric region continuously divide resulting in permanent tissue growth and movement toward the oral and aboral ends of the polyp. Here, terminal differentiation into hypostome-, tentacle-, and foot-specific cells takes place. Continuous growth also provides the tissue for asexual evagination of daughter polyps (buds) and ongoing renewal of tentacles.
In tentacle and bud evagination, epithelial tissue changes its direction of movement and is recruited toward the evaginating centre in a circular pattern (9, 10). The signals initiating this movement are unknown. The first sign of bud evagination in the presumptive budding zone of a big, budless polyp is pallisading, where ectodermal and endodermal epithelial cells enlarge their apical-basal dimension and become narrower in their planar dimension (11). Then, ≈10–20 endodermal epithelial cells decrease their apical-basal length and increase their basal membrane area. This process leads to a curvature of the endodermal layer toward the ectodermal layer (12), followed by the beginning evagination of both layers and the disruption of the regular arrangement of ectodermal and endodermal muscle fibers (13). Evaginating epithelial cells remain within their layer and retain their epithelial configuration. When bud tissue starts to elongate, epithelial cells reestablish regular fiber orientation according to the direction of the new bud axis (13).
It is generally assumed that the evagination of buds and tentacles is a morphallactic process. Evagination and elongation of the bud up to stage 6–7 are based on tissue recruitment from the mother polyp and are not a result of enhanced growth (9, 14–16). Mass tissue movement in the mother polyp toward the evaginating centre is supposed to be the driving force (11, 14). Only in later budding stages, cell proliferation starts to contribute to the maturation of the bud (9, 14). Oriented cell division has been demonstrated to have no role in evagination or elongation of buds and tentacles (17).
In the present work, we characterize a noncanonical Wnt signaling pathway in Hydra, and show that transcriptional activation of this pathway is associated with tissue evagination during the formation of buds and tentacles. Activation of noncanonical Wnt signaling genes in Hydra requires Wnt/β-Catenin signaling, which acts in patterning the major oral-aboral body axis. Our work indicates that canonical and noncanonical Wnt pathways were already separated in ancestral eumetazoans, but acted in a coordinated way in axial patterning and morphogenesis.
Results and Discussion
Evagination in Hydra Is Based on Lateral Intercalation.
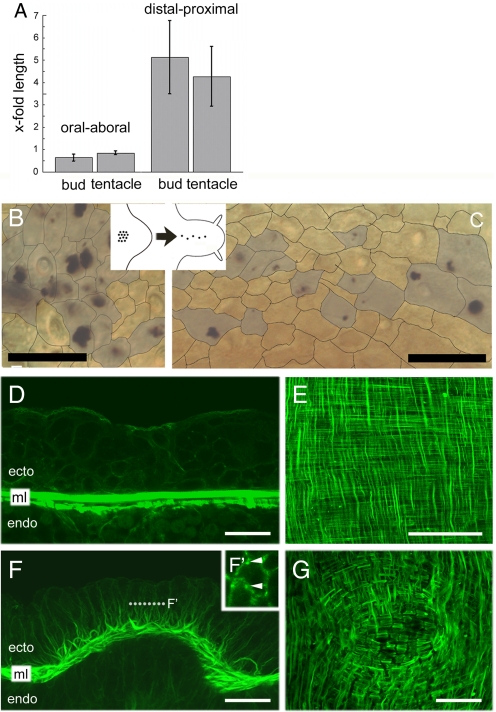
To analyze cell interaction patterns during evagination, we labeled circular clusters of ectodermal epithelial cells with carbon particles in the body column close to the border between the body column and evaginating buds and tentacles (9, 10, 18). Individual clusters were then observed during tissue recruitment and evagination. Quantitative analysis during 3 days of bud formation showed a 30–40% decrease in the oral-aboral length of carbon-labeled clusters, whereas the distal-proximal length had enlarged by ≈5-fold (Fig. 1A). Epithelial cells in elongating buds did not significantly differ in shape from those in unevaginated gastric region [supporting information (SI) Fig. S1]. After 3 days of tentacle evagination, carbon-labeled clusters exhibited a 20% decrease in their oral-aboral length and a 4–5-fold length increase along the distal-proximal axis (Fig. 1A). Ectodermal epithelial cells from the gastric column flatten and increase their planar diameters during terminal differentiation into tentacle-specific cells (Fig. S1). This shape change likely contributes to the elongation of tentacles along the distal-proximal axis and to the smaller length ratio along the oral-aboral axis as compared with evaginated buds. Three days after labeling, carbon-positive ectodermal cells exhibited significantly reduced numbers of labeled neighbors in the evaginated bud (Fig. 1 B and C). Because growth, oriented cell division, and shape changes have no major role, our data indicate that labeled epithelial cells had intercalated laterally with unlabelled cells. Comparable tissue movements based on lateral intercalation have been studied in the development of many bilaterian animals (19, 20). Prominent examples include sea urchin gastrulation (21), evagination of imaginal discs and dorsal closure in insects (7, 8), and convergence and extension in amphibians (3, 19).
Fig. 1.
Lateral cell intercalation and actin reorganization during evagination. (A) Changes in the diameters of initially circular patches of carbon-labeled ectodermal epithelial cells; oral-aboral refers to the mouth-foot axis of the mother polyp, distal-proximal to the perpendicular axis of evaginating buds and tentacles. The length ratio of individual clusters between day 3 and day 0 was calculated for each individual Hydra and plotted as x-fold length. Bars represents mean ± SD from 4 to 14 samples. (B and C) View on the plane of ectodermal epithelium of a bud showing lateral intercalation between carbon-labeled (shaded in gray) and unlabelled cells over 3 days. Dotted lines indicate lateral cell membranes. (D–G) Confocal images of rhodamine-phalloidin-stained Hydra. (D) Sagittal section through the midgastric region of a large, budless polyp; oral is to the right. ml, muscle layers formed by ectodermal and endodermal muscle fibers attached to the mesoglea. (E) View onto the plane of unevaginated epithelium at the level of the mesoglea shows an oral-aboral and circumferential orientation of muscle fibers of ectodermal and endodermal epithelial cells, respectively; oral is to the top. (F) Detachment of muscle processes from the mesoglea, visualized in a sagittal section through a stage 2 bud; oral is to the right. (F′) Optical section in the plane of the ectodermal epithelium shows the association of actin filaments (arrowheads) with lateral cell membranes. Position of the optical section is shown as a dotted line. (G) Planar view onto a stage 2 bud at the level of the mesoglea shows the beginning rearrangement of ectodermal and endodermal muscle processes; oral is to the right. (Scale bars, 50 μm.)
In vertebrates, noncanonical Wnt signaling directs tissue movements by regulating actin filaments and changing cellular polarity through a pathway similar to the Drosophila planar cell polarity (PCP) pathway (5, 22). In Hydra, evagination of buds and tentacles was associated with dramatic reorganization of the actin cytoarchitecture (Fig. 1 D–G). In unevaginated tissue, f-actin was restricted to ectodermal and endodermal muscle fibers in the basal portion of each epithelial cell running adjacent to the mesoglea (ml in Fig. 1D). A view onto the plane of unevaginated epithelium at the level of the mesoglea shows the oral-aboral and circumferential orientation of muscle fibers of ectodermal and endodermal epithelial cells, respectively (Fig. 1E). However, in evaginating tissue, these muscle fibers detached from the mesoglea and stretched out to lateral membrane domains (Fig. 1F). Also, we found large numbers of cytoplasmic actin filaments oriented in apical-basal direction along the lateral cell membranes in ectodermal epithelial cells (Fig. 1F ′). A view onto the newly evaginating bud showed the beginning rearrangement of ectodermal and endodermal muscle processes (Fig. 1G). We propose that these changes in actin filament orientation are essential to facilitate lateral membrane interactions and epithelial cell shape changes during the process of evagination.
Noncanonical Wnt Pathway Genes Are Activated in Evaginating Epithelial Cells.
We cloned Hydra orthologues encoding potential noncanonical Wnt pathway members including wnt5, wnt8, frizzled2 (fz2), strabismus(stbm)/van Gogh, and Rho-kinase (rok) (Fig. 2A and Fig. S2). Hydra dishevelled and JNK orthologues have been reported earlier (23, 24). Amino acid alignments revealed a high degree of structural conservation in the predicted protein–protein interaction domains as compared with higher bilaterians (Fig. 2A, Fig. S3a, and Fig. S4). Critical amino acid residues involved in the action of Dishevelled in Wnt/β-Catenin and PCP signaling (25) are conserved in the Hydra orthologue, suggesting that its capacity to act in canonical and noncanonical Wnt signaling was invented early in metazoan evolution.
Fig. 2.
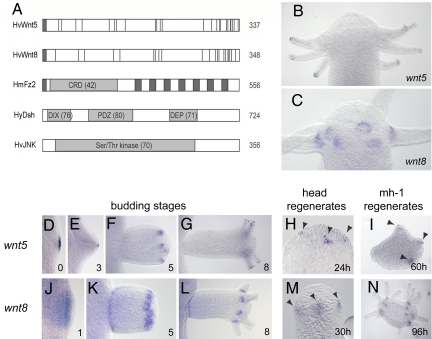
Noncanonical hvwnt5 and hvwnt8 gene activation patterns. (A) Domain structure of the predicted HvWnt5, HvWnt8, HmFz2, HyDsh, HvJNK proteins. HyDsh and HvJNK are taken from refs. 23 and 24. Numbers at the ends of the bars represent the total number of amino acids; numbers in parenthesis show amino acid identity between the Hydra and human orthologues in protein–protein interaction domains. HvWnt5 and HvWnt8 exhibit conserved cysteine residues (vertical lines). (B and C) Expression of hvwnt5 and hvwnt8 in the polyp head. (D–G) Expression dynamics of hvwnt5 in small patches of ectodermal epithelial cells that define the starting point for bud and tentacle evagination. (J–L) Expression of hvwnt8 in the pallisading zone and thereafter in evaginating epithelial cells at the base of tentacles and buds. (H, I, M, and N) Appearance of hvwnt5 and hvwnt8 expression centers during initiation of normal and ectopic tentacles in wild-type and mh-1 regenerates. Numbers define bud stages (9), or regeneration times.
In intact polyps, hvwnt5 was expressed in the distal tip of the tentacles (Fig. 2B), whereas hvwnt8, hmfz2, and hydsh showed strong local up-regulation at the base of the tentacles (Fig. 2C and Fig. S5 a and b); hvstbm and hvrok were expressed uniformly throughout intact polyps and during bud formation and regeneration (Fig. S3). In large, budless polyps, hvwnt5 expression started in a patch of ≈10 ectodermal epithelial cells in the budding zone in the lower body column (Fig. 2D), which is defined by an elevated gene expression level of β-catenin and tcf (23). Directly adjacent to the ectodermal wnt5-expressing cell cluster, endodermal epithelial cells initiated a symmetry-breaking curvature toward the ectodermal layer (Fig. S6) (12); hvwnt5 expression disappeared from the center of the bud protrusion at bud stage 3 (Fig. 2E), when a stable wnt3-expressing head organizer is established (23, 26). In stage 5 buds, patches of 5–10 hvwnt5-expressing ectodermal epithelial cells appeared in the future head region and defined the positions, where new tentacles began to evaginate (Fig. 2F and Fig. S6d). These hvwnt5-positive cells remained in the tips of outgrowing tentacles (Fig. 2G), until they were finally shed off in large mother polyps. This result and the disappearance of hvwnt5 expression from early bud stages shortly after the onset of evagination supports a view that HvWnt5 is primarily involved in the induction of tissue evagination and not in its maintenance. We can currently not decide whether Wnt5 ligands act on the canonical or noncanonical pathway or both pathways, because the hvwnt5-expressing cell clusters occur in regions where both β-catenin and tcf are expressed (23).
The hvwnt8, hmfz2, and hydsh were coexpressed during evagination of buds and tentacles. They were up-regulated in stage 1 buds in a broader domain comprising the entire pallisading area before evagination (Fig. 2J and Fig. S5 c and h). This elevated transcriptional level was retained in evaginating epithelial cells at the base of developing buds until the buds detached. Equivalent expression patterns were found at the sites of tentacle formation (Fig. 2 K and L, and Fig. S5 d, e, i, and j). It should be emphasized that the hytcf gene is not expressed at the base of evaginating buds after bud stage 3 (23). Therefore, we propose that hvwnt8, hmfz2, and hydsh act in evagination through a noncanonical pathway independently of nuclear β-Catenin/Tcf complexes.
On removal of the head from intact polyps, hvwnt5, hvwnt8, hmfz2, and hydsh expressing centers of 5–10 epithelial cells appeared in the regenerating tips after 24–30 h and defined, as in buds, the sites of tentacle evagination (Fig. 2 H and M, and Fig. S5 f and k). When small tissue pieces were excised from the upper gastric region of mh-1 polyps, which exhibit an increased head activation potential (27), ectopic tentacles regenerated at high frequency. As in normal head regenerates, formation of ectopic tentacles was associated with local activation of hvwnt5, hvwnt8, hmfz2, and hydsh genes (Fig. 2 I and N, and Fig. S5 g and l).
The results above indicate that Wnt5, Wnt8, Frizzled2, and Dishevelled are involved in bud and tentacle evagination in Hydra. In the anthozoan cnidarian Nematostella vectensis, wnt5 is also expressed at the base of developing tentacles during metamorphosis of planula larvae (28). Also, previous work has demonstrated a role for Wnt5 in noncanonical Wnt signaling in deuterostomes and insects (29). The most parsimonious explanation of all these data are that canonical and noncanonical Wnt pathways had been separated in the common cnidarian-bilaterian ancestor, and that a function of Wnt5 subfamily members in cell polarization and movement was invented in early eumetazoans and inherited by both cnidarians and bilaterians. Our finding that hvwnt8, a member of a Wnt subfamily strictly acting through β-Catenin/Tcf in vertebrates, is coexpressed with hmfz2 and hydsh in tissue evagination independently of Tcf supports a recently proposed hypothesis that Wnt ligands define the intracellular response depending on the receptor context (30).
JNK Activity and Tissue Evagination in Hydra.
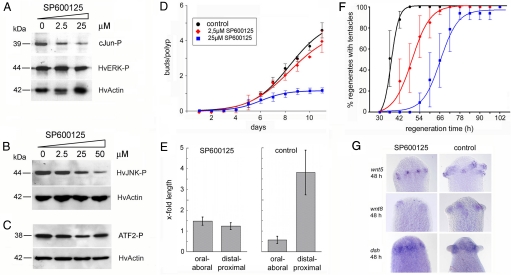
A key player downstream of bilaterian noncanonical Wnt pathways is JNK (5, 6, 31). We have previously described strong expression of the Hydra JNK orthologue during nematocyte differentiation (24). Here, by using RT-PCR and mRNA from interstitial cell-free Hydra, hvJNK mRNA was detected in epithelial cells of head and body column tissue (Fig. S7). Also, Western blottings made from epithelial cells clearly exhibited phosphorylated JNK (Fig. S7). Hydra tissue has been demonstrated to be well accessible for treatment with macromolecules such as peptides, antibodies, and small molecule inhibitors using a DMSO loading protocol (32, 33). To explore morphogenetic effects of JNK in Hydra, we used the JNK-specific inhibitor SP600125 (34). Tissue extracts from polyps treated with 2.5 and 25 μM SP600125 showed strongly reduced capacities to phosphorylate a recombinant c-Jun target (Fig. 3A). Treatment also decreased phosphorylation of Hydra JNK at 25 and 50 μM concentrations (Fig. 3B), which can be explained by JNK autophosphorylating activity as observed in other systems (34). SP600125 did not affect phosphorylation of Hydra ERK (Fig. 3A), nor did it block the ability of tissue extracts to phosphorylate the p38 target ATF2 (Fig. 3C). These results demonstrated that SP600125 acts in the Hydra JNK pathway, whereas the related p38 and ERK pathways showed no response. In vivo, continuous SP600125 treatment of freshly detached and daily fed polyps for >10 days inhibited bud formation in a dose-dependent manner (Fig. 3D). To study the action of JNK on tissue movement, cell clusters at the border between the body column and newly evaginating buds were carbon-labeled, and the polyps then continuously incubated in 25 μM SP600125. Individual clusters were observed over 3 days. Bud evagination was inhibited in SP600125-treated polyps and the initially circular carbon-labeled cell clusters showed no obvious elongation or narrowing (Fig. 3E), suggesting that JNK activity is necessary for correct lateral cell intercalation. We observed a small general increase in cluster length along the oral-aboral and distal-proximal axes (Fig. 3E), which could be due to tissue growth or a minor apical-basal flattening of ectodermal epithelial cells caused by SP600125. DMSO-treated controls showed regular bud evagination, and the carbon-labeled cell clusters exhibited elongation and narrowing equivalent to untreated controls (Fig. 3E).
Fig. 3.
Dose-dependent effects of the JNK inhibitor SP600125 in Hydra. (A) Tissue lysates from SP600125-treated Hydra show a reduced level of c-Jun phosphorylation in a JNK assay kit (Cell Signaling Technology), whereas the level of phosphorylated Hydra ERK is unaffected in these lysates. (B) SP600125 treatment decreases JNK phosphorylation in Hydra as visualized by using an anti-phospho-JNK immunoblot. (C) Tissue lysates from SP600125-treated Hydra show a constant level of ATF2 phosphorylation in a p38 MAP kinase assay kit (Cell Signaling Technology). Anti-actin immunoblotting was the loading control in all Western blottings. (D and F) Continuous SP600125 treatment of freshly detached and daily fed Hydra and head regenerates leads to dose-dependent inhibition of bud formation and tentacle evagination during regeneration. Data points represent the mean ± SEM from 3 independent experiments; 12 polyps per time point were assayed in each experiment. (E) Changes in the diameters of initially circular patches of carbon-labeled ectodermal epithelial cells of SP600125-treated buds compared with DMSO-treated control buds; oral-aboral refers to the mouth-foot axis of the mother polyp, distal-proximal to the perpendicular axis of evaginating buds. Bars represents mean ± SD from 5 to 14 samples. (G) Treatment with 25 μM SP600125 during Hydra head regeneration did not affect expression of hvwnt5, hvwnt8, and hydsh mRNA as revealed by in situ hybridizations.
SP600125 treatment also delayed the formation of tentacles during head regeneration in a dose-dependent manner (Fig. 3F). This assay was used to explore whether SP600125 affected gene expression. In head regenerates, treatment with 25 μM did not delay the formation of noncanonical wnt expression centers (Fig. 3G). Treatment also did not affect activation of the tentacle-specifc gene alx (35). These results suggest that inhibition of JNK activity blocks tentacle formation downstream of canonical and noncanonical Wnt pathway genes. Inhibition could be due either to a block of morphogenetic tissue movements or to changes in the patterning system defining the positional values for evagination along the major body axis. To test whether SP600125 treatment altered positional values, SP600125-treated tissue explants were transplanted laterally into untreated polyps and scored for their ability to induce a secondary body axes. In this experiment, SP600125 treatment did not alter the capacity to induce a second axis as compared with untreated control tissue (Fig. S8). This result supports a view that JNK does not affect positional information, but more likely interferes with morphogenesis and tissue movement similar to its known function in noncanonical Wnt signaling in bilaterians (5, 31). Whether Hydra JNK is directly regulated by Dishevelled is presently unknown. JNK can be activated by various upstream regulators such as cytokines, growth factors, and environmental stress (36). Therefore, we cannot exclude that other effectors than noncanonical Wnt signaling are involved in modulating the morphogenetic action of JNK in Hydra.
Wnt/β-Catenin Signaling Activates Noncanonical Signaling Centers.
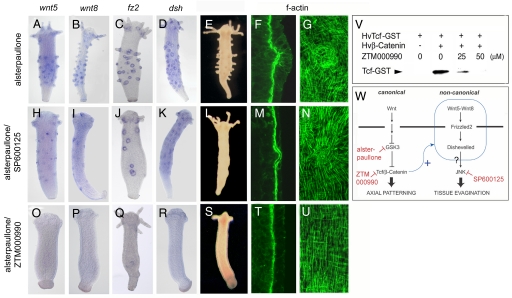
The patterning systems generating tentacles and buds are coupled to positional information along the oral-aboral axis of Hydra. Buds and tentacles emerge at defined axial positions, which are under the control of a head organizer located in the hypostome (37). Canonical Wnt signaling has been demonstrated to be a critical component of the Hydra head organizer and oral organizers in other cnidarians (23, 38–41). Also, the hytcf gene (23), and all Hydra wnt genes found in EST and genome sequence collections except hvwnt5 and hvwnt8, act in the hypostomal head organizer in patterning the oral-aboral body axis. Treatment of budless polyps with 5 μM of the GSK3-specific inhibitor alsterpaullone results in nuclear translocation and over-activation of β-Catenin, which leads to the stepwise formation of ectopic tentacles and ectopic heads throughout the entire body column (33). Fig. 4 A–E shows that multiple hvwnt5, hvwnt8, hmfz2, and hydsh expression centers occurred in alsterpaullone-treated Hydra, and these centers defined the position of ectopic tentacle evagination. Early stages of ectopic tentacle formation exhibited curvature of the muscle layers toward the ectoderm (Fig. 4F) and reorientation of ectodermal and endodermal muscle processes (Fig. 4G) as in normal tissue evagination (Fig. 1 F and G). When treatment with 5 μM alsterpaullone was combined with 25 μM of the JNK inhibitor SP600125, ectopic hvwnt5, hvwnt8, hmfz2, and hydsh expression centers appeared (Fig. 4 H–K). Also, actin stainings showed curvature of the muscle layers (Fig. 4M) and reorientation of muscle processes at the positions of these signaling centers (Fig. 4N), but ectopic tentacle formation was inhibited (Fig. 4L). As in head regeneration, JNK acted on evagination downstream of canonical and noncanonical gene activation potentially by inhibiting lateral cell intercalation, whereas particular aspects of tissue evagination such as the reorientation of muscle processes seemed to be independent of JNK activity.
Fig. 4.
The β-Catenin-induced formation of noncanonical Wnt signaling centers. (A–E) Polyps treated with 5 μM alsterpaullone show multiple ectopic hvwnt5, hvwnt8, hmfz2, and hydsh expressing cell clusters and ectopic tentacle formation. (F and G) Actin filaments show curvature of the muscle layers toward the ectoderm and reorientation of ectodermal and endodermal muscle processes equivalent to normal tissue evagination. (H–L) Cotreatment with 5 μM alsterpaullone/25 μM SP600125 results in formation of multiple ectopic cell clusters expressing noncanonical wnt genes, but not in ectopic tentacle formation. (M and N) Actin staining shows curvature of the muscle layers and beginning reorientation of muscle processes. (O–S) Cotreatment with 5 μM alsterpaullone/25 μM ZTM000990 blocks ectopic activation of noncanonical wnt genes and tentacle formation. (T and U) The arrangement of actin filaments is equivalent to unevaginated tissue. (F, M, and T) Optical sections through the sagittal plane of phalloidin-stained whole mounts. (G, N, and U) Planar view of phalloidin-stained preparations at the level of the mesoglea. Phenotypes of treated polyps were analyzed 60 h after the onset of treatment. (V) ZTM000990 inhibits the interaction between recombinant Hydra β-Catenin and Tcf proteins in a dose-dependent manner in a β-Catenin pulldown assay by using GST-tagged Tcf. (W) Model of the interaction between canonical and noncanonical Wnt signaling in Hydra.
In this assay, we also tested the β-Catenin inhibitor ZTM000990, which had previously been shown to inhibit the interaction between β-Catenin and Tcf in vertebrates (42). ZTM000990 strongly inhibited the interaction of recombinant Hydra β-Catenin and Hydra Tcf proteins in coimmunoprecipitation experiments (Fig. 4V). Also, treatment of intact polyps with 25 μM ZTM000990 led to a substantial decrease in the head activation potential in body column tissue as tested in lateral transplantation assays (Fig. S8). In polyps cotreated with 5 μM alsterpaullone and 25 μM ZTM000990, ectopic hvwnt5, hvwnt8, hmfz2, and hydsh expression centers did not form (Fig. 4 O–R and Fig. S9), and ectopic head structures did not develop (Fig. 4S). The arrangement of epithelial muscle processes was equivalent to normal, unevaginated tissue (Fig. 4 T and U). Together, our results demonstrate that nuclear β-Catenin activates expression of noncanonical pathway genes, which then induce tissue evagination (Fig. 4W). It is presently unclear, whether transcriptional activation is based on direct binding of β-Catenin/Tcf complexes to the corresponding promoters. Because alsterpaullone leads to uniform activation of the tcf gene and uniform nuclear translocation of β-Catenin along the oral-aboral axis (33), additional unknown regulation must restrict the activation of noncanonical genes to spot-like expression centers.
A notable feature of polyps cotreated with 5 μM alsterpaullone and 25 μM ZTM000990 was the formation of over-sized basal discs (Fig. 4 O–S and Fig. S10). Although gain of nuclear β-Catenin activity leads to oralization of the entire oral-aboral axis in Hydra (33), loss of nuclear β-Catenin activity seems to result in aboral fate decision. Because Wnt/β-Catenin signaling specifies head to tail polarity during embryogenesis and regeneration in all major bilaterian clades (43, 44), our observation supports the view that the cnidarian oral-aboral and the bilaterian anterior–posterior body axes are related (45, 46).
Conclusion
According to Hyman, Eumetazoa were the first animals to evolve definite and reproducible form as well as primitive body outgrowths and appendages such as buds and tentacles (47). Here, we show that evagination of such structures in the cnidarian Hydra, a representative of the most basal extant eumetazoans, is mediated by mass tissue movements based on lateral intercalation, and that noncanonical Wnt signaling acts in the initiation and maintenance of evagination. Because polarized tissue movements are regulated by noncanonical Wnt pathways throughout bilaterian model organisms, it appears likely that this mechanism represents one of the significant features of the ancestral tool kit for the development of eumetazoans. Based on the data presented here, we argue that the evolution of a rather complex Wnt signaling network that integrated axial patterning and tissue morphogenesis appeared >550 million years ago and may have been essential for the diversification of eumetazoan body plans.
Materials and Methods
Animals and Tissue Manipulations.
Hydra vulgaris strain Basel and Hydra magnipapillata mutant strain multiheaded-1 were kept in mass culture under daily feeding. Experimental animals were collected 24 h after the last feeding. For head regeneration, polyps were bisected at 80% body length. For regeneration of ectopic head structures, tissue rings of ≈1/10 of the entire body length were cut out of the distal gastric region of mh-1 polyps. All experiments were performed at 18 °C.
Carbon Labeling and Morphometric Measurements.
Clusters of ectodermal epithelial cells were vitally labeled by ink injection as described (18), and analyzed under phase contrast optics. Details are given in SI Materials and Methods.
Actin Staining.
Animals were relaxed in 2% urethane and fixed in 4% paraformaldehyde in hydra medium at 4 °C for 1 h. After 3 10-min washes in PBS and permeabilization in PBS/Triton 0.1%, staining was done with Alexa Fluor 488 phalloidin diluted 1:200 in PBS/Triton 0.1% at room temperature for 1 h. Last, preparations were washed 3 times in PBS for 10 min.
In Situ Hybridization.
For cloning details see SI Materials and Methods. Whole mount in situ hybridization with DIG-labeled RNA probes was performed as described (23).
Inhibitor Treatments.
Experimental details are provided in SI Materials and Methods.
Kinase Assays and Phospho-Specific Antibodies.
Kinase assays were performed according to the manufacturers guidelines. Details are available in SI Materials and Methods.
Immunoprecipitation of Hydra β-Catenin and Tcf.
Immunoprecipitation of recombinant proteins to Ni-NTA Sepharose was done according to standard protocols. Experimental details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank the Mishima Hydra Laboratory for providing mh-1, sf-1, and A-10 mutants; Novartis Pharma AG for providing ZTM000990; Hans Bode, Rob Steele, Dan Rokhsar, and Jarrod Chapman for giving access to unpublished Hydra EST and genome sequences; Peter Ladurner, Charles N. David, and Dirk Meyer for comments on the manuscript; 2 reviewers for their constructive criticism and the late Reinnard Rieger for his conceptual input. This work was supported by grants from the German Science Foundation (T.W.H. and B.H.) and Austrian Science Fund Grants P16685 and P19232 (to B.H.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AM263447, AM279158, EU442372, AM263457, and AM263448).
This article contains supporting information online at www.pnas.org/cgi/content/full/0812847106/DCSupplemental.
References
- 1.Lawrence PA. Last hideout of the unknown? Nature. 2004;429:247. doi: 10.1038/429247a. [DOI] [PubMed] [Google Scholar]
- 2.Gierer A. Physical aspects of tissue evagination and biological form. Quart Rev Biophys. 1977;10:529–593. doi: 10.1017/s0033583500003218. [DOI] [PubMed] [Google Scholar]
- 3.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 4.Lecuit T, Le Goff L. Orchestrating size and shape during morphogenesis. Nature. 2007;450:189–192. doi: 10.1038/nature06304. [DOI] [PubMed] [Google Scholar]
- 5.Wallingford JB, Fraser SE, Harland RM. Convergent extension: The molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 6.Steinmetz PRH, Zelada-Gonzales F, Burgtorf C, Wittbrodt J, Arendt D. Polychaete trunk neuroectoderm converges and extends by mediolateral cell intercalation. Proc Natl Acad Sci USA. 2007;104:2727–2732. doi: 10.1073/pnas.0606589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seifert JRK, Mlodzik M. Frizzled/PCP signaling: A conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 8.Fristrom D, Fristrom JW. The mechanism of evagination of imaginal discs of Drosophila melanogaster: I. General considerations. Dev Biol. 1975;43:1–23. doi: 10.1016/0012-1606(75)90127-x. [DOI] [PubMed] [Google Scholar]
- 9.Otto JJ, Campbell RD. Budding in Hydra attenuata: Bud stages and fate map. J Exp Zool. 1977;200:417–428. doi: 10.1002/jez.1402000311. [DOI] [PubMed] [Google Scholar]
- 10.Dübel S. Cell differentiation in the head of Hydra. Differentiation. 1989;41:99–109. [Google Scholar]
- 11.Graf L, Gierer A. Size, shape and orientation of cells in budding Hydra and regulation of regeneration in cell aggregates. Wilh Roux's Arch. 1980;188:141–151. doi: 10.1007/BF00848806. [DOI] [PubMed] [Google Scholar]
- 12.Gelei J von. Über die Sprossbildung bei Hydra grisea. Arch Entwmech Org. 1925;105:633–645. doi: 10.1007/BF02080918. [DOI] [PubMed] [Google Scholar]
- 13.Otto JJ. Orientation and behavior of epithelial muscle processes during Hydra budding. J Exp Zool. 1977;202:307–322. doi: 10.1002/jez.1402020303. [DOI] [PubMed] [Google Scholar]
- 14.Holstein TW, Hobmayer E, David CN. Pattern of epithelial cell cycling in Hydra. Dev Biol. 1991;148:602–611. doi: 10.1016/0012-1606(91)90277-a. [DOI] [PubMed] [Google Scholar]
- 15.Clarkson SG, Wolpert L. Bud morphogenesis in Hydra. Nature. 1967;214:780–783. doi: 10.1038/214780a0. [DOI] [PubMed] [Google Scholar]
- 16.Campbell RD. Tissue dynamics of steady state growth in Hydra littoralis. I. Patterns of cell division. Dev Biol. 1967;15:487–502. doi: 10.1016/0012-1606(67)90039-5. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu H, Bode PM, Bode HR. Patterns of oriented cell division during steady-state morphogenesis of the body column in Hydra. Dev Dyn. 1995;204:349–357. doi: 10.1002/aja.1002040402. [DOI] [PubMed] [Google Scholar]
- 18.Campbell RD. Vital marking of single cells in developing tissues: India Ink injections to trace tissue movements in Hydra. J Cell Sci. 1973;13:651–661. doi: 10.1242/jcs.13.3.651. [DOI] [PubMed] [Google Scholar]
- 19.Keller R, et al. Mechanisms of convergence and extension by cell intercalation. Phil Trans R Soc Lond B. 2000;355:897–922. doi: 10.1098/rstb.2000.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keller R, Shook D, Skoglund P. The forces that shape embryos: Physical aspects of convergent extension by cell intercalation. Phys Biol. 2008;5 doi: 10.1088/1478-3975/5/1/015007. 015007. [DOI] [PubMed] [Google Scholar]
- 21.Ettensohn CA. Gastrulation in the sea urchin embryo is accompanied by the rearrangement of invaginating epithelial cells. Dev Biol. 1985;112:389–390. doi: 10.1016/0012-1606(85)90410-5. [DOI] [PubMed] [Google Scholar]
- 22.Winter CG, et al. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytosceleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 23.Hobmayer B, et al. Wnt signaling molecules act in axis formation in the diploblastic metazoan Hydra. Nature. 2000;407:186–189. doi: 10.1038/35025063. [DOI] [PubMed] [Google Scholar]
- 24.Philipp I, Holstein TW, Hobmayer B. HvJNK, a Hydra member of the c-Jun NH2-terminal kinase gene family, is expressed during nematocyte differentiation. Gene Expr Patterns. 2005;5:397–402. doi: 10.1016/j.modgep.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Penton A, Wodarz A, Nusse RA. Mutational analysis of dishevelled in Drosophila defines novel domains in the Dishevelled protein as well as novel suppressing alleles of axin. Genetics. 2002;161:747–762. doi: 10.1093/genetics/161.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinhardt B, Broun M, Blitz IL, Bode HR. HyBmp5–8b, a BMP5–8 orthologue, acts during axial patterning and tentacle formation in hydra. Dev Biol. 2004;267:43–59. doi: 10.1016/j.ydbio.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Sugiyama T. Roles of head-activation and head inhibition potentials in pattern formation of Hydra: Analysis of a multi-headed mutant strain. Amer Zool. 1982;22:27–34. [Google Scholar]
- 28.Kusserow A, et al. Unexpected complexity of the Wnt gene family in a sea anemone. Nature. 2005;433:156–160. doi: 10.1038/nature03158. [DOI] [PubMed] [Google Scholar]
- 29.Gordon MD, Nusse R. Wnt signaling: Multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 30.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits β-Catenin-Tcf signaling depending on receptor context. PLOS Biol. 2006;4:570–582. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamanaka H, et al. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraser SE, Green CR, Bode HR, Gilula NB. Selective disruption of gap junctional communication interferes with a patterning process in Hydra. Science. 1987;237:49–55. doi: 10.1126/science.3037697. [DOI] [PubMed] [Google Scholar]
- 33.Broun M, Gee L, Reinhardt B, Bode HR. Formation of the head organizer in hydra involves the canonical Wnt pathway. Development. 2005;132:2907–2916. doi: 10.1242/dev.01848. [DOI] [PubMed] [Google Scholar]
- 34.Bennett BL, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith KM, Gee L, Bode HR. HyAlx, an aristaless-related gene, is involved in tentacle formation in hydra. Development. 2000;127:4743–4752. doi: 10.1242/dev.127.22.4743. [DOI] [PubMed] [Google Scholar]
- 36.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Op Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Meinhardt H. A model for pattern formation of hypostome, tentacles, and foot in Hydra: How to form structures close to each other, how to form them at a distance. Dev Biol. 1993;157:321–333. doi: 10.1006/dbio.1993.1138. [DOI] [PubMed] [Google Scholar]
- 38.Lee PN, Pang K, Matus DQ, Martindale MQ. A Wnt of things to come: Evolution of Wnt signaling and polarity in cnidarians. Sem Cell Dev Biol. 2006;17:157–167. doi: 10.1016/j.semcdb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Plickert G, Jacoby V, Frank U, Müller WA, Mokady O. Wnt signaling in hydroid development: Formation of the primary body axis in embryogenesis and its subsequent patterning. Dev Biol. 2006;298:368–378. doi: 10.1016/j.ydbio.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 40.Guder C, et al. The Wnt code: Enidarians signal the way. Oncogene. 2006;25:7450–7460. doi: 10.1038/sj.onc.1210052. [DOI] [PubMed] [Google Scholar]
- 41.Momose T, Derelle R, Houliston E. A maternally localized Wnt ligand required for axial patterning in the cnidarian Clytia hemisphaerica. Development. 2008;135:2105–2113. doi: 10.1242/dev.021543. [DOI] [PubMed] [Google Scholar]
- 42.Lepourcelet M, et al. Small-molecule antagonists of the oncogenic Tcf/β-catenin protein complex. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka EM, Weidinger G. Heads or tails: Can Wnt tell which one is up? Nat Cell Biol. 2008;10:122–124. doi: 10.1038/ncb0208-122. [DOI] [PubMed] [Google Scholar]
- 44.Petersen CP, Reddien PW. Smed-βcatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science. 2008;319:327–330. doi: 10.1126/science.1149943. [DOI] [PubMed] [Google Scholar]
- 45.Meinhardt H. Models of biological pattern formation: From elementary steps to the organization of embryonic axes. Curr Top Dev Biol. 2008;81:1–63. doi: 10.1016/S0070-2153(07)81001-5. [DOI] [PubMed] [Google Scholar]
- 46.Martindale MQ. The evolution of metazoan axial properties. Nat Rev Genet. 2005;6:917–927. doi: 10.1038/nrg1725. [DOI] [PubMed] [Google Scholar]
- 47.Hyman LH. The Invertebrates: Protozoa through Ctenophora. Vol 1. New York and London: McGraw–Hill; 1940. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.