Abstract
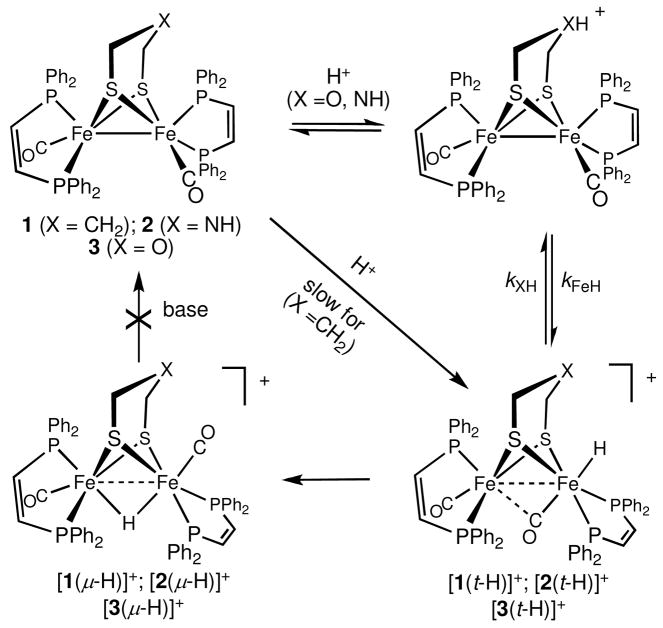
The dithiolate cofactor for the [FeFe]-hydrogenase models, Fe2(xdt)(CO)2(dppv)2 (where xdt = 1,3-propanedithiolate (pdt), azadithiolate (adt), (SCH2)2NH, and oxadithiolate (odt), (SCH2)2O; dppv = cis-1,2-bis-(diphenylphosphino)ethylene) have been probed for their functionality as proton relays enabling formation and deprotonation of terminal hydrides. Compared to the propanedithiolate derivative, the azadithiolate and oxaditiholate show enhanced rates of proton transfer between solution and the terminal site on one Fe center. The results are consistent with the heteroatom of the dithiolate serving a gating role for both protonation and deprotonation. The pKa of the transiently formed ammonium (pK CD2Cl2 5.7–8.2) or oxonium (pK CD2Cl2 −4.7–1.6) regulates the proton transfer. As consequence, only the azadithiolate is capable of yielding the terminal hydride from weak acids. The aza- and oxadithiolates manifested the advantages of proton relays: the odt derivative proved to be a faster catalyst for hydrogen evolution than the pdt derivative as indicated from cyclic voltammetry plots of ic/ip vs. [H+]. The adt derivative was capable of proton reduction from the weak acid [HPMe2Ph]BF4 (pK CD2Cl2 = 5.7). The proton relay function does not apply to the isomeric bridged-hydrides [Fe2(xdt)(μ-H)(CO)2(dppv)2]+, where the hydride is too distant and too basic to interact to be affected by the heteroatomic relay site. None of these μ-H species can be deprotonated.
The [FeFe]-hydrogenases are among the very best catalysts known for the reduction of protons to dihydrogen, with turnover frequencies estimated to be ~6000 mol H2/mol enzyme per second operating at nearly Nerstian potentials.1 The question about why the [FeFe]-hydrogenases are so efficient is topical,2 and the answer is likely related to the incompletely characterized dithiolate cofactor that bridges the diiron subunit. In 2001, Nicolet et al. proposed that this dithiolate is the azadithiolate (adt, (SCH2)2NH), wherein the amine functionality could relay protons to and from the apical site on the distal Fe center.3 It is known that, unlike typical amine bases, transition metals can be slow to protonate.4 The adt hypothesis is attractive because it potentially shows how to couple the kinetic facility of amine protonation with the redox abilities of iron hydrides. Indeed, DuBois has demonstrated that amine bases constrained within diphosphine ligands greatly accelerate both H2 uptake and production for mononuclear iron and nickel phosphine complexes.5 A recent DFT investigation suggests that the dithiolate cofactor is the oxadithiolate (odt, (SCH2)2O), which also merits evaluation since protein crystallography cannot distinguish between C, N, and O.6
Efforts to understand and exploit the possible presence of the adt cofactor have been underway for several years. The amine can be protonated independently of the diiron site.7,8 However, N-protonation has little effect on proton reduction by the catalysts of the general type Fe2(μ-H)(SR)2L6, where μ-H indicates that the hydride bridges the two Fe centers.9,10
The recent discovery that diiron(I) dithiolates initially protonate to give terminal, not bridging, hydrides opens a new and potentially significant phase in elucidating the role of the dithiolate cofactor in the catalysis.11 Terminal hydride ligands12 would be directly adjacent to the heteroatom in the dithiolate, which is therefore well positioned as a site for proton-relay. We recently demonstrated that protonations of the electronically symmetrical Fe2(pdt)(CO)2(dppv)2 (1) and Fe2(adt)(CO)2(dppv)2 (2) yield relatively stable (t1/2 ~ minutes at 25 °C), terminal hydride derivatives (dppv = cis-1,2-bis(diphenylphosphino)ethylene; pdt = 1,3-propanedithiolate; adt = 2-azapropane-1,3-dithiolate). These terminal hydrides undergo reduction at a milder potential than the isomeric bridging hydride species, are catalytically competent, and are sufficiently robust to study in detail.13 The crucial unanswered question is whether a heteroatom in the dithiolate participates in proton transfer to and from the terminal hydride. To address this question, we report results that demonstrate a functional role of oxa- and azadithiolates as proton relays. These experiments are benchmarked relative to the third crystallographically feasible dithiolate, propanedithiolate. We prepared Fe2(odt)(CO)2(dppv)2 from the hexacarbonyl14 and confirmed spectroscopically (odt = 2-oxopropane-1,3-dithiolate).
Protonation of Fe2(odt)(CO)2(dppv)2 (3) at −78 °C with the strong acid [H(Et2O)2]BAr F4 afforded the terminal hydride [3(t -H)]BArF4. 1H and 31P NMR analysis confirmed that protonation occurred at a single Fe center, characteristic of related derivatives.15 This terminal hydride was found to isomerize upon warming to give the μ-hydride complex, [3(μ-H)]BArF4 (2.6 × 10−4 s−1, −10 °C), a process following unimolecular kinetics. The isomerization rate is similar to that for [2(t-H)]BArF4 (1.4 × 10−4 s−1) but is faster than [1(t-H)]BArF4 (2.5 × 10−5 s−1).
Odt and adt Accelerate Deprotonation of [HFe2(xdt)(CO)2(diphosphine)2]+
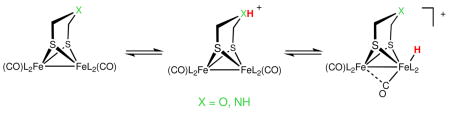
We first compared the facility with which a CD2Cl2 solution of [3(t-H)]BArF4 deprotonates. At −78 °C, [3(t-H)]BArF4 is unreactive toward base, but upon warming to ~ 0 °C two products form, [3(μ-H)]BArF4 and 3, as assayed by 1H and 31P NMR spectroscopy. The ratio of these two products was unaffected by concentration of the base as well as its pKa, as indicated by deprotonations with both strong and weak bases, respectively tetramethylguanidine (TMGH+, pKa ~23) and PPh3 ([HPPh3] BF4, pK CD2Cl2 = 1.6).16 In contrast, [1(t-H)]BArF4 cannot be deprotonated by any base, even at room temperature, where isomerization to [1(μ-H)]+ eventually occurs. Deprotonation of [2(t-H)]BArF4 is however immediate with PBu3 ([HPBu3]BF4, pK CD2Cl2 = 8.2) even at −90 °C, exclusively providing 2. The close similarity of the IR spectra in the νCO region for [1(t-H)]BArF4, [2(t-H)]BAr F4, and [3(t-H)]BAr F4 suggests that these terminal hydrides should have similar thermodynamic acidities.17 The similar thermodynamic acidities of these three hydrides indicate that the rate of deprotonation is strongly influenced by the presence of a heteroatom in the dithiolate (Scheme 1). Not only is deprotonation of the terminal hydrides strongly affected by the identity of the dithiolate ligand, the stereochemistry of the hydride also has a profound effect. The three bridging hydrides, [1(μ-H)]+, [2(μ-H)]+, and [3(μ-H)]+ are not deprotonated by NEt3 at room temperature.
Scheme 1.
Acid-base reactions of 1–3.
Heteroatom in the Dithiolate Strongly Affects the Protonation of Fe2(xdt)(CO)2(diphosphine)2
The presence of a heteroatom was found to strongly affect the rate of protonation at iron. The strong acid [H(Et2O)2]BAr F4 protonated 1, 2, and 3 quickly at −90 °C, but the billion fold weaker acid [HPMe2Ph]BF4 (pKCD2Cl2 = 5.7) protonated only 2 (−90 °C), not 1 or 3.16 The pKa of [2H]+ is bracketed by the finding that 2 is not protonated by [HPBu3]BF4. The implication that the acidity of the ammonium and terminal hydride tautomers of [2H]+ are comparable is supported by the previously reported finding that the ratio of the ammonium and hydride tautomers can be shifted by the solvent: MeOH favors the ammonium tautomer, CH2Cl2 the hydride tautomer.18
These results are consistent with a mechanism whereby hydride formation is regulated by the basicity of the heteroatom in the dithiolate: the ammonium center in 2 is easily protonated and then quickly relays protons to Fe. In contrast for complexes with weakly basic oxadithiolate (pKCD2Cl2(R2OH+) ~−4.7 to 1.6) or nonbasic propanedithiolate, the Fe site can only be protonated by strong acids, even though the basicities of these diiron centers are very similar.
Heteroatom-Containing Dithiolates Enhance Proton Reduction Catalysis
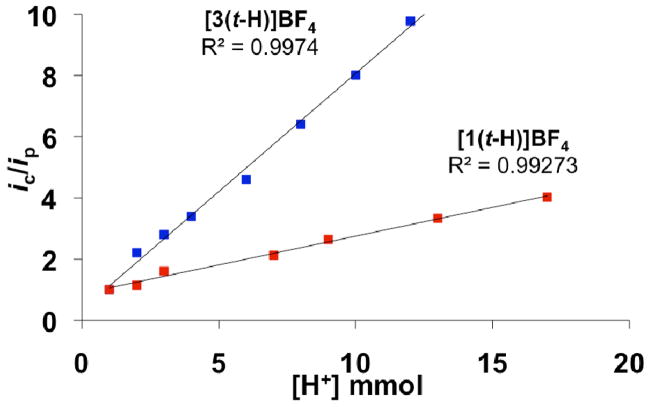
As the azadithiolate exhibits enhanced rates of protonation, this enhancement could be manifested in catalysis by accelerating the rate of proton reduction. At −40 °C, where these terminal hydrides are stable, the hydrides [1(t-H)]BF4, [2(t-H)]BF4, and [3(t-H)]BF4 all catalyze hydrogen evolution at about the same potentials, ~−1.5 V vs Fc/Fc+ (~−0.8 V vs NHE). Using [HPMe2Ph]BF4 (pKCD2Cl2 = 5.7), however, [2(t-H)]BF4 is catalytically active, but [1(t-H)]BF4 and [3(t-H)]BF4 are not. The pKCD2Cl2 of [HPMe2Ph]BF4 has been estimated to correspond to an aqueous pKa of 6.8.16 Catalysis by the amine [2(t-H)]BF4 with strong acids is complicated because protonation occurs at both the amine and terminally at Fe.7,10,18 Interestingly, for strong acids, [3(t-H)]BF4 is a significantly faster catalyst for hydrogen evolution than is [1(t-H)]BF4, which suggests that even the weakly basic ether group assists in proton relay (Figure 1).
Figure 1.
Dependence of current (ic/ip) vs of [HBF4·Et2O] for [1(t-H)]BF4 and [3(t-H)]BF4 (−40 °C, 1 mM catalyst), where ic is peak catalytic current and ip is the peak current in the absence of acid.
The results presented in this paper indicate that the presence of a heteroatom in the dithiolate bridge strongly facilitates proton transfer to and from the apical site on Fe, but only to the extent that the acid can protonate the bridgehead atom. Although both azadithiolate (in 2) and oxadithiolate (in 3) exhibit relay-like behavior, indicated by enhanced rates of proton reduction catalysis by 2 and 3, only the azadithiolate 2 enables hydride formation from weak acids, which is relevant to catalysis at low overpotentials.19
Supplementary Material
Preparative and spectroscopic details. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This research was supported by NIH and PRF.
References
- 1.Vincent KA, Parkin A, Armstrong FA. Chem Rev. 2007;107:4366–4413. doi: 10.1021/cr050191u. [DOI] [PubMed] [Google Scholar]; Fontecilla-Camps JC, Volbeda A, Cavazza C, Nicolet Y. Chem Rev. 2007;107:4273–4303. doi: 10.1021/cr050195z. [DOI] [PubMed] [Google Scholar]
- 2.Lewis NS, Nocera DG. Proc Nat Acad Sci. 2006;103:15729–15735. doi: 10.1073/pnas.0603395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicolet Y, de Lacey AL, Vernede X, Fernandez VM, Hatchikian EC, Fontecilla-Camps JC. J Am Chem Soc. 2001;123:1596–1601. doi: 10.1021/ja0020963. [DOI] [PubMed] [Google Scholar]
- 4.Kramarz KW, Norton JR. Prog Inorg Chem. 1994;42:1–65. [Google Scholar]
- 5.Henry RM, Shoemaker RK, DuBois DL, Rakowski DuBois M. J Am Chem Soc. 2006;128:3002–3010. doi: 10.1021/ja057242p. [DOI] [PubMed] [Google Scholar]; Wilson AD, Shoemaker RK, Miedaner A, Muckerman JT, DuBois DL, Rakowski Dubois M. Proc Nat Acad Sci. 2007;104:6951–6956. doi: 10.1073/pnas.0608928104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandey AS, Harris TV, Giles LJ, Peters JW, Szilagyi RK. J Am Chem Soc. 2008;130:4533–4540. doi: 10.1021/ja711187e. [DOI] [PubMed] [Google Scholar]
- 7.Eilers G, Schwartz L, Stein M, Zampella G, de Gioia L, Ott S, Lomoth R. Chem Eur J. 2007;13:7075–7084. doi: 10.1002/chem.200700019. [DOI] [PubMed] [Google Scholar]
- 8.Wang F, Wang M, Liu X, Jin K, Dong W, Li G, Åkermark B, Sun L. Chem Commun. 2005:3221–3223. doi: 10.1039/b503371c. [DOI] [PubMed] [Google Scholar]; Stanley JL, Heiden ZM, Rauchfuss TB, Wilson SR, De Gioia L, Zampella G. Organometallics. 2007;27:119–125. doi: 10.1021/om7009599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang S, Liu J, Shi Y, Wang Z, Åkermark B, Sun L. Dalton Trans. 2007:896–902. doi: 10.1039/b615037c. [DOI] [PubMed] [Google Scholar]; Wang Z, Liu JH, He CJ, Jiang S, Åkermark B, Sun LC. J Organometal Chem. 2007;692:5501–5507. [Google Scholar]
- 10.Wang F, Wang M, Liu X, Jin K, Dong W, Sun L. Dalton Trans. 2007:3812–3819. doi: 10.1039/b706178a. [DOI] [PubMed] [Google Scholar]
- 11.Capon JF, Ezzaher S, Gloaguen F, Petillon FY, Schollhammer P, Talarmin J. Chem Eur J. 2007;14:1954–1964. doi: 10.1002/chem.200701454. [DOI] [PubMed] [Google Scholar]
- 12.van der Vlugt JI, Rauchfuss TB, Whaley CM, Wilson SR. J Am Chem Soc. 2005;127:16012–16013. doi: 10.1021/ja055475a. [DOI] [PubMed] [Google Scholar]
- 13.Barton BE, Rauchfuss TB. Inorg Chem. 2008;47:2261–2263. doi: 10.1021/ic800030y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Rauchfuss TB. J Am Chem Soc. 2002;124:726–727. doi: 10.1021/ja016964n. [DOI] [PubMed] [Google Scholar]; Song LC, Yang ZY, Bian HZ, Hu QM. Organometallics. 2004;23:3082–3084. [Google Scholar]
- 15.Morvan D, Capon JF, Gloaguen F, Le Goff A, Marchivie M, Michaud F, Schollhammer P, Talarmin J, Yaouanc JJ. Organometallics. 2007;26:2042–2052. [Google Scholar]
- 16.Li T, Lough AJ, Morris RH. Chem Eur J. 2007;13:3796–3803. doi: 10.1002/chem.200601484. [DOI] [PubMed] [Google Scholar]
- 17.Landau SE, Morris RH, Lough AJ. Inorg Chem. 1999;38:6060–6068. doi: 10.1021/ic990876a. [DOI] [PubMed] [Google Scholar]
- 18.Barton BE, Rauchfuss TB. Inorg Chem. 2008;47:2261–2263. doi: 10.1021/ic800030y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felton GAN, Glass RS, Lichtenberger DL, Evans DH. Inorg Chem. 2006;45:9181–9184. doi: 10.1021/ic060984e. [DOI] [PubMed] [Google Scholar]; Felton GAN, Vannucci AK, Chen J, Lockett LT, Okumura N, Petro BJ, Zakai UI, Evans DH, Glass RS, Lichtenberger DL. J Am Chem Soc. 2007;129:12521–12530. doi: 10.1021/ja073886g. [DOI] [PubMed] [Google Scholar]; Hu XL, Brunschwig BS, Peters JC. J Am Chem Soc. 2007;129:8988–8998. doi: 10.1021/ja067876b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preparative and spectroscopic details. This material is available free of charge via the Internet at http://pubs.acs.org.