Abstract
Objective
To examine the relation between baseline fat mass and gain in bone area and bone mass in preschoolers studied prospectively for 4 y, with a focus on the role of physical activity and TV viewing.
Study design
Children were part of a longitudinal study in which measures of fat, lean and bone mass, height, weight, activity, and diet were taken every 4 months from ages 3 to 7 y. Activity was measured by accelerometer, and TV viewing by parent checklist. We included 214 children with total body dual energy x-ray absorptiometry (Hologic 4500A) scans at ages 3.5 and 7 y.
Results
Higher baseline fat mass was associated with smaller increases in bone area and bone mass over the next 3.5 y (p<0.001). More TV viewing was related to smaller gains in bone area and bone mass accounting for race, sex, and height. Activity by accelerometer was not associated with bone gains.
Conclusions
Adiposity and TV viewing are related to less bone accrual in preschoolers.
Keywords: bone mineral, preschool, fat mass, sedentary, obesity, outdoor play
While it is known that the strength of bone is its resistance to fracture and largely reflects skeletal adaptation to muscle forces, the impact of obesity on bone strength in children is unclear 1, 2. Obesity might promote increases in bone strength to support the locomotion of a heavy body mass; higher adiposity in children is related to greater bone area and BMC.3-5 If overweight children have a bone strength advantage, it might be due to the greater muscle force needed to move a larger body mass. On the other hand, in adults of similar weight, those with more fat mass have lower bone mass6 and higher fracture risk7. In studies of children, some have reported that forearm fracture risk is greater in children with more fat mass,8, 9 whereas others have not.10 Part of the increased fracture risk with greater fat mass may be due to poorer balance11 and higher impact force with falling in heavier children.12 Children with greater relative fat mass may be more prone to fracture because they have lower bone mineral content (BMC).13, 14. Muscle mass is more strongly correlated with BMC than is fat mass in children,15 so overweight children with low muscle mass may still have low BMC. Different characterization of adiposity as absolute fat mass3-5 or fat mass relative to total mass13, 14 may also contribute to the inconsistent findings.
Physical activity must be considered as a possible confounder or mediator of the relationship of bone mass with fat mass, as either low activity or high sedentary time may plausibly lead to low muscle and high fat mass, or vice versa. The inconsistent findings on the relation between adiposity and BMC, and possibly fracture risk, in children, reflect our limited understanding of the relations among activity, fat mass, and bone during growth.
A limitation of prior pediatric studies on bone health in relation to body fat is that they have included children spanning multiple stages of maturation.3, 8, 9, 13, 14, 16-19 At puberty, the levels of bone- and adiposity-promoting hormones such as estradiol and leptin,20 are changing, and thus complicating the evaluation of the relation between body fat and bone. Studies on bone health in relation to body fat were conducted in solely pre- or early-pubertal children (Tanner 1 or 2), ranging in age from 7 to 10 y. A study in 10-year-old girls showed higher baseline fat mass was associated with greater subsequent 2-year increases in total body bone area and BMC.4 Similarly, fat mass was associated with greater bone area and BMC in 7-8-year olds.5 A third study showed no difference in bone density between children with BMI above the 95th and below the 85th percentile for age and sex.21 None considered the potential confounding or mediating effects of physical activity, which may partly explain the lack of agreement of findings. Our objective was to examine the relation of adiposity with bone accrual in preschoolers, with a focus on the role of physical activity and TV viewing.
METHODS
Study population
The data for the current analyses were collected as part of a longitudinal study of children in which serial measures of fat, lean and bone mass, height, weight, physical activity, and dietary intake were taken every 4 months from ages 3 to 7 y (13 visits). The study's main aim was to determine the age at BMI rebound (the point in early childhood when body fatness reaches a nadir before increasing again). Children recruited from pediatric practices in a midwest metropolitan region were eligible if they were born at term (38-42 weeks gestation), had a birth weight that was appropriate for gestational age, were free from known chronic disease, and had at least one biological parent available to provide information. The parent(s) provided written informed consent. The study was approved by the Institutional Review Board.
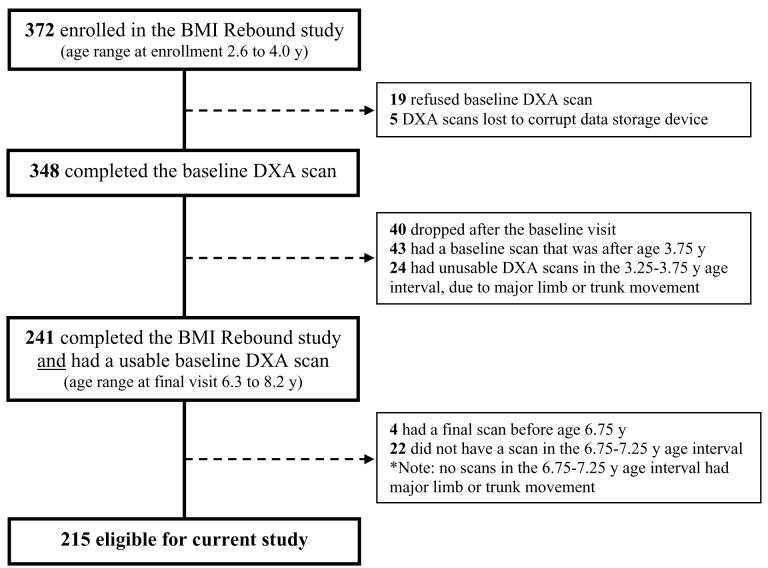
Of the 372 children enrolled in the larger study, 348 (93%) had a total body dual energy x-ray absorptiometry (DXA QDR4500, Hologic Inc, Waltham MA) scan at baseline, and 308 completed the study. To maximize the time interval between baseline and follow up scans, while maintaining a narrow age range at each scan, we only included, in this analysis, the 215 children with 2 acceptable scans, one between ages 3.25 and 3.75 y, and the other between 6.75 and 7.25 y (Figure 1; available at www.jpeds.com).
Figure 1.
Selection of children for the current study from the main study.
Anthropometrics, body composition, and bone
Height and weight were measured using a wall-mounted stadiometer and digital scale. BMI z-scores were determined using the 2000 Centers for Disease Control and Prevention growth reference.22 Total body fat mass, lean mass, bone area, and BMC were measured by DXA. We previously reported measurement error of <1.9% for fat, lean, and bone mass by DXA QDR4500 at our institution.23 The skull was excluded from the bone area and BMC data to avoid the complication of the decline in head size relative to body size over time in young children. All scans were analyzed with low-density bone edge detection algorithms (Hologic software v12.4). Trained personnel visually inspected all printed scans and those with major limb or trunk movement were excluded (n=24, Figure 1).
Physical activity and TV viewing
Parents were asked to have the child wear for 2 weekdays and 1 weekend day per visit a tri-axial accelerometer (RT3, Stay Healthy Inc., Monrovia, CA) on the right hip except when sleeping, swimming, or bathing. The tri-axial data were downloaded as vector magnitude units (i.e., “counts” based on all 3 axes). Sometimes the accelerometer was worn for more than 3 days, as the RT3 collects data for 7 days once switched on. For each day, accelerometer counts per minute was calculated following the algorithm in Table I (available at www.jpeds.com). After this process, 6348 days from our 214 children qualified for analyses. We used the average of the average counts per minute over all of the child's visits (80% with at least 10 visits) for analyses. The overall mean counts per minute for years 1 (3 visits), 2 (3 visits), 3 (3 visits), and 4 (4 visits) did not differ by a large degree (<8%), suggesting that information was not lost by averaging over all 13 visits. All usable days were included (93% of children with ≥20 usable days).
Table 1.
Algorithm for handling of RT3 accelerometer data (daily recordings) for children ages 3 to 7 years. Counts = minute-by-minute recordings based on 3 axes.
| Sequential Criteria | # of days excluded |
# of days remaining |
|---|---|---|
| 1. Determine start time: the first 5 consecutive minutes after 6:00am with ≥10 counts each. | ||
| 2. Determine stop time: the last 10 minutes of the day where 7 of the minutes had ≥10 counts, followed by 120 consecutive minutes with <10 counts each. | ||
| 3. Wearing period: Subtract start time from stop time. | 158661 | |
| 4. If wearing period <8 hours, then exclude. | 4993 | 10873 |
| Rationale: Unlikely that the full day was captured. | ||
| 5. If wearing period >20 hours, then exclude. | 150 | 10723 |
| Rationale: Unlikely that a child would have over 20 consecutive hours uninterrupted by a sleeping period of at least 2 hours. | ||
| 6. If >50% of the minutes in the wearing period have a count of <10, then exclude. | 1064 | 9659 |
| Rationale: Unlikely that a child would spend over half the wearing period completely sedentary (high likelihood of not wearing the instrument for the whole day). | ||
| 7. If the average counts per minute in the wearing period <20, then exclude. | 3 | 9656 |
| Rationale: Unlikely that the child would spend almost the entire wearing period sedentary or in extremely low activity (high likelihood of not wearing the instrument for the whole day; or instrument malfunction). | ||
| 8. If the average counts per minute in the wearing period >2000, then exclude. | 65 | 9591 |
| Rationale: Unlikely that the child would spend almost the entire wearing period in extremely high activity (high likelihood of not wearing the instrument for the whole day; or instrument malfunction). |
Total # of days recorded from the full cohort of children (n=372 enrolled) followed every 4 months for 13 visits
For 2 weekdays and 1 weekend day at each visit, TV viewing was estimated from the parent(s) check-box responses to the question: “How much time did your child spend watching TV or videotapes?” (0; 1-30; 31-60; or >60 min) from “wake-up time until noon”, “noon until 6 pm”, and “6 pm until midnight”. The check-box responses were assigned categorical values of 0, 1, 2, and 3. The sum of the categorical values for the 3 time increments was calculated (range 0-9), and the average sum across the 3 days determined. An increase of one was assumed to represent an additional 30 minutes of TV viewing (1 = 30 minutes; 2 = 60 minutes;…; 9 = 270 minutes). These estimates were averaged to obtain the hours/day spent viewing TV using all 3-day records (95% with at least 12 records). The overall mean TV viewing for years 1 (3 visits), 2 (3 visits), 3 (3 visits), and 4 (4 visits) had a narrow range (2.0-2.3 hours), suggesting that, as with counts per minute, information was not lost by averaging over all 13 visits. Outdoor playtime was measured on the same questionnaire with the same check-box method as previously described,24 giving the average hours/day spent in outdoor play over 13 visits.
Calcium intake
Parent(s) were asked to provide a 3-day diet record (2 weekdays and 1 weekend day) for the same days as the accelerometer recordings. Nutrient Data System for Research (NDS) software (v2005, Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN) was used to determine average daily calcium intake for each 3-day record. Only 10 of the 4258 records collected for the whole cohort were <3 days. The average of the 3-day averages over 13 records (97% with at least 12 records) was used to control for the potential confounding effect of calcium intake, which may be related to both bone25 and fat26 accrual in children.
Statistical methods
The percentage change in bone area (Δbone area) and ΔBMC from age 3.5 to 7 y were calculated, and linear regression used to examine the bi-variate relation of these variables with fat mass at age 3.5 y. Analysis of variance was used to describe differences by race and sex in bone area, BMC, fat mass, lean mass, height, BMI, counts per minute, TV viewing, outdoor playtime, and calcium intake.
For hypothesis testing, bone area and BMC at age 7 y were the dependent variables, and fat mass at age 3.5 y the primary independent variable. We used analysis of covariance to examine the relation between fat mass at age 3.5 y and bone area or BMC at age 7 y, adjusting for bone area or BMC at age 3.5 y, race, sex, exact age at baseline and final visit, height or lean mass at age 7 y, and calcium intake. The co-linear variables of height and lean mass were not included simultaneously. Models were run with and without the counts per minute, TV viewing, and outdoor playtime variables to test if activity explained the relation between fat mass and bone area or BMC. Interaction terms for fat mass-by-race and fat mass-by-sex were checked for significance. Parsimonious models, determined by sequential backward elimination of co-variables having a p value of >0.10, are reported. A categorical variable for quartile of fat mass at age 3.5 y was entered into the final models to obtain the least squares means for bone area and BMC at age 7 y according to fat mass quartile at age 3.5 y.
To confirm consistency of findings, we ran similar models in which Δbone area and ΔBMC were the dependent variables and included the same co-variables obtained by the primary hypothesis-testing approach (preceding paragraph). Models with bone area and BMC expressed as the absolute change from baseline (rather than %) also were examined, with baseline bone area or BMC included. Similar findings occurred with all approaches. We report only the models examining the relation between fat mass at age 3.5 y and bone area or BMC at age 7 y after adjusting for bone area or BMC at age 3.5 y.
To gain insight on whether bone area and BMC accrual were more closely linked with total body weight or just fat mass, children were classified as below or above the median for fat mass within each tertile for weight at age 3.5 y (6 fat mass-by-weight groups; n of 33 to 38 per group). The fat mass-by-weight term was entered into the final models to obtain the least squares means for bone area and BMC at age 7 y for the 6 groups.
One child with a fat mass at age 3.5 y that was >5 SD above the mean was excluded from all analyses because inclusion of this single observation resulted in a >20% change in the beta coefficient for fat mass in statistical models (final N=214).
RESULTS
Bone area, BMC, fat mass, lean mass, activity, and calcium intake at ages 3.5 and 7 years according to race and sex are shown in Table II. Reported household income was $20 000-$49 999 for 37% of white and 41% of black parents; and $50 000-$74 999 for 58% of white and 59% of black parents (p=0.6). Although the BMI z-score was lower for blacks than whites at age 3.5 years, blacks had a greater change in BMI z-score from age 3.5 to 7 years (0.64 ± 0.86 vs. 0.05 ± 0.63, p<0.001) such that the difference in BMI z-score between blacks and whites at age 7 years was not statistically significant. Fat mass at age 3.5 years was inversely associated with Δbone area (r=−0.38, p<0.001) and ΔBMC (r=−0.21, p<0.01).
Table 2.
Bone area and bone mineral content, body composition, physical activity, TV viewing, and calcium intake according to race and sex.
| Girls | Boys | |||||||
|---|---|---|---|---|---|---|---|---|
| Black n=24 |
White n=81 |
Black n=17 |
White n=92 |
|||||
| Age 3.5 y | Age 7 y | Age 3.5 y | Age 7 y | Age 3.5 y | Age 7 y | Age 3.5 y | Age 7 y | |
| Dependent variables | ||||||||
| Bone area (cm2) a,* | 739 ± 71 | 976 ± 76 | 758 ± 44 w | 972 ± 64 | 736 ± 49 | 997 ± 78 x | 735 ± 42 w | 950 ± 64 x |
| Bone mineral content (g) § | 337 ± 59 | 650 ± 90 w | 332 ± 39 | 608 ± 80 x | 344 ± 42 | 679 ± 109 x,y | 322 ± 36 | 587 ± 75 w,y |
| Independent variable | ||||||||
| Fat mass (kg) b,c,† | 4.2 ± 1.3 | 7.9 ± 3.9 w | 4.8 ± 1.0 x,y | 7.1 ± 2.4 z | 3.9 ± 0.8 y | 6.1 ± 2.8 | 4.1 ± 0.8 x | 5.7 ± 2.1 w,z |
| Co-variables | ||||||||
| Age (y) | 3.5 ± 0.1 | 7.1 ± 0.1 | 3.4 ± 0.1 | 7.0 ± 0.1 | 3.4 ± 0.1 | 7.1 ± 0.1 | 3.5 ± 0.1 | 7.0 ± 0.1 |
| Lean mass (kg) d,e,* | 11.0 ± 1.6 | 19.2 ± 3.2 | 11.0 ± 1.4 w | 18.0 ± 2.4 x | 11.8 ± 1.4 | 20.3 ± 3.4 x | 11.7 ± 1.5 w | 18.9 ± 2.7 |
| Height (cm) | 99 ± 5 | 124 ± 6 | 99 ± 4 | 123 ± 5 | 99 ± 5 | 125 ± 7 | 99 ± 4 | 123 ± 6 |
| BMI z-score † | 0.04 ± 1.22 |
0.73 ± 1.23 |
0.48 ± 0.91 | 0.53 ± 0.88 | 0.24 ± 0.71 |
0.81 ± 0.84 | 0.47 ± 1.03 | 0.52 ± 0.97 |
| Age 3 to 7 y | Age 3 to 7 y | Age 3 to 7 y | Age 3 to 7 y | |||||
| Counts per minute f,# | 471 ± 86 w,z | 541 ± 89 y,z | 513 ± 83 x | 598 ± 110 w,x,y | ||||
| TV viewing (hours/d)^ | 2.3 ± 0.1 | 2.2 ± 0.1 x | 2.7 ± 0.2 w,x | 2.2 ± 0.1 w | ||||
| Outdoor play (hours/d) g,& | 1.52 ± 0.13 | 1.59 ± 0.07 w | 1.45 ± 0.15 | 1.84 ± 0.06 w | ||||
| Calcium intake (mg/d) h,# | 706 ± 154 w | 819 ± 199 | 756 ± 165 | 893 ± 205 w | ||||
Counts per minute, TV viewing, outdoor play and calcium intake are averaged from 13 3-day measures taken every 4 months from age 3 to 7 years.
p<0.01
p<0.001 for main effect of sex (girls>boys) at age 3.5 y
p<0.001 for main effect of sex (girls>boys) at age 7 y
p<0.001 for main effect of sex (boys>girls) at age 3.5 y
p<0.05 for main effect of sex (boys>girls) at age 7 y
p<0.001
p<0.05
p<0.01 for main effect of sex (boys>girls)
p<0.05
p<0.001 for main effect of race (black>white) at age 7 y
p≤0.05 for main effect of race (white>black) at age 3.5 y
p<0.001 for main effect of race (white>black)
p=0.01 for main effect of race (black>white)
p>0.05 for main effect of race (white>black)
Matching superscripts within a row indicate significant a difference between two groups (p<0.05 Tukey HSD)
Matching superscripts within a row indicate significant a difference between two groups (p<0.05 Tukey HSD)
Matching superscripts within a row indicate significant a difference between two groups (p<0.05 Tukey HSD)
Matching superscripts within a row indicate significant a difference between two groups (p<0.05 Tukey HSD)
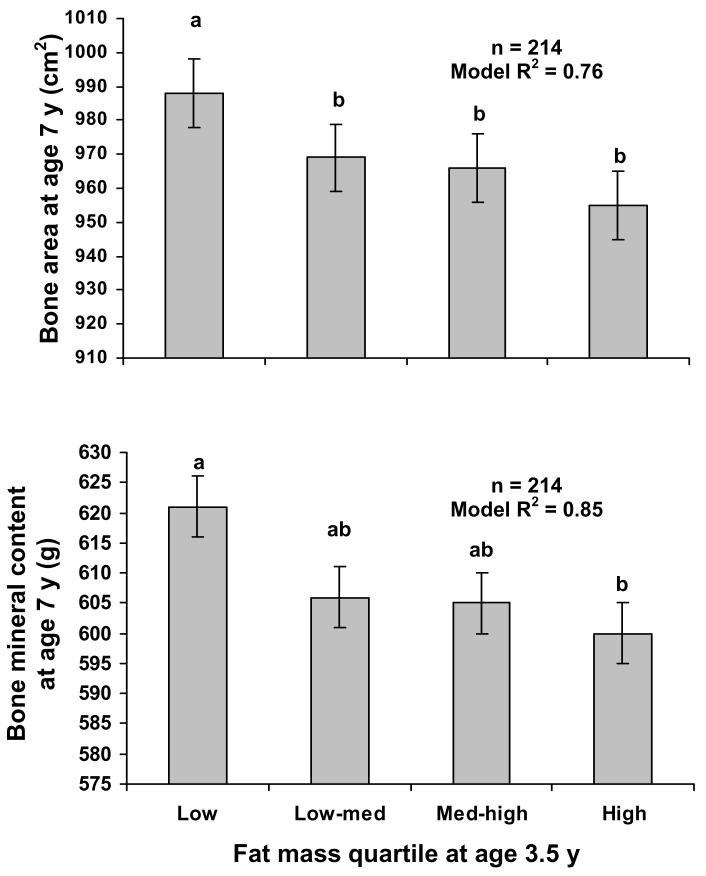
Final models are given in Table III. Briefly, fat mass at age 3.5 y was inversely related to bone area and BMC at age 7 y adjusting for bone area and BMC at age 3.5 y, race, sex, exact age at baseline and final visit, and height. Bone area and BMC at age 7 y were highest for those in the lowest quartile of fat mass at age 3.5 y (Figure 2). Counts per minute was not related to bone area or BMC at age 7 years, whether the counts per minute variable was treated as continuous or categorical [upper (n=53), inter (n=108), and lower (n=53) quartiles for counts per minute]. Outdoor playtime was not related to bone area or BMC. Children who spent <2 hours per day viewing TV (n=77) had greater bone area (least squares means: 981 ± 5 vs. 966 ± 3 cm2, p<0.01) and BMC (least squares means: 628 ± 5 vs. 617 ± 3 g, p=0.04) at age 7 y than those who spent ≥ 2 hours per day (n=137). The coefficient for fat mass at age 3.5 y was not appreciably lower when TV viewing was included (bone area: −13.23; BMC: −7.88) than when TV viewing was not included (bone area: −13.80; BMC: −8.55), suggesting that TV viewing was not a strong mediator of the relation between baseline fat mass and bone accrual. Children with < 2 hours per day spent viewing TV spent a lower proportion of daily minutes being inactive (i.e., <10 accelerometer counts) than those who spent ≥ 2 hours per day viewing TV (13.1 ± 3.9% vs. 14.5 ± 4.4%, p=0.03). TV viewing was not related to counts per minute (p=0.3) or outdoor playtime (p=0.4).
Table 3.
Parsimonious models for predicting bone area and bone mineral content at age 7 years (n = 214).
| Estimate | Standard error of the estimate | p value | ||
|---|---|---|---|---|
| Bone area at age 7 ( R2 = 0.76) | ||||
| Intercept | −367 | 144 | ||
| Fat mass at age 3.5 y (kg) | −13.23 | 2.81 | <0.001 | |
| Bone area at age 3.5 y (cm2) | 0.74 | 0.07 | <0.001 | |
| Race [white (0), black (1)] | 31.91 | 9.22 | ||
| Sex [boy (0), girl (1)] | 7.43 | 5.56 | ||
| Race*sex [white boy (0), white girl (0), | −24.86 | 12.11 | 0.04 | |
| black boy (0), black girl (1)] a | ||||
| Exact age at baseline (y) | −50.23 | 20.25 | 0.01 | |
| Exact age at final visit (y) | 36.46 | 18.99 | 0.06 | |
| Height at age 7 y (cm) b | 6.16 | 0.57 | <0.001 | |
| TV viewing hours/day [<2 (0), ≥2 (1)] | −14.31 | 5.07 | <0.01 | |
| BMC at age 7 y (R2 = 0.85) | ||||
| Intercept | −201 | 99 | ||
| Fat mass at age 3.5 y (kg) | −7.88 | 2.81 | <0.01 | |
| BMC at age 3.5 y (g) | 1.31 | 0.09 | <0.001 | |
| Race [white (0), black (1)] | 46.65 | 9.66 | ||
| Sex [boy (0), girl (1)] | 4.54 | 5.62 | ||
| Race*sex [white boy (0), white girl (0), black boy (0), black girl (1)] c | −19.95 | 12.26 | 0.10 | |
| Exact age at baseline (y) | −84.14 | 20.51 | <0.001 | |
| Height at age 7 y (cm) b | 5.84 | 0.63 | <0.001 | |
| Calcium intake (mg/day) | −0.02 | 0.01 | 0.08 | |
| TV viewing hours/day [<2 (0), ≥2 (1)] | −10.74 | 5.09 | 0.04 | |
TV viewing and calcium intake are averaged from 13 3-day measures taken every 4 months from age 3 to 7 years.
p<0.05 for Tukey HSD between least squares means (black boys > white boys).
Similar results occurred when height at age 7 y was replaced by lean mass at age 7 y.
p<0.05 for Tukey HSD between least squares means (black boys > white girls and white boys; black girls > white girls and white boys).
Figure 2.
Least squares means ± SEM for bone area (top) and BMC (bottom) at age 7 y by quartile of fat mass at age 3.5 y, adjusted for bone area or BMC at age 3.5 y, race, sex, exact age, height at age 7 y; and TV viewing.
Levels not connected by the same letter are significantly different (p<0.05 Tukey HSD between least squares means).
p<0.001 (top) and p<0.01 (bottom) for the effect of the continuous variable for fat mass at age 3.5 y in the final model (Table II).
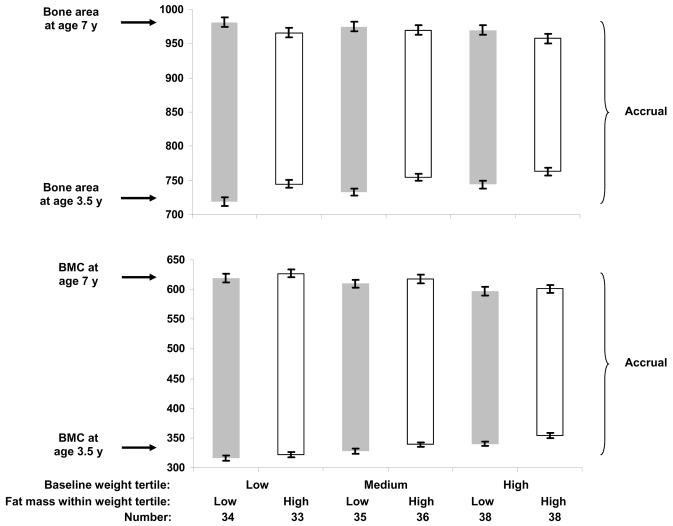
Figure 3 shows results by baseline weight tertile and fat mass (high or low) within tertile. Those with high fat mass tended to gain less bone area within each baseline weight tertile (white bars shorter than gray bars), but this relation was not present for BMC. For BMC, children who were heavier at baseline gained less BMC regardless of fat mass.
Figure 3.
The upper edge of each bar represents the least squares mean ± SEM for bone area (top) and BMC (bottom) at age 7 y, adjusted for bone area or BMC at age 3.5 y, race, sex, exact age at baseline and final visit, height at age 7 y, and TV viewing. The lower edge of each bar represents the least squares mean ± SEM for bone area (top) and BMC (bottom) at age 3.5 y, adjusted for race, sex, age, and height.
The left two, middle two, and right two bars are the low, medium, and high tertiles for weight at age 3.5 y. Within each tertile, children were classified as above- and below-the-median for fat mass at age 3.5 y.
Top: p=0.05 for effect of fat mass group and p=0.5 for effect of weight tertile.
Bottom: p=0.2 for effect of fat mass group and p=0.02 for effect of weight tertile.
The interaction of fat mass group by weight tertile was not significant for either bone area or BMC (both p>0.7).
DISCUSSION
We found that higher fat mass at age 3.5 y was associated with smaller increases in bone area and BMC over 3.5 years of follow-up, after accounting for race, sex, height, activity, and calcium intake. To our knowledge, this is the first such study to completely encompass the preschool years. Our findings contradict a prior report among older pre-pubertal children,4 but support other reports of lower bone mass13, 14 or higher fracture risk8, 9, 17, 19 in children of varied ages with more adiposity. It is difficult to separately assess the effects of fat mass and weight on bone because fat mass is a determinant of weight. However, we explored whether the relation between baseline fat mass and gain in bone area and BMC differed by baseline weight. The smaller gain in bone area (but not BMC) in children with more body fat at baseline regardless of their baseline weight may indicate that adipose-related effects on bone size (but not mass) occur in young children with a relatively high amount of body fat, but who may not be identified clinically as overweight. All else held equal, if two children have similar gains in BMC, the child with the larger concurrent gain in bone size will likely have a bone strength advantage because of a larger bone diameter. Since heavier children gained less BMC than lighter children, those who are heaviest and have the highest amount of body fat will likely have the smallest gain in bone strength. For both bone area and BMC, the long term ramifications, if any, of less accrual in early childhood on fracture risk or eventual bone strength are unknown. The 1-2% decrease in bone area and BMC per 1 kg increase in baseline fat mass that we observed likely has clinical relevance, considering that pre-pubertal children's rate of BMC gain is approximately 1-2% per year.27
A strength of our study is the narrow age span for children. Further, we had frequent, closely-spaced measures of the potential confounders or mediators of physical activity and TV viewing, important under the assumption that high fat mass may lead to inactivity or vice versa. We found no relation between physical activity by accelerometer and change in bone area and BMC. A striking finding was that children who spent less than two hours per day viewing TV had greater gains in bone area and BMC, but TV viewing did not explain the relation between fat mass and bone area and BMC. Thus, both fat mass and TV viewing appear to be related to bone accrual. Our study agrees with prior reports of a negative association between sedentary activity in pre-puberty and later bone mass,28 and higher forearm fracture risk with more TV viewing in 9- to 16-year-olds.29 We found that TV viewing was not related to counts per minute, which is not surprising as others have shown that sedentary time is not a strong correlate of physical activity.30 Our findings suggest TV viewing is a better measure of inactivity than activity, and is not likely a surrogate measure for lack of outdoor playtime. However, we did not find the proportion of daily minutes spent inactive to be related to bone area or BMC gain (data not shown). The possibility that TV viewing during early pre-puberty has a deleterious impact on bone accrual has clear public health implications as >60% of US preschoolers spend at least 2 hours per day viewing TV.31
Wide differences in maturation-related hormones among children in this study are unlikely, and all presumably had low sex steroid concentrations given their young age. Nevertheless, small differences in estradiol concentrations between pre-pubertal children with greater and lesser fat mass may lead to different bone accrual patterns. High estradiol is related to decreased periosteal and increased endosteal bone apposition,32, 33 generally giving smaller bone area (i.e., bone deposited on the “outside” periosteal surface increases bone area whereas bone deposited on the “inside” endosteal surface next to the marrow cavity does not). It is intriguing that high fat mass might be related to an inhibition of periosteal expansion that manifests as early as the preschool age. Further, the elevated leptin production in children with a high amount of body fat34 might induce a state of leptin resistance that has a deleterious effect on cortical bone formation and therefore total skeletal mass.35 It is unclear whether or to what extent early-childhood-onset leptin resistance occurs, and whether there is a corresponding biologically relevant impact on accrual of bone size, mass, or strength. Alternatively, there may be adiposity-promoting genetic and environmental factors that precede a high fat mass condition; the high fat mass condition leads to increased adipocytokine secretion; and this combination promotes a biologic environment that favors nutrient partitioning for fat rather than lean and bone accrual. Obese pre-pubertal children have normal serum insulin-like growth factor (IGF) concentrations, but decreased growth hormone (GH) secretion, which returns to normal (compared with non-obese controls) with a 25% reduction in BMI.36 However, the low serum IGF binding proteins, high GH binding protein, and high insulin of obese children do not normalize even with a 50% BMI reduction.36 The considerable disruption to the GH-IGF axis may involve an increase in tissue IGF-I availability, explaining obese children's normal growth despite low GH.37 The impact of such hormonal shifts on bone accrual is unknown. Investigation into the possible role of predisposing factors, and on the role of adipocytokines, on the relation between fat and bone accrual in children, is needed.
There are some limitations to our study. First, our instrument for parent report of TV viewing has not been validated, although similar TV records have been found to correlate highly with video assessment of TV viewing in preschoolers,38 and our TV variable was associated with our own objective measure of inactivity. Because under-reporting of TV viewing by parents of overweight children would likely result in an attenuation of the statistical relationship between TV viewing and bone, our interpretations are conservative. Second, we were not able to determine average time spent in moderate and vigorous physical activity because there are no such published counts per minute cut-points for preschoolers using the RT3. However, we did show that children in the highest quartile of daily counts per minute did not have greater bone area or BMC gains than other children. Third, we lacked 3-dimensional bone measures such as those provided by peripheral quantitative computed tomography (pQCT), and so could not examine baseline fat mass in relation to subsequent changes in trabecular bone and cortical thickness, volumetric density, and strength. A recent pQCT study39 showed smaller bone dimensions and lesser bone material at the radius and tibia in teenage females with excess fat mass than those with normal fat mass. A similar study in young children is needed to investigate the possibility that predisposing factors leading to high fat mass, and/or increases in adipocytokines due to high fat mass, are related to an inhibition of periosteal expansion or diminished bone strength as early as pre-puberty.
We found that higher fat mass at baseline, and more TV viewing, were associated with smaller increases in bone area and BMC from age 3.5 to 7 y, adjusting for other significant factors. Our findings give further compelling reasons to reduce obesity and TV viewing early in life.
ACKNOWLEDGMENTS
We are especially grateful to Karen Munson and Marcia Schmidt for their extraordinary work in the completion of this study. Most importantly, we sincerely thank the children and their families for their dedicated participation.
This project was supported by grant #R01HL064022 (PI: SRD) from the National Heart, Lung, and Blood Institute.
ABBREVIATIONS
- BMC
bone mineral content, bone mass
- BMI
body mass index
- DXA
dual energy x-ray absorptiometry
- NDS
Nutrient Data System for Research
- IGF
insulin-like growth factor
- GH
growth hormone
- pQCT
peripheral quantitative computed tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No reprints requested.
The authors declare no conflicts of interest.
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Frost HM. On our age-related bone loss: insights from a new paradigm. J Bone Miner Res. 1997;12:1539–46. doi: 10.1359/jbmr.1997.12.10.1539. [DOI] [PubMed] [Google Scholar]
- 3.Arabi A, Tamim H, Nabulsi M, Maalouf J, Khalife H, Choucair M, et al. Sex differences in the effect of body-composition variables on bone mass in healthy children and adolescents. Am J Clin Nutr. 2004;80:1428–35. doi: 10.1093/ajcn/80.5.1428. [DOI] [PubMed] [Google Scholar]
- 4.Clark EM, Ness AR, Tobias JH. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrin Metab. 2006;91:2534–41. doi: 10.1210/jc.2006-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garnett SP, Hogler W, Blades B, Baur LA, Peat J, Lee J, et al. Relation between hormones and body composition, including bone, in prepubertal children. Am J Clin Nutr. 2004;80:966–72. doi: 10.1093/ajcn/80.4.966. [DOI] [PubMed] [Google Scholar]
- 6.Zhao L, Liu Y, Liu P, Hamilton J, Recker RR, Deng H. Relationship of obesity with osteoporosis. J Clin Endocrin Metab. 2007;92:1640–6. doi: 10.1210/jc.2006-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu Y, Venners SA, Terwedow HA, Feng Y, Niu T, Li Z, et al. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006;83:146–54. doi: 10.1093/ajcn/83.1.146. [DOI] [PubMed] [Google Scholar]
- 8.Goulding A, Grant AM, Williams SM. Bone and body composition of children and adolescents with repeated forearm fractures. J Bone Miner Res. 2005;20:2090–6. doi: 10.1359/JBMR.050820. [DOI] [PubMed] [Google Scholar]
- 9.Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ. Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy x-ray absorptiometry study. J Pediatr. 2001;139:509–15. doi: 10.1067/mpd.2001.116297. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari SL, Chevalley T, Bonjour JP, Rizzoli R. Childhood fractures are associated with decreased bone mass gain during puberty: an early marker of persistent bone fragility? J Bone Miner Res. 2006;21:501–7. doi: 10.1359/jbmr.051215. [DOI] [PubMed] [Google Scholar]
- 11.Goulding A, Jones IE, Taylor RW, Piggot JM, Taylor D. Dynamic and static tests of balance and postural sway in boys: effects of previous wrist bone fractures and high adiposity. Gait Posture. 2003;17:136–41. doi: 10.1016/s0966-6362(02)00161-3. [DOI] [PubMed] [Google Scholar]
- 12.Davidson PL, Goulding A, Chalmers DJ. Biomechanical analysis of arm fracture in obese boys. J Paediatr Child Health. 2003;39:657–64. doi: 10.1046/j.1440-1754.2003.00243.x. [DOI] [PubMed] [Google Scholar]
- 13.Lazcano-Ponce E, Tamayo J, Cruz-Valdez A, Diaz R, Hernandez B, Del Cueto R, et al. Peak bone mineral area density and determinants among females aged 9 to 24 years in Mexico. Osteoporos Int. 2003;14:539–47. doi: 10.1007/s00198-002-1363-2. [DOI] [PubMed] [Google Scholar]
- 14.Weiler HA, Janzen L, Green K, Grabowski J, Seshia MM, Yuen KC. Percent body fat and bone mass in healthy Canadian females 10 to 19 years of age. Bone. 2000;27:203–7. doi: 10.1016/s8756-3282(00)00314-8. [DOI] [PubMed] [Google Scholar]
- 15.Crabtree NJ, Kibirige MS, Fordham JN, Banks LM, Muntoni F, Chinn D, et al. The relationship between lean body mass and bone mineral content in paediatric health and disease. Bone. 2004;35:965–72. doi: 10.1016/j.bone.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Goulding A, Jones IE, Taylor RW, Manning PJ, Williams SM. More broken bones: a 4-year double cohort study of young girls with and without distal forearm fractures. 2000;15:2011–8. doi: 10.1359/jbmr.2000.15.10.2011. 2000. [DOI] [PubMed] [Google Scholar]
- 17.Goulding A, Taylor RW, Jones IE, MacAuley KA, Manning PJ, Williams SM. Overweight and obese children have low bone mass and area for their weight. In J Obes Relat Metab Disord. 2000;24:627–32. doi: 10.1038/sj.ijo.0801207. [DOI] [PubMed] [Google Scholar]
- 18.Petit MA, Beck TJ, Shults J, Zemel BS, Foster BJ, Leonard MB. Proximal femur bone geometry is appropriately adapted to lean mass in overweight children and adolescents. Bone. 2005;36:568–76. doi: 10.1016/j.bone.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Skaggs DL, Loro ML, Pitukcheewanont P, Tolo V, Gilsanz V. Increased body weight and decreased radial cross-sectional dimensions in girls with forearm fractures. J Bone Miner Res. 2001;16:1337–42. doi: 10.1359/jbmr.2001.16.7.1337. [DOI] [PubMed] [Google Scholar]
- 20.Matkovic V, Ilich JZ, Skugor M, Badenhop NE, Goel P, Clairmont A, et al. Leptin is inversely related to age at menarche in human females. J Clin Endocrinol Metab. 1997;82:3239–45. doi: 10.1210/jcem.82.10.4280. [DOI] [PubMed] [Google Scholar]
- 21.Klein KO, Larmore KA, de Lancey E, Brown JM, Considine RV, Hassink SG. Effect of obesity on estradiol level, and its relationship to leptin, bone maturation, and bone mineral density in children. J Clin Endocrinol Metab. 1998;83:3469–75. doi: 10.1210/jcem.83.10.5204. [DOI] [PubMed] [Google Scholar]
- 22.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 23.Wosje KS, Knipstein BL, Kalkwarf HJ. Measurement error of DXA: interpretation of fat and lean mass changes in obese and non-obese children. J Clin Densitom. 2006;9:335–40. doi: 10.1016/j.jocd.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Burdette HL, Whitaker RC, Daniels SR. Parental report of outdoor playtime as a measure of physical activity in preschool-aged children. Arch Pediatr Adolesc Med. 2004;158:353–7. doi: 10.1001/archpedi.158.4.353. [DOI] [PubMed] [Google Scholar]
- 25.Winzenberg T, Shaw K, Fryer J, Jones G. Effects of calcium supplementation on bone density in healthy children: meta-analysis of randomised controlled trials. BMJ. 2006;333:775. doi: 10.1136/bmj.38950.561400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skinner JD, Bounds W, Carruth BR, Ziegler P. Longitudinal calcium intake is negatively related to children's body fat indexes. J Am Diet Assoc. 2003;103:1626–31. doi: 10.1016/j.jada.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrin Metab. 2007;92:2087–99. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Crawford PB, Hudes M, Van Loan MD, Siemering K, Bachrach LK. Diet in midpuberty and sedentary activity in prepuberty predict peak bone mass. Am J Clin Nutr. 2003;77:495–503. doi: 10.1093/ajcn/77.2.495. [DOI] [PubMed] [Google Scholar]
- 29.Ma D, Jones G. Television, computer, and video viewing; physical activity; and upper limb fracture risk in children: a population-based case control study. J Bone Miner Res. 2003;18:1970–7. doi: 10.1359/jbmr.2003.18.11.1970. [DOI] [PubMed] [Google Scholar]
- 30.Sallis JF, Prochaska JJ, Taylor WC. A review of correlates of physical activity of children and adolescents. Med Sci Sports Exerc. 2000;32:963–75. doi: 10.1097/00005768-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Mendoza JA, Zimmerman FJ, Christakis DA. Television viewing, computer use, obesity, and adiposity in US preschool children. Int J Behav Nutr Phys Act. 2007;25:44. doi: 10.1186/1479-5868-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner RT, Hannon KS, Demers LM, Buchanan J, Bell NH. Differential effects of gonadal function on bone histomorphometry in male and female rats. J Bone Miner Res. 1989;4:557–63. doi: 10.1002/jbmr.5650040415. [DOI] [PubMed] [Google Scholar]
- 33.Zhang XZ, Kalu DN, Erbas B, Hopper JL, Seeman E. The effects of gonadectomy on bone size, mass, and volumetric density in growing rats are gender-, site-, and growth hormone-specific. J Bone Miner Res. 1999;14:802–9. doi: 10.1359/jbmr.1999.14.5.802. [DOI] [PubMed] [Google Scholar]
- 34.Fleisch AF, Agarwal A, Roberts MD, Han JC, Theim KR, Vexler A, et al. Influence of serum leptin on weight and body fat growth in children at high risk for adult obesity. J Clin Endocrin Metab. 2007;92:948–54. doi: 10.1210/jc.2006-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34:376–83. doi: 10.1016/j.bone.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Argente J, Caballo N, Barrios V, Pozo J, Munoz MT, Chowen JA, et al. Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in prepubertal children with exogenous obesity: effect of short- and long-term weight reduction. J Clin Endocrin Metab. 1997;82:2076–83. doi: 10.1210/jcem.82.7.4089. [DOI] [PubMed] [Google Scholar]
- 37.Ballerini MG, Ropelato MG, Domene HM, Pennisi P, Heinrich JJ, Jasper HG. Differential impact of simple childhood obesity on the components of the growth hormone-insulin-like growth factor (IGF)-IGF binding proteins axis. J Pediatr Endocrinol Metab. 2004;17:749–57. doi: 10.1515/jpem.2004.17.5.749. [DOI] [PubMed] [Google Scholar]
- 38.Anderson DR, Field DE, Collins PA, Lorch EP, Nathan JG. Estimates of young children's time with television: a methodological comparison of parent reports with time-lapse video home observation. Child Dev. 1985;56:1345–57. doi: 10.1111/j.1467-8624.1985.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 39.Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am J Clin Nutr. 2007;86:1530–8. doi: 10.1093/ajcn/86.5.1530. [DOI] [PubMed] [Google Scholar]