Abstract
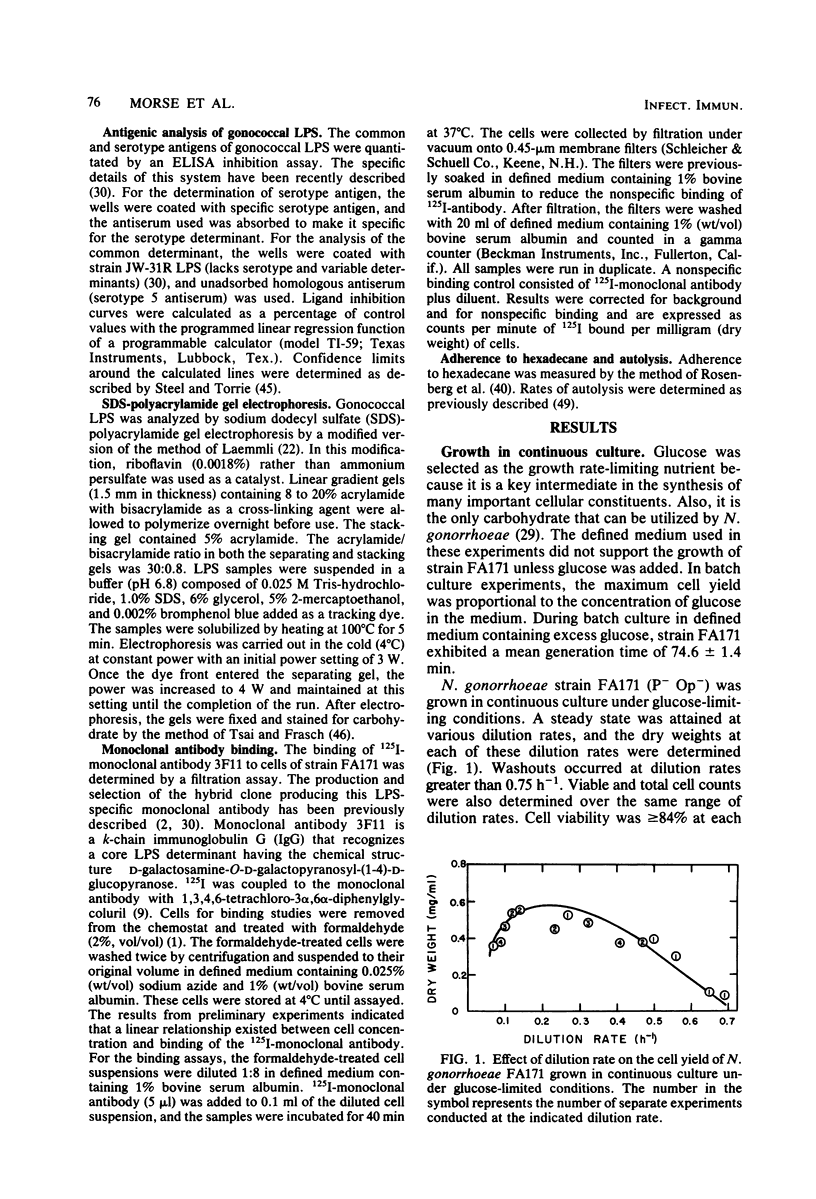
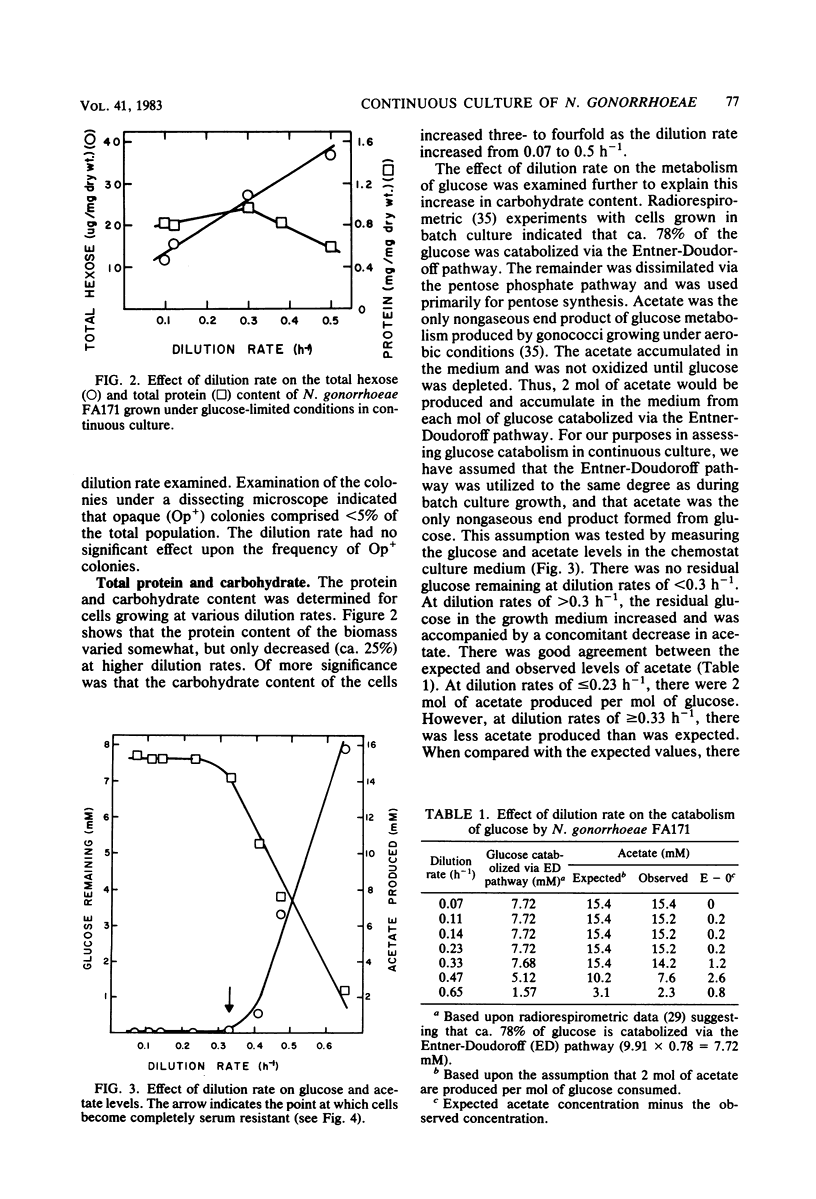
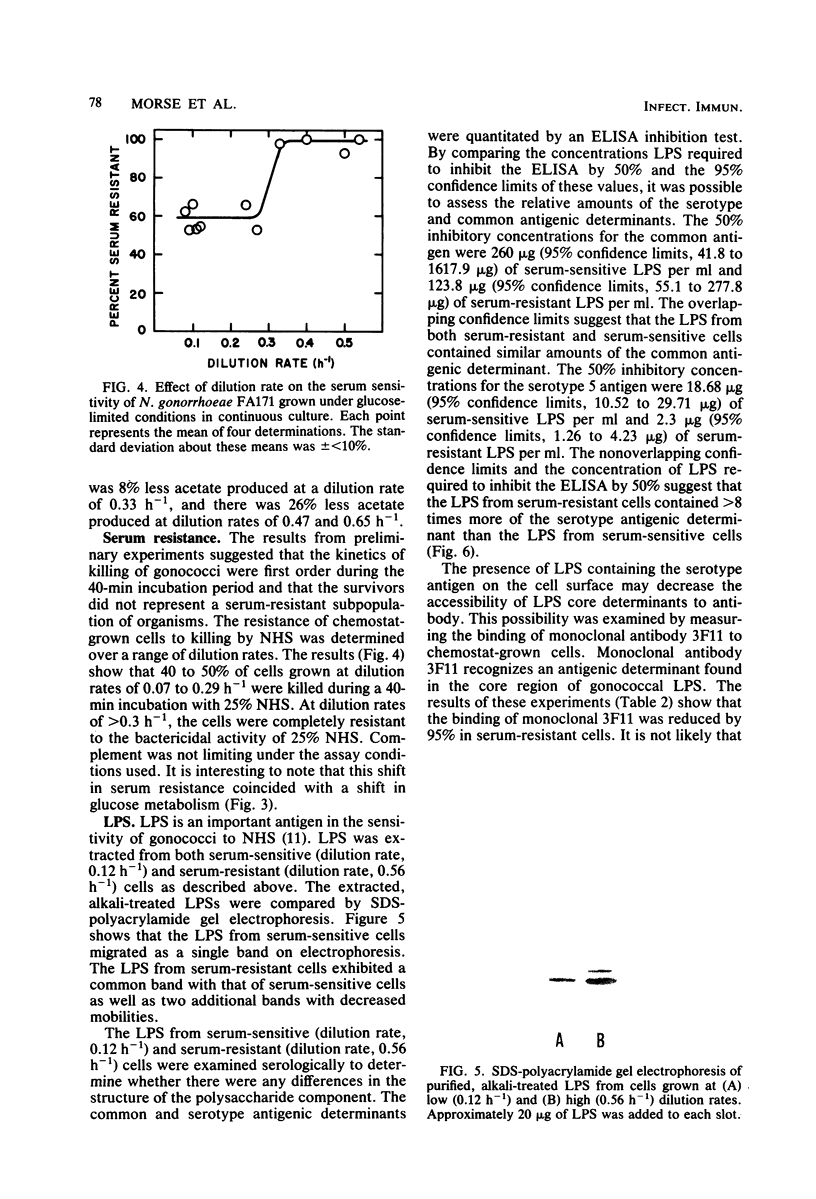
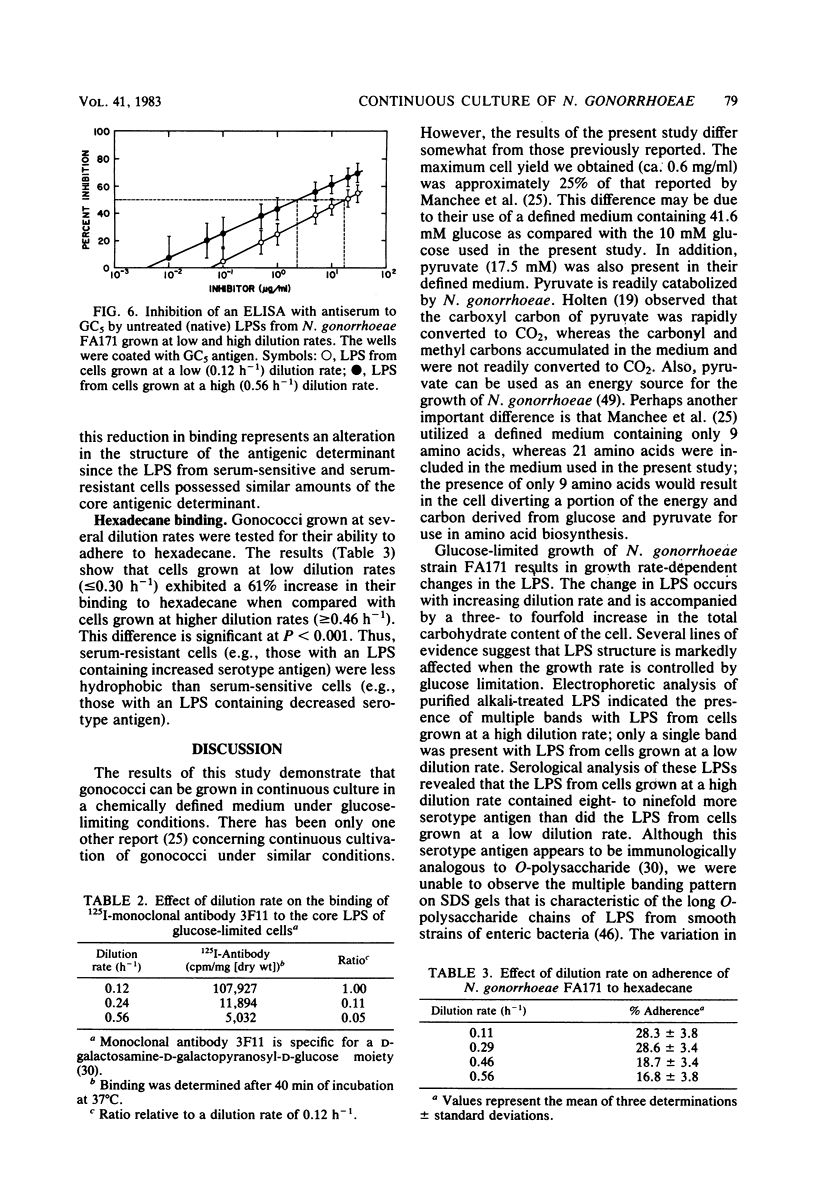
Growth of Neisseria gonorrhoeae strain FA171 in continuous culture under glucose-limiting conditions resulted in a growth-rate-dependent change in the lipopolysaccharide (LPS). The evidence for this change is an alteration in the mobility of purified alkali-treated LPS on sodium dodecyl sulfate-polyacrylamide gels and a quantitative difference in the amount of the LPS serotype antigen. The LPS from cells grown at a low dilution rate (0.12 h-1) contained ca. eightfold less serotype antigen than the LPS from cells grown at a high dilution rate (0.56 h-1). The decrease in LPS serotype antigen was associated with an increase in sensitivity to the bactericidal activity of normal human serum and an increase in cell surface hydrophobicity. An increase in the amount of serotype antigen was associated with a reduction in the accessibility of a monoclonal antibody to a core LPS determinant, an increase in resistance to normal human serum, and a decrease in cell surface hydrophobicity. The microheterogeneity of gonococcal LPS with respect to the content of serotype antigen may result from an alteration in the metabolism of glucose.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. Z., Connelly M. C., Apicella M. A. Interaction of lectins with Neisseria gonorrhoeae. Can J Microbiol. 1980 Apr;26(4):468–474. doi: 10.1139/m80-078. [DOI] [PubMed] [Google Scholar]
- Apicella M. A., Bennett K. M., Hermerath C. A., Roberts D. E. Monoclonal antibody analysis of lipopolysaccharide from Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1981 Dec;34(3):751–756. doi: 10.1128/iai.34.3.751-756.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M. A., Breen J. F., Gagliardi N. C. Degradation of the polysaccharide component of gonococcal lipopolysaccharide by gonococcal and meningococcal sonic extracts. Infect Immun. 1978 Apr;20(1):228–234. doi: 10.1128/iai.20.1.228-234.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braude A. I. Maxwell Finland lecture. Resistance to infection with the gonococcus. J Infect Dis. 1982 May;145(5):623–624. doi: 10.1093/infdis/145.2.623. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Lee T. J., Guymon L. F., Sparling P. F. Genetics of serum resistance in Neisseria gonorrhoeae: the sac-1 genetic locus. Infect Immun. 1981 May;32(2):547–552. doi: 10.1128/iai.32.2.547-552.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Connelly M. C., Stein D. C., Young F. E., Morse S. A., Allen P. Z. Interaction with lectins and differential wheat germ agglutinin binding of pyocin 103-sensitive and -resistant Neisseria gonorrhoeae. J Bacteriol. 1981 Dec;148(3):796–803. doi: 10.1128/jb.148.3.796-803.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gibbs D. L., Roberts R. B. The interaction in vitro between human polymorphonuclear leukocytes and Neisseria gonorrhoeae cultivated in the chick embryo. J Exp Med. 1975 Jan 1;141(1):155–171. doi: 10.1084/jem.141.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A., Ward M. E. Nature and Heterogeneity of the Antigens of Neisseria gonorrhoeae Involved in the Serum Bactericidal Reaction. Infect Immun. 1970 Aug;2(2):162–168. doi: 10.1128/iai.2.2.162-168.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymon L. F., Esser M., Shafer W. M. Pyocin-resistant lipopolysaccharide mutans of Neisseria gonorrhoeae: alterations in sensitivity to normal human serum and polymyxin B. Infect Immun. 1982 May;36(2):541–547. doi: 10.1128/iai.36.2.541-547.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Morse S. A. Physiology and metabolism of pathogenic neisseria: tricarboxylic acid cycle activity in Neisseria gonorrhoeae. J Bacteriol. 1976 Oct;128(1):192–201. doi: 10.1128/jb.128.1.192-201.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempfling W. P., Mainzer S. E. Effects of varying the carbon source limiting growth on yield and maintenance characteristics of Escherichia coli in continuous culture. J Bacteriol. 1975 Sep;123(3):1076–1087. doi: 10.1128/jb.123.3.1076-1087.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holten E. Radiorespirometric studies in genus Neisseria. 3. The catabolism of pyruvate and acetate. Acta Pathol Microbiol Scand B. 1976 Feb;84(1):9–16. [PubMed] [Google Scholar]
- James J. F., Zurlinden E., Lammel C. J., Brooks G. F. Relation of protein I and colony opacity to serum killing of Neisseria gonorrhoeae. J Infect Dis. 1982 Jan;145(1):37–44. doi: 10.1093/infdis/145.1.37. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee T. J., Utsinger P. D., Snyderman R., Yount W. J., Sparling P. F. Familial deficiency of the seventh component of complement associated with recurrent bacteremic infections due to Neisseria. J Infect Dis. 1978 Sep;138(3):359–368. doi: 10.1093/infdis/138.3.359. [DOI] [PubMed] [Google Scholar]
- Maw J., Meynell G. G. The true division and death rates of Salmonella typhimurium in the mouse spleen determined with superinfecting phage P22. Br J Exp Pathol. 1968 Dec;49(6):597–613. [PMC free article] [PubMed] [Google Scholar]
- McDonald I. J., Adams G. A. Influence of cultural conditions on the lipopolysaccharide composition of Neisseria sicca. J Gen Microbiol. 1971 Feb;65(2):201–207. doi: 10.1099/00221287-65-2-201. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Apicella M. A. Isolation of a lipopolysaccharide mutant of Neisseria gonorrhoeae: an analysis of the antigenic and biologic difference. J Infect Dis. 1982 Feb;145(2):206–216. doi: 10.1093/infdis/145.2.206. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974 Apr;145(4):1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Purine metabolism in Neisseria gonorrhoeae: the requirement for hypoxanthine. Can J Microbiol. 1980 Jan;26(1):13–20. doi: 10.1139/m80-003. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Hebeler B. H. Effect of pH on the growth and glucose metabolism of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):87–95. doi: 10.1128/iai.21.1.87-95.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Lysko P. G., McFarland L., Knapp J. S., Sandstrom E., Critchlow C., Holmes K. K. Gonococcal strains from homosexual men have outer membranes with reduced permeability to hydrophobic molecules. Infect Immun. 1982 Aug;37(2):432–438. doi: 10.1128/iai.37.2.432-438.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Stein S., Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974 Nov;120(2):702–714. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A. The biology of the gonococcus. CRC Crit Rev Microbiol. 1978;7(2):93–189. doi: 10.3109/10408417909083071. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Perry M. B., Daoust V. The lipopolysaccharides of Neisseria gonorrhoeae colony types 1 and 4. Can J Biochem. 1975 May;53(5):623–629. doi: 10.1139/o75-084. [DOI] [PubMed] [Google Scholar]
- Polk H. C., Miles A. A. The decisive period in the primary infection of muscle by Escherichia coli. Br J Exp Pathol. 1973 Feb;54(1):99–109. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Perry A., Bayer E. A., Gutnick D. L., Rosenberg E., Ofek I. Adherence of Acinetobacter calcoaceticus RAG-1 to human epithelial cells and to hexadecane. Infect Immun. 1981 Jul;33(1):29–33. doi: 10.1128/iai.33.1.29-33.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Buchanan T. M., Holmes K. K. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J Clin Invest. 1976 Nov;58(5):1163–1173. doi: 10.1172/JCI108569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Guymon L. F., Sparling P. F. Identification of a new genetic site (sac-3+) in Neisseria gonorrhoeae that affects sensitivity to normal human serum. Infect Immun. 1982 Mar;35(3):764–769. doi: 10.1128/iai.35.3.764-769.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt S. K., Jones F., Shockley T. E., Jackson J. H. Cotransformation of a serum resistance phenotype with genes for arginine biosynthesis in Neisseria gonorrhoeae. Infect Immun. 1980 Jul;29(1):287–289. doi: 10.1128/iai.29.1.287-289.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wegener W. S., Hebeler B. H., Morse S. A. Cell envelope of Neisseria gonorrhoeae: relationship between autolysis in buffer and the hydrolysis of peptidoglycan. Infect Immun. 1977 Oct;18(1):210–219. doi: 10.1128/iai.18.1.210-219.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oss C. J. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]



