Abstract
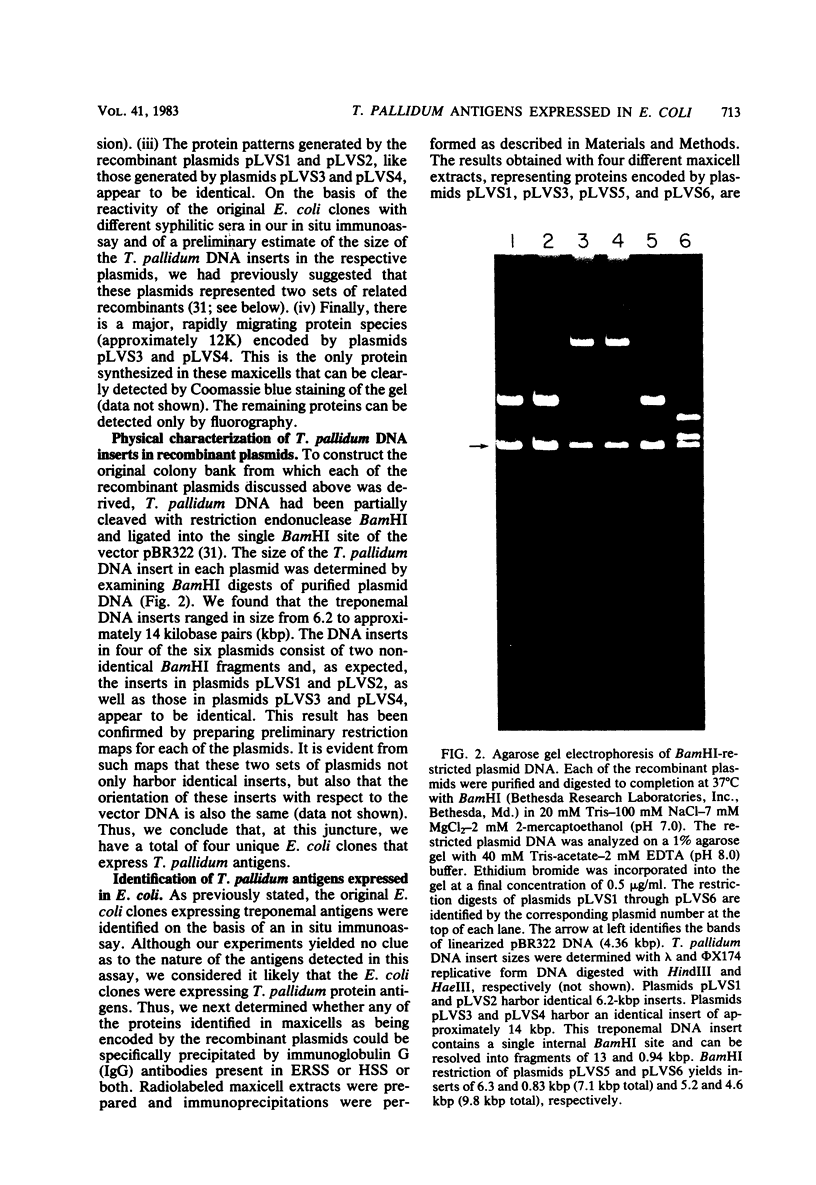
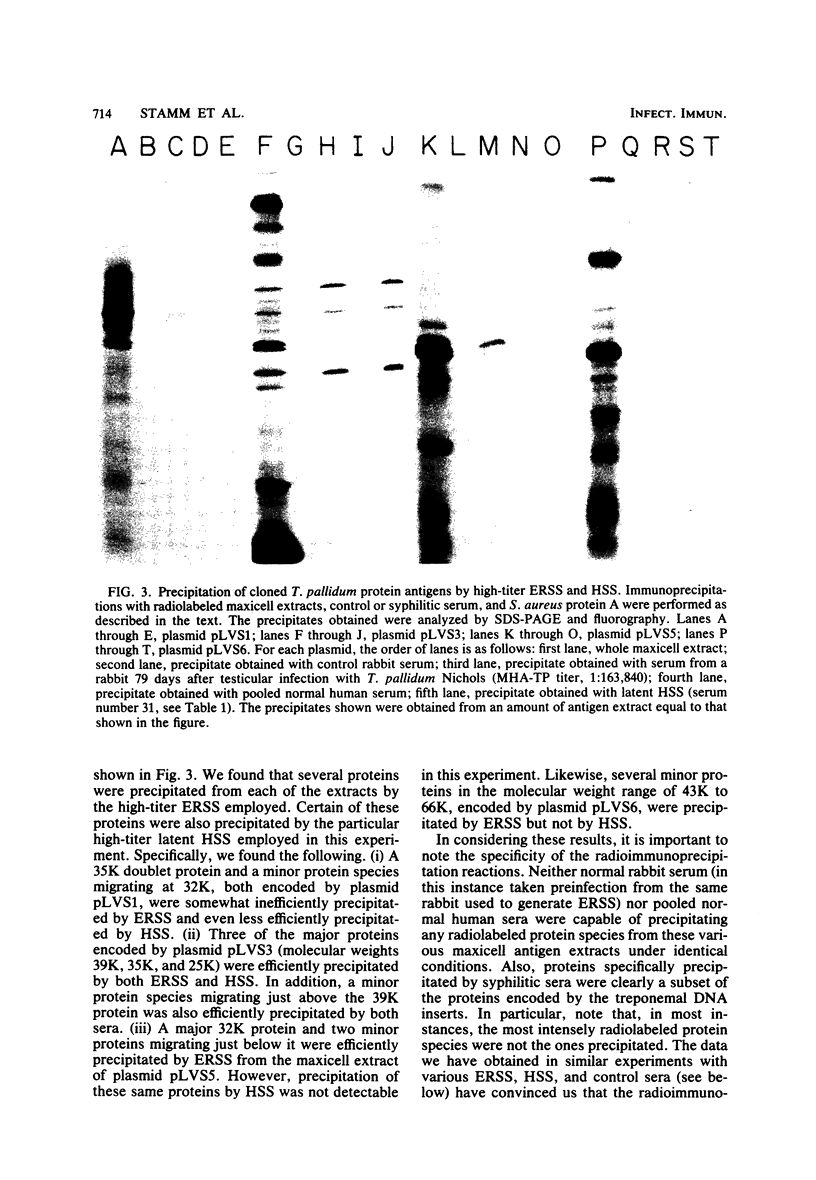
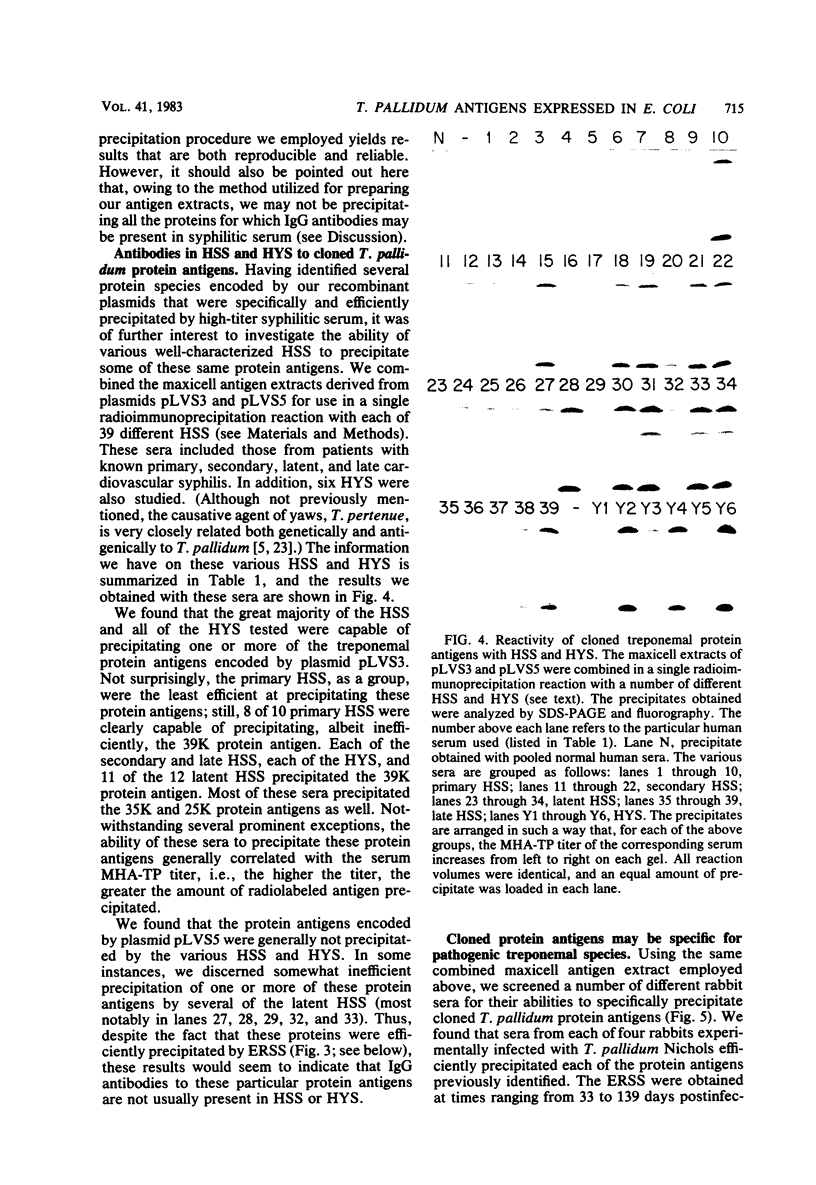
We have previously described the construction in Escherichia coli K-12 of a hybrid plasmid colony bank of Treponema pallidum (Nichols strain) genomic DNA. By screening a portion of this bank with an in situ immunoassay, we identified six E. coli clones that express T. pallidum antigens. In this study, the recombinant plasmids from each of these clones have been analyzed in E. coli maxicells and have been found to encode a number of proteins that are not of vector pBR322 origin and are, therefore, of treponemal origin. In each case, several of these proteins can be specifically precipitated from solubilized maxicell extracts by high-titer experimental rabbit syphilitic serum. Certain of these proteins are also precipitated by high-titer latent human syphilitic sera (HSS). The T. pallidum DNA inserts in these plasmids range in size from 6.2 to 14 kilobase pairs, and from the restriction patterns of the inserts and the protein profiles generated by each plasmid in maxicells, it is apparent that we have recovered a total of four unique clones from our colony bank. Recombinant plasmids pLVS3 and pLVS5 were of particular interest. Plasmid pLVS3 encodes three major protein antigens with molecular weights of 39,000, 35,000, and 25,000. These three proteins, which were not recognized by pooled normal human sera, were efficiently precipitated by most secondary HSS, latent HSS, and late HSS tested. These proteins were also precipitated, although somewhat inefficiently, by most primary HSS tested. Plasmid pLVS5 encodes a major protein antigen with a molecular weight of 32,000 and several minor protein antigens that, although efficiently precipitated by experimental rabbit syphilitic serum, were generally not recognized by the various HSS tested. Evidence is presented indicating that the protein antigens encoded by plasmids pLVS3 and pLVS5 are specific for pathogenic treponemal species. We have also demonstrated that immunoglobulin G antibodies directed against these protein antigens can be detected in rabbits experimentally infected with T. pallidum Nichols as early as 11 days postinfection.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Analysis of serum IgG against Treponema pallidum protein antigens in experimentally infected rabbits. Br J Vener Dis. 1981 Oct;57(5):302–308. doi: 10.1136/sti.57.5.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Baseman J. B. Surface characterization of virulent Treponema pallidum. Infect Immun. 1980 Dec;30(3):814–823. doi: 10.1128/iai.30.3.814-823.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Baseman J. B. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979 Dec;26(3):1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes E. C. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J Exp Med. 1980 Mar 1;151(3):573–586. doi: 10.1084/jem.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Nichols J. C., Rumpp J. W., Hayes N. S. Purification of Treponema pallidum from Infected Rabbit Tissue: Resolution into Two Treponemal Populations. Infect Immun. 1974 Nov;10(5):1062–1067. doi: 10.1128/iai.10.5.1062-1067.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Silhavy T. J., Beckwith J. R. Use of gene fusion to study secretion of maltose-binding protein into Escherichia coli periplasm. J Bacteriol. 1979 Jul;139(1):19–31. doi: 10.1128/jb.139.1.19-31.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. II. The relationship of neutralizing factors in immune serum to acquired resistance. J Immunol. 1976 Jul;117(1):197–207. [PubMed] [Google Scholar]
- Covarrubias L., Cervantes L., Covarrubias A., Soberón X., Vichido I., Blanco A., Kupersztoch-Portnoy Y. M., Bolivar F. Construction and characterization of new cloning vehicles. V. Mobilization and coding properties of pBR322 and several deletion derivatives including pBR327 and pBR328. Gene. 1981 Jan-Feb;13(1):25–35. doi: 10.1016/0378-1119(81)90040-8. [DOI] [PubMed] [Google Scholar]
- DEACON W. E., HUNTER E. F. Treponemal antigens as related to identification and syphilis serology. Proc Soc Exp Biol Med. 1962 Jun;110:352–356. doi: 10.3181/00379727-110-27515. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Fieldsteel A. H., Cox D. L., Moeckli R. A. Further studies on replication of virulent Treponema pallidum in tissue cultures of Sf1Ep cells. Infect Immun. 1982 Feb;35(2):449–455. doi: 10.1128/iai.35.2.449-455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J. Pathogenesis and immunology of Treponema pallidum. Annu Rev Microbiol. 1981;35:29–54. doi: 10.1146/annurev.mi.35.100181.000333. [DOI] [PubMed] [Google Scholar]
- HUNTER E. F., DEACON W. E., MEYER P. E. AN IMPROVED FTA TEST FOR SYPHILIS, THE ABSORPTION PROCEDURE (FTA-ABS). Public Health Rep. 1964 May;79:410–412. [PMC free article] [PubMed] [Google Scholar]
- Hanff P. A., Fehniger T. E., Miller J. N., Lovett M. A. Humoral immune response in human syphilis to polypeptides of Treponema pallidum. J Immunol. 1982 Sep;129(3):1287–1291. [PubMed] [Google Scholar]
- Hayes N. S., Muse K. E., Collier A. M., Baseman J. B. Parasitism by virulent Treponema pallidum of host cell surfaces. Infect Immun. 1977 Jul;17(1):174–186. doi: 10.1128/iai.17.1.174-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt B. Studies of the Treponema pallidum immobilizing activity in normal human serum. I. A method. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Apr;82(2):185–200. doi: 10.1111/j.1699-0463.1974.tb02311.x. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kronvall G., Williams R. C., Jr Differences in anti-protein A activity among IgG subgroups. J Immunol. 1969 Oct;103(4):828–833. [PubMed] [Google Scholar]
- Lind I., Mansa B. Further investigation of specific and non-specific adsorption of serum globulins to Staphylococcus aureus. Acta Pathol Microbiol Scand. 1968;73(4):637–645. doi: 10.1111/j.1699-0463.1968.tb03221.x. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Gubish E. R., Jr Identification of Treponema pallidum antigens: comparison with a nonpathogenic treponeme. J Immunol. 1982 Aug;129(2):833–838. [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Lloyd R. M., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980 Jan;124(1):461–467. [PubMed] [Google Scholar]
- Miao R. M., Fieldsteel A. H. Genetic relationship between Treponema pallidum and Treponema pertenue, two noncultivable human pathogens. J Bacteriol. 1980 Jan;141(1):427–429. doi: 10.1128/jb.141.1.427-429.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R., Fieldsteel A. H. Genetics of Treponema: relationship between Treponema pallidum and five cultivable treponemes. J Bacteriol. 1978 Jan;133(1):101–107. doi: 10.1128/jb.133.1.101-107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. N., de Bruijn J. H., Bekker J. H., Onvlee P. C. The antigenic structure of Treponema pallidum, Nichols strain. I. The demonstration, nature and location of specific and shared antigens. J Immunol. 1966 Mar;96(3):450–456. [PubMed] [Google Scholar]
- Norgard M. V. Rapid and simple removal of contaminating RNA from plasmid DNA without the use of RNase. Anal Biochem. 1981 May 1;113(1):34–42. doi: 10.1016/0003-2697(81)90040-3. [DOI] [PubMed] [Google Scholar]
- Pedersen N. S., Axelsen N. H., Jørgensen B. B., Petersen C. S. Antibodies in secondary syphilis against five of forty Reiter treponeme antigens. Scand J Immunol. 1980;11(6):629–633. doi: 10.1111/j.1365-3083.1980.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Pedersen N. S., Axelsen N. H., Petersen C. S. Antigenic analysis of Treponema pallidum: cross-reactions between individual antigens of T. pallidum and T. Reiter. Scand J Immunol. 1981;13(2):143–150. doi: 10.1111/j.1365-3083.1981.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Pedersen N. S., Petersen C. S., Vejtorp M., Axelsen N. H. Serodiagnosis of syphilis by an enzyme-linked immunosorbent assay for IgG antibodies against the Reiter treponeme flagellum. Scand J Immunol. 1982 Apr;15(4):341–348. doi: 10.1111/j.1365-3083.1982.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Pepose J. S., Bishop N. H., Feigenbaum S., Miller J. N., Zeltzer P. M. The humoral immune response in rabbits infected with Treponema pallidum: Comparison of antibody levels measured by the staphylococcal protein A-IgG (SPA-TP) microassay with VDRL, FTA-Abs, and TPI antibody responses during the development of acquired resistance to challenge. Sex Transm Dis. 1980 Jul-Sep;7(3):125–129. [PubMed] [Google Scholar]
- Petersen C. S., Pedersen N. S., Axelsen N. H. Purification of a Reiter treponemal protein antigen that is immunologically related to an antigen in Treponema pallidum. Infect Immun. 1982 Mar;35(3):974–978. doi: 10.1128/iai.35.3.974-978.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm L. V., Bassford P. J., Jr Cloning and expression of Treponema pallidum protein antigens in Escherichia coli. DNA. 1982;1(4):329–333. doi: 10.1089/dna.1982.1.329. [DOI] [PubMed] [Google Scholar]
- Stamm L. V., Folds J. D., Bassford P. J., Jr Expression of Treponema pallidum antigens in Escherichia coli K-12. Infect Immun. 1982 Jun;36(3):1238–1241. doi: 10.1128/iai.36.3.1238-1241.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Hall M. N., Enquist L., Silhavy T. J. Identification of OmpR: a positive regulatory protein controlling expression of the major outer membrane matrix porin proteins of Escherichia coli K-12. J Bacteriol. 1981 Jul;147(1):255–258. doi: 10.1128/jb.147.1.255-258.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. L., Clark J. W., Jr, Cline G. B., Anderson N. G., Russell H. Separation of Treponema pallidum from tissue substances by continuous-flow zonal centrifugation. Appl Microbiol. 1972 Apr;23(4):714–720. doi: 10.1128/am.23.4.714-720.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa T., Kasamatsu S., Yamaya S. I. Usefulness of the hemagglutination test using Treponema pallidum antigen (TPHA) for the serodiagnosis of syphilis. Jpn J Med Sci Biol. 1969 Dec;22(6):341–350. doi: 10.7883/yoken1952.22.341. [DOI] [PubMed] [Google Scholar]
- Zeltzer P. M., Pepose J. S., Bishop N. H., Miller J. N. Microassay for immunoglobulin G antibodies to Treponema pallidum with radioiodinated protein A from staphylococcus aureus: immunoglobulin G response in experimental syphilis in rabbits. Infect Immun. 1978 Jul;21(1):163–170. doi: 10.1128/iai.21.1.163-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]