Abstract
A novel and integral approach to the understanding of human neurodegenerative diseases (HNDDs) and cancer based upon the disruption of the intracellular dynamics of the hydrogen ion (H+) and its physiopathology, is advanced. From an etiopathological perspective, the activity and/or deficiency of different growth factors (GFs) in these pathologies are studied, and their relationships to intracellular acid-base homeostasis reviewed. Growth and trophic factor withdrawal in HNDDs indicate the need to further investigate the potential utilization of certain GFs in the treatment of Alzheimer disease and other neurodegenerative diseases. Platelet abnormalities and the therapeutic potential of platelet-derived growth factors in these pathologies, either through platelet transfusions or other clinical methods, are considered. Finally, the etiopathogenic mechanisms of apoptosis and antiapoptosis in HNDDs and cancer are viewed as opposite biochemical and biological disorders of cellular acid-base balance and their secondary effects on intracellular signaling pathways and aberrant cell metabolism are considered in the light of the both the seminal and most recent data available. The “trophic factor withdrawal syndrome” is described for the first time in English-speaking medical literature, as well as a Darwinian-like interpretation of cellular behavior related to specific and nonspecific aspects of cell biology.
Keywords: neurodegenerative diseases and growth factors, Alzheimer’s Disease, human neurodegenerative diseases, cancer, intracellular acid-base homeostasis, apoptosis, antiapoptosis, etiopathogenesis and treatment
“We can only cure what we can understand first”
Otto Warburg
Introduction
Modern medicine faces an almost total lack of success both in the prevention and treatment of human neurodegenerative diseases (HNDDs), like Alzheimer’s disease (AD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), Hungtington’s disease (HD), retinitis pigmentosa (RP), and others. Symptomatic treatments are available for other processes, such as Parkinson’s disease (PD). The main problem seems to lie in the fact that it has not been so far possible to prevent, stop or reverse the premature and spontaneous process of progressive cell death or programmed cell death (PCD) via apoptosis or related mechanisms in HNDDs (paraptosis, autophagy, etc) (Harguindey et al 2007). New conceptual approaches appear most necessary in order to discover novel therapies for the prevention and treatment of these processes (Sperandio et al 2000; Lagadic-Gossman et al 2004; Bröcker et al 2005).
Attempts to advance an integral and unifying hypothesis on the etiopathogenesis of HNDDs have been proposed for decades. This has mainly focused on the lack in growth factor (GF) stimulation, mainly on deficiencies in nerve growth factor (NGF) (Appel 1983; Hefti 1983; Schultze-Herbrüggen et al 2008). PCD in HNDDs, either apoptosis-mediated or not, can be induced either by: A) Depletion, spontaneous or induced, of different GFs, either from neural origin or otherwise (trophic factor withdrawal, or TFW) (Rebollo et al 1995; Khaled et al 2001; Tatton et al 2002; Resnick et al 2004), and/or B) The activation or overexpression of mechanisms that stimulate PCD (ie, caspases, etc.), which can also be secondary to TFW-mediated mechanisms (Sperandio et al 2000; Tatton et al 2002; Liu et al 2004; Lavrik et al 2005;Riedl et al 2005). While acting upon mechanism B is to a great deal still controversial, mechanism A can be to a certain extent counteracted through the therapeutic utilization of a wide array of trophic and/or human GFs in order to control and/or even suppress pathological cell death programs (PCDP). The utilization of GFs, besides preventing cell death, is used to stimulate cell growth and proliferation in a wide array of clinical settings in modern clinical practice (Table 1) (Varon 1981; Barbin 1987; Khaled et al 2001; Tatton et al 2002; Anitua et al 2004, 2007b; Anitua 2005). Recently, several plasma signaling proteins have been found to be significantly deficient even in preclinical and asymptomatic stages of AD, revealing a decrease in the circulation of different hematopoiesis-dependent GFs, like platelet-derived growth factor (PDGF), transforming growth factor-1 (TGF-1), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), etc., in this disease (Ray et al 2007). Since several of them, like PDGF, TGF-1, EGF, VEGF, bFGF, HGF, bone morphogenetic protein-2 (BMP-2), -4, -6, connective tissue growth factor (CTGF), etc. (Table 2) (Anitua et al 2004; Kut et al 2007), are contained in high concentrations in young functional platelets and/or leukocytes, it becomes surprising that while highly sophisticated methods are being investigated to induce and increase the activity of a cohort of growth factors, either from neural origin or not, in different models of HNDDs (Storkebaum et al 2004; Vende Velde et al 2005; Wagner et al 2006), at least to our knowledge no previous mention has been made on the possibility of using young and healthy platelets and, perhaps to a lesser degree, leukocyte transfusions, their most widespread natural source, in the treatment of certain HNDDs. The apparent lack of specificity in the stimulation of tissue regeneration by a cohort of several trophic factors and other cytokines contained in young and healthy platelets (Table 2) (Anitua et al 2004, 2005, 2006, 2007b, 2008a) led us to introduce the concept of a general “trophic factor withdrawal syndrome” in recent publications of our group (Anitua et al 2007b; Harguindey et al 2007, 2008). Finally, since epigenetic and genetic factors can primarily influence the susceptibility to neurodegeneration, the implementation of personalized treatments based on pharmacogenetic principles are also acquiring an increasing importance in order to optimize the so far limited therapeutic resources nowadays available in these complex neurodegenerative disorders (Cacabelos 2005; Cacabelos et al 2006).
Table 1.
Use of platelet-based technology in modern clinical medicine
| Objective | Results | References |
|---|---|---|
| Gastric ulcer healing | Treatment with platelet-rich plasma significantly accelerated ulcer healing | Wallace et al 2006 |
| Chronic ulcer treatment | At 8 weeks, the mean percentage of surface healed in the PRGF group was significantly higher than in the control group (73% versus 21.4%) (P < 0.05) | Anitua et al 2007 |
| Bone regeneration in sinus floor augmentation | Bone densitometric values and bone amount were higher in sites treated with PRP | Consolo et al 2007 |
| Nerve regeneration | The best results were obtained when the nerves were sutured and PRP was added to the sutures, showing a neurotrophic effect | Farrag et al 2007 |
| Repair of chronic elbow tendinosis | At final follow-up (mean 25.6 months), PRP patients reported 93% reduction in pain compared with before the treatment | Mishra et al 2007 |
| Repair of anterior cruciate ligament | Collagen-PRP mixture resulted in significant improvements in load at yield, maximum load, and linear stiffness at 4 weeks post-treatment | Murray et al 2007 |
| Expansion of mesenchymal stem cells (MSCs) | MSCs maintained their osteogenic, chondrogenic, and adipogenic differentiation properties and their immuno-suppressive activity | Doucet et al 2005 |
| Dry eye symptoms | Symptoms improved significantly in 89% of the 18 patients. Improvement on lachrymal meniscus and conjunctival hyperemia were observed | Alio et al 2007 |
Abbreviations: PRGF, preparation rich in growth factors; PRP, platelet-rich plasma; MSCs, mesenchymal stem cells. (For further details see Anitua et al 2007b).
Table 2.
Growth factors contained in normal platelets
| Growth factors | Function |
|---|---|
| PDGF, TGF-b1 and-2, EGF, VEGF (A y C) | Chemotaxis, cell proliferation |
| bFGF; HGF, BMP-2, -4, -6, CTGF | Cell trophism, coagulation, angiogenesis, etc. |
Abbreviations: PDGF, platelet-derived growth factors; TGF-b1 and -2, transforming growth factor; EGF, epidermal growth factor; VEGF, vasoendothelial growth factor; bFGF, fibroblastic growth factor; HGF, hepatocyte growth factor; BMP-2, -4, -6, bone morphogenetic protein; CTGF, connective tissue growth factor. (For further details see Anitua et al 2004).
The trophic factor withdrawal syndrome (TFWS) in the etiopathogenesis of human neurodegenerative diseases (HNDDs)
Large scale apoptosis induced by trophic hormone withdrawal was first reported in hormone-dependant tumors (Kerr et al 1972). Microenvironmental depletion or functional inability of a wide array of growth factors whose stimulus is necessary for cell survival, such as insulin-like growth factor-1 (IGF-1), PDGF, VEGF, EGF, FGF, and neurotrophins like NGF or others (Barbin 1987;Desmuck et al 1997; Guo et al 1997;Counts et al 2000; Estévez et al 2000; Poser et al 2003; Chao et al 2006), apart from cytokines like interleukin-2 (IL-2), IL-3, IL-7 (Khaled et al 2001), a downregulated Bcl-2 (Shacka et al 2005), or an upregulated p53, etc., is sufficient to induce massive neural cell apoptosis (Rebollo et al 1995; Tatton et al 2003) (Table 3). In the central nervous system (CNS), a functional deficiency of certain growth factors in the microenvironment can be secondary to: A) a systemic failure of growth factor production, this is, lack of availability; B) inadequate activity of certain trophic molecules in the cell membranes of target tissues of neural origin; C) transmembrane receptor down-regulation or binding deficiency, and/or D) abnormalities in the intracellular signaling cascade. An altered function of any of these circumstances may have a key etiopathogenic role in the pathological cell death of different HNDDs (Politi et al 2001; Tatton et al 2002; Alvarez et al 2007; Corzo et al 2007; Ray et al 2007). Apoptotic or parapototic cell suicide can be initiated by removal of different growth factors, while trophic factor withdrawal is a key factor in inducing an apparently sine qua non intracellular acidification that seems necessary in mediating the different cell death programs in both neural cells and cancer cells alike (Burns et al 1983; Vincent et al 1999). Thus, the systematic clinical utilization of different GFs, alone or in combination, opens new possibilities in the prevention and treatment of HNDDs (Bröker et al 2005). This review is an exercise in both translational and transversal research between neurology, hematology, and oncology research in order to propose new pathways towards a better understanding of the pathogenesis of HNDDs to discover new therapeutic approaches through the clinical utilization of GFs and other similar measures in both the prevention and treatment of these processes.
Table 3.
Opposed trends of apoptotic-related parameters and therapeutic directions in human neurodegenerative diseases (HNDDs) and cancer
| HNDDs (Alzheimer’s disease, etc) | Cancer | |
|---|---|---|
| Factors involved: | ||
| Apoptosis | ↑ (pro-apoptosis, pathological) | ↓ (antiapoptosis, pathological) |
| Trophic factors | ↓ | ↑ |
| Caspase activity | ↑ | ↓ |
| Bax activity | ↑ | ↓ |
| p53 activity | ↑ | ↓ (or mutated) |
| Bcl-2 | ↓ | ↑ |
| Na+/H+ exchanger | ↓(?) | ↑ |
| pHi | ↓ | ↑ |
| Oxidative stress | ↑ | ↓ |
| Therapeutic directions | ||
| GFs | To stimulate (PDGF, VEGF, NGF) | To inhibit (IDN-5370, z-VAD-fmk) |
| Caspases | To inhibit | To stimulate |
| c-Jun protein kinase | To inhibit | To stimulate |
| Apoptosis | To inhibit (antiapoptosis) | To stimulate (pro-apoptosis) |
| Antioxidants | Indicated | Contraindicated (?) |
| Na+/H+ Antiporter | To stimulate (Bcl-2, cloroquine, imidazole, GFs (G-CSF, PDGFs, etc.): Platelet transfusions | To inhibit (amiloride, Il-2, LAK, lovastatin, squalamine, staurosporine, etc.) |
Note: For further details see Harguindey et al 2007.
Mediating mechanisms in the pathogenesis of HNDDs. The universal role of Na+/H+ exchange and intracellular pH in the action of growth and trophic factors. Cellular Darwinism and the law of specificity and nonspecificity in biology
The role of Na+/H+ exchange and intracellular pH as universal mediators and in the activation and inhibition of growth and trophic factors in HNNDs and cancer
Some of the main areas of cancer research, from etiopathogenesis to treatment at both the basic and clinical levels (malignant transformation, growth and proliferation, cell migration, angiogenesis, the metastatic process, multiple drug resistance to chemotherapy (MDR), oncogene expression, growth factor activity, tumor glycolysis, cell cycling, DNA synthesis, apoptosis, etc.), have been recently integrated under an Unitarian perspective based upon the dynamics of the hydrogen ion and Na+/H+ antiporter (Harguindey et al 2005). Similar aspects of cancer have lead some researchers to consider that “cancer Achilles’s heel” can hide within the frame of H+ dynamics and its secondary effects on cellular metabolism (J. Poúyssegür, personal communication). Furthermore, the most active proton pump inhibitors (PPI) of the amiloride series and beyond, increasingly appear as cancer “magic bullet” because their selective potential to induce a selective metabolic collapse and cell death, apoptosis-mediated or otherwise, of cancer cells and tissues irrespective of origin and cell lineage (Rich et al 2000; Torigoe et al 2002; Izumi et al 2003; Harguindey at al 2005; Poüyssegúr et al 2006).
While human and trophic growth factors have different origins and specific target cells, their mediating mechanisms of action at the level of the cell membrane and intracellular signaling show common final pathways in all kinds of cells (Harguindey et al 2008). First, pro- and antiapoptotic intracellular signaling pathways are already known to a considerable extent, and maps of those intracellular pathways as possible therapeutic targets in different pathologies are already available (Hanahan 2000; Reed 2004; Lavrik et al 2005). Secondly, we have learned from experimental and translational oncology research that a key feature mediating the molecular mechanisms of action in the stimulation by the majority of, if not all, growth factors, is an increase in the rate of exchange of Na+ and H+. An over-expressed extrusion of H+ ions, which is mainly mediated by the membrane-bound Na+-H+ exchanger, induces an intracellular (IC) alkalinization and the disruption of both the IC–extracellular (EC) homeostasis of the cell (Burns et al 1983; Moolenar et al 1983; Harguindey et al 1995, 2005; Reshkin et al 2000, 2003; Khaled et al 2001;Harguindey 2003; Orive et al 2003; Cardone et al 2005; Di Sario et al 2007). This phenomenon is recognized as a key and specific feature not only in malignant cell transformation but also in the growth and invasion of cancer cells of all lineages and origins, as well as in the activation the metastatic process, through the creation of an abnormal IC–EC proton gradient (the so called “H+-gradient reversal”) (Belaud-Rotureau et al 2000; Reshkin et al 2000; Khaled et al 2001; Cardone et al 2005; Harguindey et al 2005; Poüysségur et al 2006).
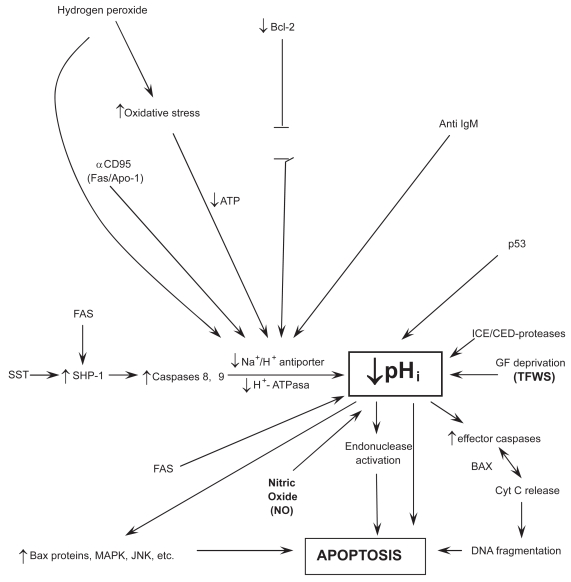
The NHE, mainly the NHE-1 isoform, plays an essential and pivotal role in the IC signal transduction pathways of a multiplicity of different hormones, membrane signals, as well as trophic and growth factors (for a review, see Harguindey et al 2005). The stimulation of this final common pathway by all the different kinds of mitogenic factors results in the activation of the Na+/H+ antiporter and its secondary induction of an elevation of intracellular pH (Burns et al 1983; Di Sario et al 2001; Cardone et al 2005; Harguindey et al 2005). This phenomenon has been known for some time to represent an early and essential step in mediating cell proliferation and DNA synthesis (Burns et al 1983; Moolenar et al 1983; L’ Allemain et al 1984; Grinstein et al 1989; Harguindey et al 1995; Reshkin et al 2000). Indeed, trophic and growth factors with clear-cut antiapoptotic activity always stimulate the NHE-1 and increase pHi, this being a fundamental homeostatic mechanism that protects cells against a pathological fall in pHi (Burns et al 1983; Di Sario et al 2007; Harguindey et al 2007). In this way, cell death programs, either through apoptosis and/or parallel mechanisms, can be counteracted (Table 4) (Rich et al 2000; Khaled et al 2001; Reshkin et al 2003; Harguindey et al 2005). Among these antiapoptotic–antiacidification factors are the Bcl-2 family, Bax deletion, certain oncogenes, a dysfunctional p53 suppressor gene, etc. (DiGiammarino et al 2000; Marches et al 2001;Thangaraju et al 2001). The main bulk of available literature points to the conclusion that cellular neuroprotection is also mediated by a pHi-sustaining effect on IC metabolism and homeostasis of an acid-base nature (therapeutic antiapoptosis) (Vincent et al 1999), in a similar manner that the survival mechanisms described for cancer antiapoptosis, mainly in multiple drug resistance to chemotherapy (MDR) (pathological antiapoptosis) (Hamilton et al 1993; Roepe et al 1993; Gottlieb et al 1996; Vincent et al 1999;Simon 2000; Harguindey et al 2005). Most significantly, in trophic under-stimulation and/or in growth factor deprivation the cell signals to detect IC acidification appear to be the same ones as for spontaneous apoptosis (Hamilton et al 1993; Roepe et al 1993; Gottlieb et al 1996; Furlong et al 1997; Famulski et al 1999; Park et al 1999; Thangaraju et al 1999; Belaud-Rotureau et al 2000; Matsuyama et al 2000; Rich et al 2000; Di Sario et al 2001; Marches et al 2001; Simon 2001; Lagadic-Gossmann et al 2004; Di Sario 2007) (Figure 1). In this line, antiapoptotic Bcl-2 or a deleted pro-apoptotic Bax protein have been shown to directly prevent cellular acidification and thus, cellular injury (Park et al 1999; Thangaraju et al 1999; Belaud-Rotureau et al 2000; Brodski et al 2002; Tafani et al 2003). This IC homeostatic acid-base approach opens new possibilities in the strategy to implement new preventive and/or therapeutic measures in HNDDs, hitting as a key and pivotal therapeutic target pHi homeostasis with any drug or biological compound that, alone or in combination (imidazole, chloroquine, neurotrophic or other growth factors, Bcl-2, cytokines, oncogenes, cell proteins, gene products, platelet transfusions) that activate the NHE-1 and/or decrease nitric oxide (NO) production, thus sustaining pHi within normal limits and allowing IC proton homeostasis and cell function as a whole to be kept within a physiological range (Table 3).
Table 4.
pHi, apoptosis and antiapoptosis in HNDDs and cancer
| HNDDs | Cancer | |
|---|---|---|
| ↑pHi | Therapeutic antiapoptosis | Pathological antiapoptosis |
| ↓pHi | Pathological apoptosis | Therapeutic apoptosis |
Figure 1.
Relationships among intracellular signaling factors, pHi, and poptosis.
Abbreviations:↑, stimulation;↓, inhibition; ST, somatostatin; SMP-1, tyrosinephospatase; MDR, multiple drug resistance; GFs, growth factors; Cyt C, cytochrome C; NO, nitric oxide; TFWS, Trophic factor withdrawal syndrome.
Notes: For further details see Rideout et al 2001; Reed 2002, 2004; Waldeimer 2003; Waldeimer et al 2004;Broker et al 2005; Harguindey et al 2007.
Parallel processes of the pathological apoptosis in HNDDs and the overcoming of the resistance to apoptosis (pathological antiapoptosis) in cancer therapeutics
What spontaneously takes place in HNDDs (pathological apoptosis) (Raina et al 2003), is the same phenomenon that cancer therapeutics attempt to selectively induce through a wide array of different approaches and methods (therapeutic apoptosis) (Figure 1). Similarly, it has been shown that one of the main mechanisms by which cancer cells are resistant to chemotherapeutic treatment (MDR) is secondary to the fact that most cancer cells posses concerted defensive mechanisms that allow them to maintain their intracellular acid-base situation well above normal levels through all kinds of damaging microenvironmental circumstances. This has been called “the neostrategy of cancer cells and tissues” (Harguindey et al 2005). Otherwise, a successful treatment in cancer is mediated by the activation of a low pHi-mediated apoptotic chain reaction cascade ending in cancer cell death (therapeutic apoptosis) (Famulski et al 1999; Park et al 1999; Williams et al 1999; Harguindey et al 2005; Di Sario et al 2007; Letai 2008). Some of these mechanisms are mediated by executionary caspases or by the therapeutic activation of a JNK apoptotic pathway in cases of successful chemotherapy (Perona et al 2007). Most interestingly, a parallel phenomenon, namely, an oxidative stress-mediated activation of the c-Jun -N-terminal kinase, takes place in AD (see Figure 1). Furthermore, JNK activation is also mediated by directly inducing a low pHi and by amiloride treatment (Lachapelle et al 2007). Other mechanisms of successful cancer treatment include overcoming blockades to cancer cell apoptosis by inhibiting the Bcl-2 antiapoptotic family, whose activity is not surprising that is mediated by IC alkalinization and inhibited by cell acidification (Reynolds et al 1996; Yang et al 1997; Ishaque et al 1998;Shimuzu et al 1998; Zanke et al 1998;Zhiuza et al 1998; Thangaraju et al 1999; Matsuyama et al 2000; Takashaki et al 2004; Waibel et al 2007). Resistance to chemotherapy (MDR) can also be achieved by similar pH-related methods (Roepe et al 2001; Harguindey et al 2005). A large deal of accumulated data in this area indicates that the study of Bcl-2 as an antiapototic agent seems secondary to an alkalinizing pHi effect, another feature that can be integrated within the H+-mediated model (Reynolds et al 1996; Thangaraju et al 1999; Matsuyama et al 2000). This makes it highly surprising that even the more recent reviews on cell death mechanisms and resistance to them, either in cancer and HNDDs, either completely ignore the dynamics of the hydrogen ion in these situations or they are granted a meaningless secondary role at the most (Raina et al 2003; Waldeimer 2003; Reed 2004; Szakátz et al 2006). Even more, malignant cell apoptosis can be selectively induced by proton pump inhibitors (PPI) like HMA (5-(N, N-hexamethylene)-amiloride) (Rich et al 2000), or acid-mediated stimulation of p53 (Williams et al 1999) (Table 4 and Figure 1).
Multiple drug resistance to different chemotherapeutic agents (MDR) can be overcome by HMA or other PPI of the amiloride series, another low pHi-mediated phenomena (Roepe et al 2001; Harguindey et al 2005; Miraglia et al 2005, Di Sario et al 2007). In summary, all the available evidence indicates that the more reductionistic and fragmented model represented by the detailed study of any individual factors as well as the intricate abnormalities of the intracellular signaling pathways can be inserted within a more comprehensive and integral homeostatic paradigm of an acid-base nature as represented by the IC–EC dynamics of the hydrogen ion (H+). This acquires further significance when attention is paid to the fact that the dynamics of proton movements in and out of the cell and its transmembrane gradients seem to be the ultimately responsible factor and mechanism for the apoptosis-antiapoptosis machinery in both HNDDs and cancer (Gottlieb et al 1996; Furlong et al 1997; Famulski et al 1999; Park et al 1999; Vincent el al 1999; Williams et al 1999; Harguindey et al 2000, 2005, 2007; Matsuyama et al 2000; Raina et al 2003; Tafani et al 2003; Waldeimer 2003; Lagadic-Gossmann et al 2004; Reed 2004; Letai 2008).
In spite that cancer cells seem able to evade apoptosis by trophic factor deprivation in many cases (Tang et al 1998; Thompson et al 2005), antihormonal and antigrowth factors are being tried in different therapeutic attempts in the oncology setting (Shegal et al 1994; Taetle et al 1994). These and other parallel considerations relating H+ dynamics and GF activity allow to place the biochemistry and molecular biology of HNDDs and cancer at both ends of an apoptotic-antiapoptotic metabolic spectrum (Harguindey et al 1992b, 2007) (Tables 3 and 4). The same unitarian perspective that has made possible to integrate the main areas of basic and clinical oncology research (Harguindey 1992a; Harguindey et al 1995, 2005), now allows to advance another new concept: that what is pathological and damaging for some diseases (the spontaneous apoptosis of HNDDs) can be therapeutic and beneficial for their metabolic and homeostatic opposites (the antiapoptosis of malignancy) (Table 4). Thus, a primordial approach to IC homeostasis may open new and untrodden ways towards entirely new therapeutic approaches, aiming at the Na+/H+ antiporter, other membrane proton pumps and NO production and control as primordial therapeutic targets, either in the induction of selective low pH-mediated apoptosis in cancer treatment through the utilization of proton transport inhibitors (therapeutic apoptosis) or, in the opposite direction, counteracting IC acidification in HNDDs (therapeutic antiapoptosis) (Troy et al 1996; Rich et al 2000; Torigoe et al 2002; Izumi et al 2003; Harguindey et al 2005;Poüysségur et al 2007).
Terminal pathogenesis of apoptotic or para-apoptotic cell death in HNDDs. NO, oxidative stress, and cellular pHi
When deprived of trophic factors (TFWS), motor neurons undergo a programmed cell death program, apparently through nitric oxide-dependent apoptosis (Hirakura et al 1999; Vincent et al 1999; Estévez et al 2002; Wilkins et al 2004; Estévez et al 2006), and/or superoxide production (Lieberthal et al 1998) and/or caspase activation (Troy et al 1996; McCarthy et al 1997; Chan et al 1999; Deshmukh et al 2000; Choi et al 2002). The induction of NO-related mechanisms share a common final pathway that involves nitric oxide synthase (NOS) and peroxynitrite formation. NO has been linked, partly because to its oxidative role, to the development of several neurodegenerative disorders, seemingly by activating a pHi-related apoptotic cascade (Corzo et al 2007). Furthermore, the formation of NO by astrocytes has been suggested to contribute to the neurodegenerative process, while NO production is significantly elevated in platelets from AD patients (Fernandez-Vizarra et al 2004). This very same mechanism proves to be of value in inducing apoptosis, via necrosis, in neoplastic cells (Rigas et al 2008) (Table 3).
The seminal work of Vincent and colleagues (1999) in neurons has shown that lowering pHi from 7.36 to 7.09/7.00 through exposure to NO sets in motion a programmed cell death (PCDP) program, increasing DNA fragmentation and decreasing neuronal survival (low pHi-mediated metabolic collapse). This phenomenon is induced by the activation of three low pHi-dependent endonucleases responsible for neuronal injury (Figure 1). Furthermore, the formation of pHi-lowering pro-apoptotic NO by astrocytes suggests a potential for NO inhibitors in the treatment of this disease, most likely through the stabilization of IC acid-base homeostasis and the prevention of a deadly drop in pHi (Harguindey et al 2003; Arianna et al 2007) (Figure 1). Other compounds with different anti-NO properties like superoxide dismutase, minocycline, NGF, etc, have also shown neuroprotective effects (Kirkland et al 2003; Wilkins et al 2004; Estévez et al 2006). The stimulation of oxidative stress by NO has been involved in the pathogenesis of dementia (Corzo et al 2007), while oxidative stress and/or reactive oxygen species (ROS) have been widely considered as a key factor in neuronal degradation (Harguindey et al 2007). Superoxide (SO) formation induced by growth factor deprivation induces apoptosis (Lieberthal et al 1998). This phenomenon is further increased after reacting SO with NO to form peroxynitrite (Estévez et al 2006). In contrast, low or physiological concentrations of NO prevents apoptosis (Choi et al 2002). In summary, the dynamics of the killing mechanism (see also Figure 1), are:
The study of the molecular biology of cancer cells has shown that the inhibition of the electroneutral membrane exchanger NHE-1 induces intracellular acidification by entrapping hydrogen ions within the cell (Li et al 1995; Rebollo et al 1995;Harguindey et al 2003). A severe intracellular acidosis below a certain threshold (pHi:±6.8) is an essential factor in setting up a cascade-like chain reaction ending up in the metabolic collapse preceding apoptosis, either in cancer cells (therapeutic apoptosis) (Rich et al 2000, Di Sario et al 2007) or in dying cells of neural origin (pathological or degenerative apoptosis) (Figure 1) (Hamilton et al 1993; Roepe et al 1993; Gottlieb et al 1996; Harguindey et al 1995; Li et al 1995; Rebollo et al 1995; Park et al 1999, 2005; Vincent et al 1999; Thangaraju et al 1999; Matsuyama et al 2000; Rich et al 2000; Di Sario et al 2001; Simon 2001; Wahl et al 2002; Lagadic-Gossmann et al 2004).
Cellular Darwinism. The law of specificity and nonspecificity in biology
From the point of view of biological evolution, the simplest way (and to follow Darwinian rules nature always chooses the simplest possible way in order to solve its biological problems and preserve the stability and continuation of life) is that a wide array of trophic, growth factors and hormones have different kinds of target cells with specific receptors for different cell lineages. This represents a qualitative and specific variation. At the same time, cellular physiology has a very small number of transduction mechanisms from the membrane to the interior of the cell to influence intra-cellular signaling pathways that are shared by all kinds of cells. These mechanisms may only vary from one another in some quantitative, nonspecific aspects. The procedure of choosing the kind of response is through a selective, however variable distribution of specific receptors in the different cell lineages concerted with a few number of membrane-bound signals, as the family of the proton pump transporting systems, and from there on to mostly shared IC signaling pathways. This is the simplest possible way that can be imagined. Much more difficult would be that nature would have chosen that all cells would have the same or similar receptors. Should this have been the case, all cells would be equally sensitive to all different kinds of stimulating or inhibiting hormones and growth factors in order to induce numberless and different effects in each cell or cell lineage. In this case it would have been necessary to select the kind of response through a great number and diversity of signals and/or intracellular transduction mechanisms in the different cells and/or lineages. This complication is so great that it rules itself out. It is much more logical that evolution selects target cells providing them with specific receptors, which are the mediating mechanisms responsible for selecting the kind cellular response, than to have numberless IC signaling factors and pathways for each lineage or cell (Meléndez Hevia 2001; Harguindey et al 2008). In this way, a countless diversification of effects is reduced to the qualitative and quantitative distribution of membrane receptors, which would decrease the information and stimulus-response systems of cells to the smallest number and the least possible degree of complication. All the above-mentioned features can be represented through the following scheme:
| Systemic circulation | Cell membrane | Intracellular signaling |
| Multiplicity of stimulus (hormones, FC, etc.) | Specificityof receptors (qualitative) Same electro- chemical mechanisms | Similar IC pathways (quantitative/nonspecific) |
The fact that all the different kinds of cells of living organisms and a multiplicity of mitogenic stimuli, hormones and growth factors share the same universal transport systems, mainly the Na+/H+ antiporter but also a few other membrane-bound proton pumps at the cell membrane (Harguindey et al 2005), allows to formulate a law that unifies and combines specificity and nonspecificity in biology. Similar examples of this law at the level of intermediary metabolism, are:
Glucose transporters sensitive to insulin (GLUT 4), that are only present in fat, muscle, and heart tissues.
Adrenalin receptors for the stimulation of glucogenolysis, that are only present in muscle and liver.
Glucagon receptors for glucogenolysis, only present in liver.
In all these cases, the mechanism of intracellular stimulation is always the same one: activation of adenylate cyclase to produce cAMP) (Meléndez Hevia 2001). If we apply this law to neuronal neuroprotection, any trophic factors with a clear-cut antiapoptotic activity also stimulate the NHE-1 exchanger (Moolenar et al 1983; L’ Allemain et al 1984). Among the different membrane-bound proton pumps, the NHE1 appears to be the main homeostatic mechanism that protects cells against a pathological decrease in pHi and so, against cell death through cytosolic acidification. Thus, in many cases cellular protection seems to be ultimately mediated by a pHi-sustaining effect responsible for a physiological equilibrium and intracellular homeostasis of an acid-base nature. We have called this phenomena “therapeutic antiapoptosis”, a parallel concept to the “pathological antiapoptosis” which is characteristic to cancer cells, mainly in cases of multiple drug resistance to antichemotherapuetic agents (MDR) (Roepe et al 2003; Harguindey et al 2008) (Table 4). In summary, a primordial approach to the dynamics of cellular proton homeostasis gives way to new therapeutic possibilities, strategies and targets both in the treatment of HNDDs and neoplastic diseases (Rich et al 2000; Harguindey et al 2005, 2007).
The pHi factor and platelet abnormalities in AD. Therapeutic possibilities of human growth factors in the treatment of the trophic factor withdrawal syndrome in HNDDs
Intracellular homeostasis. The pHi factor
The final common pathway mediating cellular responses to different mitogenic stimulus, growth factors and other membrane signals such as hormones, has been repeatedly recognized to be secondary to the induction of an elevation of pHi (Burns et al 1983; Moolenar et al 1983; L’ Allemain et al 1984; Harguindey et al 1995; Cardone et al 2005). Contrariwise, apoptosis, either in HNDDs or other settings, is largely mediated by IC acidification (Vincent et al 1999; Harguindey et al 2005). Contrariwise, maintaining IC alkaline levels at the level of pHi =7.60 prevents neuronal injury, not only implying pHi as a critical factor in PCD but also indicating a possible role for therapeutic cellular alkalinization in the prevention and treatment of neuronal degeneration, as shown by Vincent and colleagues (1999). These authors and others (Harguindey et al 2007) have concluded that counteracting a drop in pHi through trophic and/or peptide growth factors is a most attractive therapeutic target against neuronal degeneration and pathological death. Furthermore, inhibiting NO production by NO inhibitors, like mefenamic acid and thiadiazolidinones, and so IC acidification, has been correctly proposed as a therapeutic option in the treatment of AD (Vincent et al 1999; Arianna et al 2007; Harguindey et al 2007). Thus, beyond multiple etiological causes and early EC factors that can be involved, a universal mechanism of an acid-base nature appears to be the pivotal and key event in downregulating caspase-mediated or caspase-independent IC signaling pathways leading in many cases to cell death in HNDDs and, similarly, in different lineages of cancer cells (Hamilton 1993; Li et al 1995; Pérez-Sala et al 1995; Rebollo et al 1995; Gottlieb et al 1996; Reynolds et al 1996; Furlong et al 1997; Hirakura 1999; Park et al 1999, 2005; Vincent et al 1999; Thangaraju et al 1999; Williams et al 1999; Liu et al 2000; Matsuyama et al 2000; Rich et al 2000; Hirpara 2001; Marches et al 2001; Renz et al 2001; Reshkin et al 2003; Lagadic-Gossmann et al 2004; Riedl et al 2004;Vaghefiet al 2004; Lavrik et al 2005; Bredesen et al 2006, Di Sario et al 2007) (Figure 1). In summary, any therapeutic effort to keep IC acid-base homeostasis within normal or higher than normal levels, is likely to be of significant benefit in the prevention and treatment of HNDDs.
Platelet abnormalities in AD: Interdisciplinary hematology to neurology transversal research
Evidence for a role of platelet dysfunction, structural to functional, in AD patients arises from different studies (Zubenko et al 1987a, 1987b). Arianna and colleagues (2007) and Corzo and colleagues (2007) have reported that abnormal platelet function related to an increase in NO-producing systems play an important role in the pathogenesis of AD and in neuronal degeneration. From the structural side, the presence of abnormal coated-platelets with the capacity to retain amyloid precursor protein in their surface has been shown in early stage AD and MCI (mild cognitive impairment), a feature that also shows a significant relationship with disease progression (Prodan et al 2007). Such abnormalities directly relate platelet abnormalities with the pathogenesis of at least some HNDDs, while at the same time they suggest that the supply of healthy exogenous platelets with high contents of PDGF and other platelet-derived GFs may have a significant therapeutic effect in at least certain HNDDs like AD (Figure 1 and Table 2).
Growth factors in neuronal protection
If one of the main trophic factors that stimulate growth and metabolism of muscle cells is plain exercise, the nervous system is stimulated by certain GFs, neurotrophic, platelet-derived, and beyond. The lack of cell trophism in different degenerative diseases makes it highly advisable to undertake clinical studies to detect the presence, decrease or even absence of either systemic, microenvironmental and/or cellular growth factors and other antiapoptotic substances (PDGF, VEGF, NGF, tyrosine kinases, Bcl-2, Bcl-X, etc.), not only in HNDDs but also in other systemic and nonneural degenerative diseases (Table 1) (Rideout 2001; Waldeimer et al 2003; Waldeimer 2004; Anitua et al 2005, 2006, 2007a, 2007b, 2008a, 2008b; Wallace et al 2006; Farrag et al 2007). Ultrastructural-morphological studies and determinations of the content and activity of α-granules in platelets of patients with HNDDs, as well as measuring the concentration of the different PDGFs in the platelets of patients with AD and other HNDDs represent an unexplored field of research that may lead to improving the understanding of the pathogenesis of HNDDs (Solerte et al 2005). In order to do this, the technology necessary to perform these measurements is now available (Anitua et al 2007a, 2007b).
Among the different PDGF, recent evidence points to a pivotal role for VEGF in neuronal protection (Jin 2002; Cao et al 2004; Storkebaum et al 2004a, 2004b; Solerte et al 2005). Decreased VEGF activity has been shown in different HNDDs. Recently, serum VEGF levels of patients with AD were compared with control subjects (Mateo et al 2007). The mean concentration of VEGF in the patient group was significantly lower than in the controls. These authors concluded that a decrease in serum levels of VEGF could contribute to the neurodegenerative process in AD. While no differences in serum VEGF levels between AD patients and controls have been found in other studies, low VEGF activity has also been reported in patients with ALS (Del Bo et al 2005). The VEGF deficiency trophic effect can be related to a decrease of VEGF secretion from coated-platelets secondary to already described abnormalities related to the retention of APP protein on their surface (Arianna et al 2007; Prodan et al 2007). Other authors have found co-accumulation of VEGF with β-amyloid in AD, as well as a strong binding of VEGF to it, suggesting that secondary VEGF deficiency under hypoperfusion may contribute to neurodegeneration and vascular dysfunction in the progression of AD (Del Bo et al 2005).
A decreased secretion and release of VEGF in the supernatants of circulating natural killer (NK) immune cells in patients with AD compared to normal controls and patients with other types of senile dementias has also been reported. This down-regulation of VEGF production has been demonstrated in peripheral immune cells of patients suffering from AD (Solerte et al 2005). These findings become even more significant when the pivotal importance of VEGF in brain angiogenesis, neuroprotection, and cerebrovascular exchange of nutrients is considered (Zhu et al 2002; Storkebaum et al 2004a; Yang et al 2004; Del Bo et al 2005; Solerte et al 2005) From a therapeutic point of view, direct application of VEGF delays the onset of paralysis, improves motor function and increases survival in a rat model of AD (Storkebaum et al 2004b; Vende Velde et al 2005). Since the highest known accumulation of VEGF can be found in platelets and leukocytes (Kut et al 2007), cyclic transfusions of healthy young platelets and, perhaps, leukocytes too, may represent the easiest and most natural available source of VEGF and other trophic growth factors and a significant new therapeutic approach to certain HNDDs (Harguindey et al 2007).
Similarly, mice lacking epidermal growth factor receptor (EGFR) develop neurodegeneration through Akt-caspase dependent apoptosis of the frontal cortex (Wagner et al 2006), this representing a further example of neuronal degeneration as part of the trophic factor withdrawal syndrome (TFWS). Finally, the emerging role of other GFs, like FGF, mainly through its interaction with its receptors, has been reported to present a significant role in brain neuronal trophism, repair and protection, and may also play a role in the treatment of certain mental disorders (Riva et al 2005).
The therapeutic potential of different neurotrophic factors in HNDDs has been studied for more than two decades (Hefty et al 1983; Varon et al 1987; Tatton et al 2003, Counts et al 2005). Barbin (1987) initially showed that different neurotrophins induce growth and proliferation of cells of neural origin. The inhibition of NGF has been considered to be involved in the pathogenesis and treatment of the prodromal stages of AD (Tatton et al 2003; Counts et al 2005, Schulte-Herbrügen et al 2008). However, more recent clinical trials using NGF have failed to show significant results. Besides, the intracerebral local application of NGF faces many technical problems (Brodski et al 2002; Ebert et al 2005; Dass et al 2006). However, when glial cell line neurotrophic factor (GDNF) is infused via encapsulated cells in animal models of PD some positive results are obtained (Sajadi et al 2005). Furthermore, it has been reported that the local application of NGF into the putamen has produced sustained benefits in a small series of patients with PD (Slevin et al 2006). Other neurotrophic factors like pigment epithelium-derived factor (PEGF) protects retinal ganglion cells (Pang et al 2007). Gene transfer factors and antiapoptotic promoting factors like Bcl-2 or other GFs have also been tried (Dass et al 2006; Sortwell et al 2007). Finally, hormones like thyrotrophin-releasing hormone (TRH) and its derivatives, as well as testosterone or estrogens, also play the role of GFs in protecting cells from neural injury in a similar manner than certain antioxidants like N-acetylcysteine (Yan et al 1995; Bialek et al 2004: Faden et al 2004). GFs besides VEGF and EGF, like IGF-1, whose deficiency has also been related to the pathogenesis of AD (Alvarez et al 2007), are able to promote neural survival and differentiation (Politi et al 2001). Finally, different antiapoptotic therapies for neurodegenerative diseases based upon the application of exogenous products that stimulate cell trophism, such as propargylamines, alpha-2-adrenergic receptor activators, minocycline, etc, are at the present time in preclinical stages of research (Tatton et al 2002, 2003; Lavrik 2005).
Use of PDGFs in clinical medicine and potential utilization of platelet transfusions in the treatment of HNDDs
One possibility of counteracting programmed cell death programs (PCDP) driven by deprivation of trophic and/or growth factors, would be to study the therapeutic potential of PDGF in HNDDs, as it has been recently tried in nonneural pathology (Lavrik 2005; Anitua et al 2007b; Ray et al 2007) (Table 1). Neurons, glial cells, and the pigmentary epithelial cells of the retina all contain platelet-derived growth factor receptors (PDGFR), making them classic therapeutic targets for PDGF. The cyclic administration of PDGFs in HNDDs through the exogenous supply of healthy platelets should be considered in a similar way that a wide variety of human pathologies are nowadays successfully treated through loco-regional application of autologous platelet releseates obtained by simple clinical procedures (Fedi et al 1997; Anitua et al 2004). This new research field allows for important and exciting new perspectives in opening untrodden areas of research and treatment of degenerative diseases, either from neural origin or otherwise, where a lack of one or more trophic factors is involved in their pathogenesis, including any kind of degenerative pathology where stimulation of new tissue growth or regrowth is necessary. Nowadays, clot preparations containing high concentrations of PDGF and metabolites that stimulate cell trophism, growth and proliferation, are being used with increasing frequency in a wide range of medical and surgical contexts apart from neurology, from dentistry and oral implantology to orthopaedics, gastric and skin ulcer treatment, eye disorders, etc, successfully inducing new tissue formation and accelerated tissue repair in different tissues and locations (Anitua et al 2004). Table 1 summarizes some of the therapeutic applications of platelet-based technology in modern clinical medicine. These platelet-releseates contain high concentrations of a wide array of mitogenic and pro-angiogenic factors, mainly platelet-derived growth factor PDGF, TGF-β1, EGF, VEGF, PDEGF, IGF-1, and HGF, as well as cytokines PF4 and CD40L, apart from lower concentrations of other factors like β-FGF (Anitua et al 2007b). Some of these factors show a potent stimulating effect on cell growth, proliferation and viability, to a large extent because of their positive effects on angiogenesis. The possibility of translating these successful clinical results to the prevention and treatment of HNDDs is one of the main medical challenges for the next few years.
Conclusions
The main conclusions of this study are:
The exogenous utilization of human growth factors, platelet-derived or otherwise, present a therapeutic potential in the treatment of certain human neurodegenerative diseases (HNDDs).
There is a large deal of both theoretical and experimental evidence, both at basic and clinical levels, to consider the cyclic and systemic use of healthy platelet transfusions in early stages of certain HNDDs like AD.
The therapeutic failure in the prevention and treatment of HNDDs can be secondary to a certain extent to lack of knowledge about the implications of the different trophic factors in the pathogenesis of HNDDs within the frame of the TFWS.
Every effort to maintain intracellular acid-base homeostasis within the physiological range in HNDDs, thus preventing the metabolic collapse induced by cell acidification and its secondary activation of cell death programs, will help to better understand, prevent and treat HNDDs.
In the prevention and treatment of HNDDs, a more effective pharmacological inhibition of the formation of NO by neural cells is needed.
Pathological and spontaneous programmed cell death programs in HNDDs, apoptotic or otherwise, are processes that dwell at opposite ends of an acid-base/homeostatic spectrum when compared with the resistance to the induction of apoptotsis that characterizes malignant cells and tissues (pathological antiapoptosis).
Acknowledgments
This work was supported by a grant of the Castresana Foundation, Vitoria, Spain. We thank Dr. Miriam L. Wahl, for her comments, suggestions and corrections. Moreover, the authors apologize to all investigators who have significantly contributed to the different fields of research reviewed in this contribution, but whose work we were unable to include in our review. The authors report no conflicts of interest in this work.
References
- Alio JL, Colecha JR, Pastor S, et al. Symptomatic dry eye treatment with autologous platelet-rich plasma. Ophthalmic Res. 2007;39:124–9. doi: 10.1159/000100933. [DOI] [PubMed] [Google Scholar]
- Anitua E, Andia I, Ardanza B, et al. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;9:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- Anitua E, Andia I, Sanchez M, et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res. 2005;23:281–6. doi: 10.1016/j.orthres.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Anitua E, Sanchez M, Nurden AT, et al. Autologous fibrin matrices: a potential source of biological mediators that modulate tendon cell activities. J Biomed Mater Res. 2006;77:285–93. doi: 10.1002/jbm.a.30585. [DOI] [PubMed] [Google Scholar]
- Anitua E, Sanchez M, Nurden AT, et al. Reciprocal actions of platelet-secreted TGF-β1 on the production of VEGF and HGF by human tendon cells. Plastic Reconstruct Surg. 2007a;119:950–9. doi: 10.1097/01.prs.0000255543.43695.1d. [DOI] [PubMed] [Google Scholar]
- Anitua E, Sánchez M, Orive G, Andía I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials. 2007b;28:4551–60. doi: 10.1016/j.biomaterials.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Anitua E, Aguirre JJ, Algorta J, et al. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J Biomed Mater Res B. 2008a;84:415–21. doi: 10.1002/jbm.b.30886. [DOI] [PubMed] [Google Scholar]
- Anitua E, Sánchez M, Orive G, et al. Delivering growth factors for therapeutics. Trends Pharmacol Sci. 2008b;29:37–41. doi: 10.1016/j.tips.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Alvarez A, Cacabelos R, Sanpedro C, et al. Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiol Aging. 2007;28:533–6. doi: 10.1016/j.neurobiolaging.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Appel SH. A unifying hypothesis for the cause of amyotrophic lateral sclerosis, Parkinsonism and Alzehimer’s disease. Ann Neurol. 1983;10:499–505. doi: 10.1002/ana.410100602. 1981. [DOI] [PubMed] [Google Scholar]
- Araki W, Wurtman RJ. Increased expression of amyloid precursor protein and amyloid precursor-like protein 2 during trophic factor withdrawal-induced death of neuronal PC12 cells. Mol Brain Res. 1998;56:169–77. doi: 10.1016/s0169-328x(98)00050-3. [DOI] [PubMed] [Google Scholar]
- Arianna V, Laura N, Cinzia M, et al. Modification of platelet from Alzheimer disease patients: a possible relation between membrane properties and NO metabolites. Neurobiol Aging. 2007;28:987–94. doi: 10.1016/j.neurobiolaging.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Barbin G. Le viellissement cerebral normal and pathologique. In: Maloine SA, editor. Colloques de la Fondation Nationale de Gerontologie. 1987. pp. 114–23. [Google Scholar]
- Belaud-Rotureau MA, Leducq N, de Gannes FMP. Early transitory rise in intracellular pH leads to Bax conformation change during ceramide-induced apoptosis. Apoptosis. 2000;5:551–60. doi: 10.1023/a:1009693630664. [DOI] [PubMed] [Google Scholar]
- Bialek M, Zaremba P, Borowicz KK, et al. Neuroprotective role of testosterone in the nervous system. Polish J Pharmacol. 2004;56:509–18. 2004. [PubMed] [Google Scholar]
- Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006;443:796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodski C, Vogt Weisenhorn MV, Dechant G. Therapy of neurodegenerative diseases using neurotrophic factors: cell biological perspective. Expert Rev Neurotherapeutics. 2002;2:89–98. doi: 10.1586/14737175.2.3.417. [DOI] [PubMed] [Google Scholar]
- Bröker LE, Kruyt FAE, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res. 2005;11:3155–62. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- Burns CP, Rozengurt E. Serum, Platelet-derived growth factor, vasopressin and phorbol esters increase intracellular pH in swiss 3T3 cells. Biochem Biophs Res Comm. 1983;116:931–8. doi: 10.1016/s0006-291x(83)80231-9. [DOI] [PubMed] [Google Scholar]
- Cacabelos R. Pharmacogenomics, nutrigenomics and therapeutic optimization in Alzheimer’s disease. Aging Health. 2005;1:303–48. [Google Scholar]
- Cacabelos R, Takeda M. Pharmacogenomics, nutrigenomics and future therapeutics in Alzheimer’s disease. Drugs Future. 2006;31(Suppl B):5–146. [Google Scholar]
- Cao L, Jiao X, Zuzga DS, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nature Genetics. 2004;36:827–35. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nature Revs Cancer. 2005;5:786–95. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- Consolo U, Zaffe D, Bertoldi C, et al. Platelet-rich plasma activity on maxillary sinus floor augmentation by autologous bone. Clin Oral Implants Res. 2007;18:252–62. doi: 10.1111/j.1600-0501.2006.01330.x. [DOI] [PubMed] [Google Scholar]
- Corzo L, Zas R, Rodríguez, et al. Decreased levels of serum nitric oxide in different forms of dementia. Neurosci Lett. 2007;420:263–7. doi: 10.1016/j.neulet.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Counts SE, Mufson EJ. The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J Neuropathol. 2005;64:263–72. doi: 10.1093/jnen/64.4.263. [DOI] [PubMed] [Google Scholar]
- Chao MV, Rajagopal R, Lee FS. Neurothropin signalling in health and disease. Clin Sci. 2006;110:167–73. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- Chan SL, Tammariello SP, Estus S, et al. Prostate apoptosis response-4 mediates trophic factor withdrawal-induced apoptosis of hippocampal neurones: actions prior to mitochondrial dysfunction and caspase activity. J Neurochem. 1999;73:502–12. doi: 10.1046/j.1471-4159.1999.0730502.x. [DOI] [PubMed] [Google Scholar]
- Choi B-M, Pae H-O, Jang SIl, et al. Nitric oxide as pro-apoptotic as well as anti-apoptotic modulator. J Biochem Mol Biol. 2002;35:116–26. doi: 10.5483/bmbrep.2002.35.1.116. [DOI] [PubMed] [Google Scholar]
- Dass B, Warren Olanov C, Kordover JH. Gene transfer of trophic factors and stem cells grafting as treatments for Parkinson’s disease. Neurology. 2006;66:S89–S103. doi: 10.1212/wnl.66.10_suppl_4.s89. [DOI] [PubMed] [Google Scholar]
- Del Bo R, Scarlato M, Ghezzi S, et al. Vascular endothelial growth factor gene variability is associated with increased risk for AD. Ann Neurol. 2005;57:373–80. doi: 10.1002/ana.20390. [DOI] [PubMed] [Google Scholar]
- Deshmukh M, Johnson EM. Programmed cell death in neurons: focus on the pathway of nerve growth factor deprivation-induced death of sympathetic neurones. Mol Pharmacol. 1997;51:897–906. doi: 10.1124/mol.51.6.897. [DOI] [PubMed] [Google Scholar]
- Deshmukh M, Kuida K, Johnson EM. Caspase inhibition extends the commitment to neural death beyond cytochrome C release to the point of mitochondrial depolarization. J Cell Biol. 2000;150:131–43. doi: 10.1083/jcb.150.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiammarino J, Lee ADS, Cadwell C, et al. A novel mechanism of tumorogenesis involving pH-dependent desestabilization of a mutant p53 tetramer. Nature Struct Biol. 2000;9:12–16. doi: 10.1038/nsb730. [DOI] [PubMed] [Google Scholar]
- Di Sario A, Svegliati Baroni G, Bendia E, et al. Intracellular pH regulation and Na+/H+ exchange activity in human hepatic stellate cells: effect of platelet-derived growth factor, insulin-like growth factor 1 and insulin. J Hepatology. 2001;34:378–85. doi: 10.1016/s0168-8278(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Di Sario A, Bendia E, Omenetti A, et al. Selective inhibition of ion transport mechanisms regulating intracellular pH reduces proliferation and induces apoptosis in cholangiocarcinoma cells. Digestive Liver Dis. 2007;39:60–9. doi: 10.1016/j.dld.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Ebert AD, Svendsen CN. A new tool in the battle against Alzheimer’s disease and aging: ex vivo gene therapy. Rejuvenation Res. 2005;8:131–4. doi: 10.1089/rej.2005.8.131. [DOI] [PubMed] [Google Scholar]
- Estévez AG, Sampson JB, Zhuang YX, et al. Liposome-derived superoxide dismutase prevents nitric oxide-dependent motor neuron death induced by trophic factor withdrawal. Free Rad Biol Med. 2000;28:437–46. doi: 10.1016/s0891-5849(99)00261-0. [DOI] [PubMed] [Google Scholar]
- Estévez A, Jordán J. Nitric acid and superoxide, a deadly cocktail. Ann NYAS. 2002;962:207–11. doi: 10.1111/j.1749-6632.2002.tb04069.x. [DOI] [PubMed] [Google Scholar]
- Estévez A, Sahawneh MA, Lange PS, et al. Arginase 1 regulation of nitric oxide production is key to survival of trophic factor-deprived motor neurons. J Neurosci. 2006;26:8512–6. doi: 10.1523/JNEUROSCI.0728-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden AI, Knoblach SM, Movsesyan VA, et al. Novel small peptides with neuroprotective and nootropic properties. J Alzheimer Dis. 2004;6:S93–S97. doi: 10.3233/jad-2004-6s603. [DOI] [PubMed] [Google Scholar]
- Famulski KE, MacDonald D, Paterson MC, et al. Activation of low pH-dependent nuclease by apoptotic agents. Cell Death Differ. 1999;6:281–9. doi: 10.1038/sj.cdd.4400495. [DOI] [PubMed] [Google Scholar]
- Farrag TY, Lehar M, Verhaegen P, et al. Effect of platelet rich plasma and fibrin sealant on facial nerve regeneration in a rat model. Laryngoscope. 2007;117:157–65. doi: 10.1097/01.mlg.0000249726.98801.77. [DOI] [PubMed] [Google Scholar]
- Fedi P, Tronick SR, Aaronson SA. Growth factors. In: Holland JF, Bast RC, Morton DL, et al., editors. Cancer Medicine. Baltimore: Williams and Wilkins; 1997. pp. 47–74. [Google Scholar]
- Fernández-Vizarra P, Fernández AP, Castro-Blanco S, et al. Expression of nitric oxide system in clinically evaluated cases of Alzheimer disease. Neurobiol Dis. 2004;15:287–305. doi: 10.1016/j.nbd.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Furlong IJ, Ascaso R, Lopez Rivas A, et al. Intracellular acidification induces apoptosis by stimulating ICE-like protease activity. J Cell Sci. 1997;110:653–61. doi: 10.1242/jcs.110.5.653. [DOI] [PubMed] [Google Scholar]
- Gottlieb RA, Giesing HA, Zhu JY, et al. Cell acidification and apoptosis: granulocyte colony-stimulating factor delays programmed cell death by up-regulating the vacuolar ATPase. Proc Natl Acad Sci U S A. 1995;92:5965–8. doi: 10.1073/pnas.92.13.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb RA, Nordberg J, Skowronski E, et al. Apoptosis induced in Jurkat cells by several agents is preceded by intracellular acidification. Proc Natl Acad Sci U S A. 1996;93:654–8. doi: 10.1073/pnas.93.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S, Rotin D, Mason MJ. Na+/H+ exchange and growth factor-induced cytosolic changes. Role in cellular proliferation. Biochem Biophys Acta. 1989;988:73–97. doi: 10.1016/0304-4157(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Guo Q, Sopher BJ, Furukawa K, et al. Alzheimer presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and β-amiloid peptide: involvement of calcium and oxyradicals. J Neurosci. 1997;17:4212–22. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton G, Cosentini EP, Teleky B, et al. The multidrug-resistance modifiers verapamil, cyclosporine A and tamoxifen induce an intracellular acidification in colon carcinoma cell lines in vitro. Anticancer Res. 1993;13(6A):2059–63. [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Harguindey S. Use of Na+/H+ antiporter inhibitors as a novel approach to cancer treatment. In: Cragoe EJ Jr, Kleyman ThR, Simchowitz L, editors. Amiloride and its analogs: unique cation transport inhibitors. New York: VCH Publishers Inc; 1992a. pp. 317–34. [Google Scholar]
- Harguindey S. Integrating fields of cancer research through pivotal mechanisms and synthetic final pathways: A unifying and creative overview. Med Hypotheses. 2002;58:444–52. doi: 10.1054/mehy.2001.1415. Erratum. 2003. Med Hypotheses, 61:318–19. [DOI] [PubMed] [Google Scholar]
- Harguindey S, Cragoe EJ., Jr The Na+/H+ antiporter in oncology in the light of the spontaneous regression of cancer and cell metabolism. Med Hypotheses. 1992b;39:229–37. doi: 10.1016/0306-9877(92)90114-r. [DOI] [PubMed] [Google Scholar]
- Harguindey S, Pedraz JL, García Cañero R, et al. Hydrogen ion-dependent oncogenesis and parallel new avenues to cancer prevention and treatment using a H+-mediated unifying approach: pH-related and pH-unrelated mechanisms. Critical Rev Oncog. 1995;6:1–33. doi: 10.1615/critrevoncog.v6.i1.20. [DOI] [PubMed] [Google Scholar]
- Harguindey S, Pedraz JL, García Cañero R, et al. Edelfosine, apoptosis, MDR and Na+/H+ exchanger: induction mechanisms and treatment implications. Apoptosis. 2000;5:87–9. doi: 10.1023/a:1009645927931. [DOI] [PubMed] [Google Scholar]
- Harguindey S, Orive G, Pedraz JL, et al. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin-one single nature. Bioch Biopyhs Acta Revs Cancer. 2005;1756:1–24. doi: 10.1016/j.bbcan.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Harguindey S, Reshkin SJ, Orive G, et al. Growth and trophic factors and the pH, Na+/H+ exchanger in Alzheimer’s disease, other neurodegenerative diseases and cancer: new therapeutic possibilities and potential dangers. Curr Alzheimer Res. 2007;4:53–65. doi: 10.2174/156720507779939841. [DOI] [PubMed] [Google Scholar]
- Harguindey S, Orive G, Anitua E, et al. Hacia un nuevo enfoque integrado del tratamiento de las enfermedades neurodegenerativas (ENDs): de etiopatogenia a tratamiento. El síndrome de deficiencia de factores tróficos y de crecimiento (SDFC) Gen T. 2008;3:82–9. [Google Scholar]
- Hefti F. Alzheimer’s disease caused by a lack of nerve growth factor? Ann Neurol. 1983;13:109–10. doi: 10.1002/ana.410130127. [DOI] [PubMed] [Google Scholar]
- Hirakura Y, Lin M-Ch, Kagan BL. Alzheimer amyloid aβ1-42 channels: effect of solvent, pH, and congo red. J Neurosci Res. 1999;57:458–66. [PubMed] [Google Scholar]
- Hirpara J, Clément M-V, Pervaiz S. Intracellular acidification triggered by mitochondrial-derived hydrogen peroxide is an effector mechanism for drug-induced apoptosis in tumor cells. J Biol Chem. 2001;276:514–21. doi: 10.1074/jbc.M004687200. [DOI] [PubMed] [Google Scholar]
- Ishaque A, Al-Rubeai M. Use of intracellular pH and annexin V flow cytometric assays to monitor apoptosis and its suppression by bcl-2 over-expression in hybridoma cell culture. J Immunol Methods. 1998;221:43–57. doi: 10.1016/s0022-1759(98)00166-5. [DOI] [PubMed] [Google Scholar]
- Izumi HT, Torigoe T, Ishiguchi H, et al. Cellular pH regulators: potentially promising molecular targets of cancer chemotherapy. Cancer Treat Rev. 2003;29:541–9. doi: 10.1016/s0305-7372(03)00106-3. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–50. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled AR, Moor AN, Li A, et al. Thropic factor withdrawal: p38 mitogen-activated protein kinase activates NHE1, which induces intracellular alkalinization. Mol Cell Biol. 2001;21:7545–57. doi: 10.1128/MCB.21.22.7545-7557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland RA, Franklin JI. Prooxidant effects of NGF withdrawal and MEK inhibition in sympathetic neurones. Antioxid Redox Signal. 2003;5:635–9. doi: 10.1089/152308603770310301. [DOI] [PubMed] [Google Scholar]
- Kut C, Mac Gabhann F, Popel S. Where is VEGf in the body? A meta-analysis of EGF distribution in cancer. Br J Cancer. 2007;97:978–85. doi: 10.1038/sj.bjc.6603923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagadic-Gossmann D, Huc L, Lecureur V. Alterations of intracellular pH homeostasis in apoptosis: origins and roles. Cell Death Differ. 2004;11:953–61. doi: 10.1038/sj.cdd.4401466. [DOI] [PubMed] [Google Scholar]
- L’Allemain G, Franchi A, Cragoe EJ, et al. Blockade of the Na+/H+ antiport abolishes growth factor-induced DNA synthesis in fibroblasts. J Biol Chem. 1984;259:4313–9. [PubMed] [Google Scholar]
- Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. J Clin Invest. 2005;115:2665–72. doi: 10.1172/JCI26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts D, Storkebaum E, Morimoto M, et al. VEGF is a modifier of amyothropic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. 2003;34:357–8. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- Letai AG. Diagnosing and exploiting cancer’s addition to blocks in apoptosis. Nature Revs Cancer. 2008;8:121–32. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- Li J, Eastman A. Apoptosis in an interleukin-2-dependent cytotoxic T lympohcyte cell line is associated with intracellular acidification. Role of the Na(+)/H(+)-antiport. J Biol Chem. 1995;270:3203–11. doi: 10.1074/jbc.270.7.3203. [DOI] [PubMed] [Google Scholar]
- Lieberthal W, Triaca V, Koh JS, et al. Role of superoxide in apoptosis induced by growth factor withdrawal. Am J Physiol. 1998;275:F691–F702. doi: 10.1152/ajprenal.1998.275.5.F691. [DOI] [PubMed] [Google Scholar]
- Liu D, Martino G, Thangaraju M, et al. Caspase-8-mediated intracellular acidification preceds mitochondrial dysfunction in somatostatin-induced apoptosis. J Biol Chem. 2000;275:9244–50. doi: 10.1074/jbc.275.13.9244. [DOI] [PubMed] [Google Scholar]
- Marches R, Vitetta ES, Uhr JW. A role for intracellular pH in membrane IgM-mediated cell death of human B lymphomas. Proc Natl Acad Sci U S A. 2001;98:3434–9. doi: 10.1073/pnas.061028998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo I, Llorca I, Infante J, et al. Low serum VEGF levels are associated with Alzheimer’s disease. Acta Neurol Scand. 2007;116:56–8. doi: 10.1111/j.1600-0404.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Llopis J, Deveraux QL, et al. Changes in intramitchondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nature Cell Biol. 2000;2:318–25. doi: 10.1038/35014006. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Reed JC. Mitochondria-dependent apoptosis and cellular pH regulation. Cell Death Differ. 2000;7:1155–65. doi: 10.1038/sj.cdd.4400779. 2000. [DOI] [PubMed] [Google Scholar]
- McCarthy MJ, Rubin LL, Philott KL. Involvement of caspases in sympathetic neuron apoptosis. J Cell Sci. 1997;110:2165–73. doi: 10.1242/jcs.110.18.2165. [DOI] [PubMed] [Google Scholar]
- Meléndez Hevia E. Natural selection and thermodynamics in biological evolution: from the origin of life to cancer. Servicio de Publicaciones de la Universidad de La Laguna; Tenerife, Spain: 2001. pp. 1–88. [Google Scholar]
- Miraglia E, Viarisio D, Riganti CH, et al. Na+/H+ exchanger activity is increased in doxorubicin-resistant human colon cancer cells and its modulation modifies the sensitivity of the cells to doxorubicin. Int J Cancer. 2005;115:924–9. doi: 10.1002/ijc.20959. [DOI] [PubMed] [Google Scholar]
- Moolenar WH, Mummery CL, van der Saag, et al. Na+/H+ exchange and cytoplasmatic pH in the action of growth factors in human fibroblasts. Nature. 1983;304:645–8. doi: 10.1038/304645a0. [DOI] [PubMed] [Google Scholar]
- Orive G, Reshkin SJ, Harguindey S, et al. Hydrogen ion dynamics and the Na+/H+ antiporter in cancer angiogenesis and antiangiogenesis. Br J Cancer. 2003;89:395–9. doi: 10.1038/sj.bjc.6601286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Lee BK, Park S. Effects of sabiporide, a specific Na+/H+ exchange inhibitor on neuronal cell death and brain ischemia. Brain Res. 2005;1061:67–71. doi: 10.1016/j.brainres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Pérez-Sala D, Collado-Escobar D, Mollinedo F. Intracellular alkalinization supresses lovastatin-induced apoptosis in HL60 cells through the inactivation of a pH-dependent endonuclease. J Biol Chem. 1995;270:6235–42. doi: 10.1074/jbc.270.11.6235. [DOI] [PubMed] [Google Scholar]
- Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2007;34:1774–8. doi: 10.1177/0363546506288850. [DOI] [PubMed] [Google Scholar]
- Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- Pang I-H, Zeng H, Fleenor D, et al. Pigment epithelium-derived factor protects retinal ganglion cells. BMC Neurosci. 2007;8:1–11. doi: 10.1186/1471-2202-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Lyons JC, Ohtsubo, et al. Acidic environment causes apoptosis by increasing caspase activity. Br J Cancer. 1999;80:1892–7. doi: 10.1038/sj.bjc.6690617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona R, Sánchez-Pérez I. Signalling pathways involved in clinical responses to chemotherapy. Clin Transl Oncol. 2007;9:625–33. doi: 10.1007/s12094-007-0115-3. [DOI] [PubMed] [Google Scholar]
- Politi LE, Rotstein NP, Salvador G, et al. Insulin-like growth factor- I is a potential trophic factor for amacrine cells. J Neurochem. 2001;76:1199–211. doi: 10.1046/j.1471-4159.2001.00128.x. [DOI] [PubMed] [Google Scholar]
- Poser S, Impey S, Xia Z, et al. Brain-derived neurotrophic factor protection of cortical neurones from serum withdrawal-induced apoptosis is inhibited by cAMP. J Neurosci. 2003;23:4420–7. doi: 10.1523/JNEUROSCI.23-11-04420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poüysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumor regression. Nature. 2006;441:437–43. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- Prodan CI, Ross ED, Vincent AS, et al. Coated-platelets correlate with disease progression in Alzheimer’s disease. J Neurol. 2007;254:548–9. doi: 10.1007/s00415-006-0323-8. [DOI] [PubMed] [Google Scholar]
- Raina AK, Zhu X, Rottkamp CA, et al. Cyclin’ towards dementia: cell cycle abnormalities and abortive oncogenesis in Alzheimer’s disease. J Neurosci Res. 2000;61:128–33. doi: 10.1002/1097-4547(20000715)61:2<128::AID-JNR2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Raina AK, Hochman A, Ickes H, et al. Apoptotic promoters and inhibitors in Alzheimer’s disease: who wins out? Prog Neuropsychopharmacol Biol Psych. 2003;27:251–4. doi: 10.1016/S0278-5846(03)00020-4. [DOI] [PubMed] [Google Scholar]
- Ray S, Britschgi M, Herbert CH, et al. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nature Med. 2007;13:1359–62. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- Rebollo A, Gómez J, de Aragón AM, et al. Apoptosis induced by IL-2 withdrawal is associated with an intracellular acidification. Exp Cell Res. 1995;218:581–5. doi: 10.1006/excr.1995.1195. [DOI] [PubMed] [Google Scholar]
- Reed JC. Apoptosis-based therapies. Nat Revs Drug Discov. 2002;1:111–21. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- Reed JC. Apoptosis mechanisms: Implications for cancer drug discovery. Oncology. 2004;18:11–20. [PubMed] [Google Scholar]
- Renz A, Berdel WE, Kreuter M, et al. Rapid extracellular release of cytochrome c is specific for apoptosis and marks cell death in vivo. Blood. 2001;98:1542–8. doi: 10.1182/blood.v98.5.1542. [DOI] [PubMed] [Google Scholar]
- Reshkin SJ, Bellizzi A, Caldeira S, et al. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 2000;14:2185–97. doi: 10.1096/fj.00-0029com. [DOI] [PubMed] [Google Scholar]
- Reshkin SJ, Bellizzi A, Cardone M. Paclitaxel induces apoptosis via Protein Kinase A- and p38 Mitogen-activated Protein-dependent inhibition of the Na+/H+ Exchanger (NHE) Isoform 1 in Human Breast cancer cells. Clinical Cancer Res. 2003;9:2366–73. [PubMed] [Google Scholar]
- Resnick L, Fennell M. Targeting JNK3 for the treatment of neurodegenerative disorders. Drug Disc Today. 2004;9:932–9. doi: 10.1016/S1359-6446(04)03251-9. [DOI] [PubMed] [Google Scholar]
- Reynolds JE, Li JF, Craig RW, et al. BCL-2 and MCL-1 expression in chinese hamster ovary cells inhibits intracellular acidification and apoptosis induced by staurosporine. Exp Cell Res. 1996;225:430–6. doi: 10.1006/excr.1996.0194. [DOI] [PubMed] [Google Scholar]
- Rich IR, Worthington-White D, Garden OA, et al. Apoptosis of leukemic cells accompanies reduction in intracellular pH after targeted inhibition of the Na+/H+ exchanger. Blood. 2000;95:1427–34. [PubMed] [Google Scholar]
- Rideout HJ, Stefanis L. Caspase inhibition: a potential strategy in neurological diseases. Histol Histopathol. 2001;6:895–908. doi: 10.14670/HH-16.895. [DOI] [PubMed] [Google Scholar]
- Riedl AJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nature Revs Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- Rigas B, Sun Y. Induction of oxidative stress as a mechanism of action of chemopreventive agents against cancer. Br J Cancer. 2008;98:1157–60. doi: 10.1038/sj.bjc.6604225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva M, Molteni R, Bedogni F, et al. Emerging role of the FGF system in psychiatric disorders. Trends Pharmacol Sci. 2005;26:228–31. doi: 10.1016/j.tips.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Roepe PD, Wei LY, Cruz J, et al. Lower electrical membrane and altered pHi homeostasis in multidrug-resistant (MDR) cells: further characterization of a series of MDR cell lines expressing different levels of P-glycoprotein. Biochemistry. 1993;32:11042–56. doi: 10.1021/bi00092a014. [DOI] [PubMed] [Google Scholar]
- Roepe PD. pH and multidrug resistance. In: Gillies RG, editor. The tumor microenvironment: causes and consequences of hypoxia and acidity. Chichester, New York: John Wiley and Sons Ltd; 2001. pp. 232–47. Novartis Foundation Symposium, No. 240. [Google Scholar]
- Sajadi A, Bensadoun J-Ch, Schneider BL, et al. Transient striatal delivery of GNDF via encapsulated cells to sustained behavioral improvement in a bilateral model of Parkinson’s disease. Neurobiol Dis. 2006;22:119–29. doi: 10.1016/j.nbd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Schulte-Herbrüggen O, Jockers-Scherübi MC, Hellweg R. Neurotropins: from physiopathology to treatment of Alzheimers’s disease. Curr Alz Res. 2008;5:38–44. doi: 10.2174/156720508783884620. [DOI] [PubMed] [Google Scholar]
- Shacka JJ, Roth KA. Regulation of neural cell death and neurodegeneration by members of the Bcl-2 family: therapeutic implications. Curr Drug Targets CNS Neurol Dis. 2005;4:25–39. doi: 10.2174/1568007053005127. [DOI] [PubMed] [Google Scholar]
- Sehgal I, Powers S, Huntley B, et al. Neurotensin is an autocrine trophic factor stimulated by androgen withdrawal in human prostate cancer. Proc Natl Acad Sci U S A. 1994;91:4673–7. doi: 10.1073/pnas.91.11.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. The multiple mechanisms of drug resistance and cellular pH. In: Gillies R, editor. The tumor microenvironment: causes and consequences of hypoxia and acidity. Chichester, New York: John Wiley and Sons, Ltd; 2001. pp. 269–81. Novartis Foundation Symposium No. 240. [Google Scholar]
- Slevin JT, Gash DM, Smith CD, et al. Unilateral intraputaminal glial cell line-derived neurotrophic factor in patients with Parkinson’s disease: response to 1 year each of treatment and withdrawal. Neurosurg Focus. 2006;20:E1. doi: 10.3171/foc.2006.20.5.2. [DOI] [PubMed] [Google Scholar]
- Solerte SB, Ferrari E, Cuzzoni G, et al. Decrease release of the angiogenic peptide vascular endothelial growth factor in Alzheimer’s disease: recovering effect with insulin and DHEA sulphate. Dement Geriatr Cogn Disord. 2005;19:1–10. doi: 10.1159/000080963. [DOI] [PubMed] [Google Scholar]
- Sortwell CE, Bowers WJ, Counts SE, et al. Effect of ex vivo transduction of mesencephalic reagggregates with bcl-2 on grafted dopamine neuron survival. Brain Res. 2007;1134:33–4. doi: 10.1016/j.brainres.2006.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio S, de Belle I, Bredesen DE. An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci U S A. 2000;97:14376–81. doi: 10.1073/pnas.97.26.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkebaum E, Carmeliet P. VEGF: a critical player in neurodegeneration. J Clin Invest. 2004a;113:14–18. doi: 10.1172/JCI200420682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkebaum E, Lambrechts D, Dewerchin M, et al. Treatment of motoneuron degeneration by intraventricular delivery of VEGF in a rat model of ALS. Nature Neurosci. 2004b;8:85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- Szakács G, Paterson JK, Ludwig JA, et al. Targeting multidrug resistance in cancer. Nature Revs. 2006;5:219–31. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- Tafani M, Cohn JA, Karpinich NO, et al. Regulation of intracellular pH mediates Bax activation in HeLa cells treated with staurosporine on tumor necrosis factor-alpha. J Biol Chem. 2003;277:49569–76. doi: 10.1074/jbc.M208915200. [DOI] [PubMed] [Google Scholar]
- Taetle R, Dos Santos B, Ohsuigi Y, et al. Effect of combined antigrowth factor receptor treatment on in vitro growth of multiple myeloma. J Natl Cancer Inst. 1994;86:450–5. doi: 10.1093/jnci/86.6.450. [DOI] [PubMed] [Google Scholar]
- Takashaki A, Masuda, Sun M, et al. Oxidative stress-induced apoptosis is associated with alterations in mitochondrial caspase activity and Bcl-2-dependent alterations in mitochondrial pH (pHm) Brain Res Bull. 2004;62:497–504. doi: 10.1016/j.brainresbull.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Tang DG, Li L, Chopra DP, et al. Extended survivability of prostate cancer cells in the absence of trophic factors: increased proliferation, evasion of apoptosis, and the role of apoptosis proteins. Cancer Res. 1998;58:3466–79. [PubMed] [Google Scholar]
- Tatton WG, Chamblers-Redman R, Ju WJH, et al. Propargylamines induce antiapoptotic new protein synthesis in serum- and nerve growth factor (NGF)-withdrawn, NGF-differentiated PC-12 cells. J Pharm Exp Therap. 2002;301:753–64. doi: 10.1124/jpet.301.2.753. [DOI] [PubMed] [Google Scholar]
- Tatton W, Chen D, Chamblers-Redman R, et al. Hypotheses for a common basis for neuroprotection in glaucoma and Alzheimer’s disease: antiapoptosis by alpha-2-adrenergic receptor activation. Survey Ophtalmol. 2003;48(Suppl 1):s25–s37. doi: 10.1016/s0039-6257(03)00005-5. [DOI] [PubMed] [Google Scholar]
- Thangaraju M, Sharma K, Leber B, et al. Regulation of acidification and apoptosis by SHP-1 and BCL-2. J Biol Chem. 1999;274:29549–57. doi: 10.1074/jbc.274.41.29549. [DOI] [PubMed] [Google Scholar]
- Thompson CB, Bauer DE, Lum JJ, et al. How do cancer cells acquire the fuel needed to support cell growth? Cold Spring Harbor Symp Quant Biol. 2005;70:357–62. doi: 10.1101/sqb.2005.70.011. [DOI] [PubMed] [Google Scholar]
- Torigoe TU, Izumi H, Ise T, et al. Vacuolar H+-ATPase: functional mechanisms and potential as a target for cancer chemotherapy. Anticancer Drugs. 2002;13:237–43. doi: 10.1097/00001813-200203000-00005. [DOI] [PubMed] [Google Scholar]
- Troy CM, Stefanis L, Prochiantz A, et al. The contrasting roles of ICE family proteases and inteleukin-1β in apoptosis induced by trophic factor withdrawal and by copper/zinc superoxide dismutase down-regulation. Proc Natl Acad Sci U S A. 1996;93:5635–40. doi: 10.1073/pnas.93.11.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaghefi H, Hughes Al, Neet KE. Nerve growth factor withdrawal-mediated apoptosis in naïve and differentiated PC12 cells through p53/caspase-3-dependent and -independent pathways. J Biol Chem. 2004;279:15604–14. doi: 10.1074/jbc.M311500200. [DOI] [PubMed] [Google Scholar]
- Varon S, Manthorpe M, Williams LR. Neuronotrophic and neurite-promoting factors and their clinical potentials. Dev Neurosci. 1983;6:73–86. doi: 10.1159/000112334. [DOI] [PubMed] [Google Scholar]
- Vende Velde Ch, Cleveland DW. VEGF: multitasking in ALS. Nature Neurosci. 2005;8:5–7. doi: 10.1038/nn0105-5. [DOI] [PubMed] [Google Scholar]
- Vincent AM, TenBroecke M, Maiese K. Neuronal intracellular pH directly mediates nitric oxide-induced programmed cell death. J Neurobiol. 1999;40:171–84. doi: 10.1002/(sici)1097-4695(199908)40:2<171::aid-neu4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Wagner B, Natajaran A, Grünaug S, et al. Neuronal survival depends on EGFR signalling in cortical but not in midbrain astrocytes. EMBO J. 2006;25:752–62. doi: 10.1038/sj.emboj.7600988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl ML, Owen JA, Burd R, et al. Regulation of intracellular pH in human melanoma: potential therapeutic implications. Mol Cancer Therap. 2002;1:617–28. [PubMed] [Google Scholar]
- Waibel M, Kramer S, Lauder K, et al. Mitochondria are not required for death-receptor mediated cytosolic acidificaction during apoptosis. Apoptosis. 2007;12:623–30. doi: 10.1007/s10495-006-0006-z. [DOI] [PubMed] [Google Scholar]
- Waldeimer PC, Tatton W. Interrupting apoptosis in neurodegenerative disease: potential for effective therapy? Drug Discover Today. 2004;9:210–8. doi: 10.1016/S1359-6446(03)03000-9. [DOI] [PubMed] [Google Scholar]
- Waldeimer PC. Prospects for antiapoptotic drug therapy of neurodegenerative diseases. Prog Neuropsychopharm Biol Psychiatry. 2003;27:303–21. doi: 10.1016/S0278-5846(03)00025-3. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Dicay M, McKnight W, et al. Platelets accelerate gastric ulcer healing through presentation of vascular endothelial growth factor. Br J Pharmacol. 2006;148:274–8. doi: 10.1038/sj.bjp.0706722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A, Nikodemova M, Compston A, et al. Minocycline attenuates nitric oxide-mediated neuronal axonal destruction in vitro. Neuron Glia Biol. 2004;1:297–305. doi: 10.1017/S1740925X05000104. [DOI] [PubMed] [Google Scholar]
- Williams ACT, Collard J, Paraskeva C. An acidic environment leads to p53 dependent induction of apoptosis in human adenoma and carcinoma cell lines: implications for clonal selection during colorectal carcinogenesis. Oncogene. 1999;18:3199–204. doi: 10.1038/sj.onc.1202660. [DOI] [PubMed] [Google Scholar]
- Yan CY, Ferrari G, Greene LA. N-acetylcysteine-promotes survival of PC12 cells is gluthation-independent but transcription-dependent. Am Soc Biochem Mol Biol. 1995;270:26827–32. doi: 10.1074/jbc.270.45.26827. [DOI] [PubMed] [Google Scholar]
- Yang SP, Bae AG, Kang HJ, et al. Co-accumulation of vascular endothelial growth factor with β-amyloid in the brain of patients with Alzheimer’s disease. Neurobiol Aging. 2004;25:283–90. doi: 10.1016/S0197-4580(03)00111-8. [DOI] [PubMed] [Google Scholar]
- Yang J, Xuesong Liu, Kapil Bhalla, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;75:1129–32. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Yang S-P, Dong-Goo Bae, Kang HJ, et al. Co-accumulation of vascular endothelial growth factor with β-amyloid in the brain of patients with Alzheimer’s disease. Neurobiol Aging. 2004;25:283–90. doi: 10.1016/S0197-4580(03)00111-8. [DOI] [PubMed] [Google Scholar]
- Zanke BW, Lee C, Arab S, et al. Death of tumor cells after intracellular acidification is dependent on stress-activated protein kinases (SAPK/JNK) pathway activation and can not be inhibited by Bcl-2 expression or inteleukin 1β-converting enzyme inhibition. Cancer Res. 1998;58:2801–8. [PubMed] [Google Scholar]
- Zhiuha X, Schendel S, Matsuyama S, et al. Acidic pH promotes dimerization of Bcl-2 family proteins. Biochemistry. 1998;37:6410–18. doi: 10.1021/bi973052i. [DOI] [PubMed] [Google Scholar]
- Zhu JK, Sun Y, Mao XO, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–50. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Ogawa O, Wang Y, et al. JKK1, an upstream activator of JNK/SAPK, is activated in Alzheimer’s disease. J Neurochem. 2003;85:87–93. doi: 10.1046/j.1471-4159.2003.01645.x. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Cohen BM, Reynolds CF. Platelet membrane fluidity in Alzheimer’s disease and major depression. Am J Psychiatry. 1987a;144:860–8. doi: 10.1176/ajp.144.7.860. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Wusylko M, Cohen BM, et al. Family study of platelet membrane fluidity in Alzheimer’s disease. Science. 1987b;238:539–42. doi: 10.1126/science.3659926. [DOI] [PubMed] [Google Scholar]