Abstract
The ability of clinical and carrier isolates of Neisseria meningitidis to adhere to human buccal epithelial cells and erythrocytes was investigated. Four of the 10 fimbriated strains were able to hemagglutinate. Serial subculture of three of these strains resulted in a loss of ability to hemagglutinate and was coincident with a loss of fimbriation. Other fimbriated strains were unable to hemagglutinate but did adhere to buccal epithelial cells. Subculture of one of these strains for as many as 42 passages did not result in loss of fimbriation or ability to adhere to buccal epithelial cells. The attachment of this strain to buccal epithelial cells was inhibited by glycoconjugates. Further, pH exerted different influences on the attachment of hemagglutinating and non-hemagglutinating fimbriated strains to buccal epithelial cells and erythrocytes. The results suggest that different fimbrial mechanisms are involved in the attachment of N. meningitidis to different cell types and that hemagglutination is not an absolute test for fimbriae.
Full text
PDF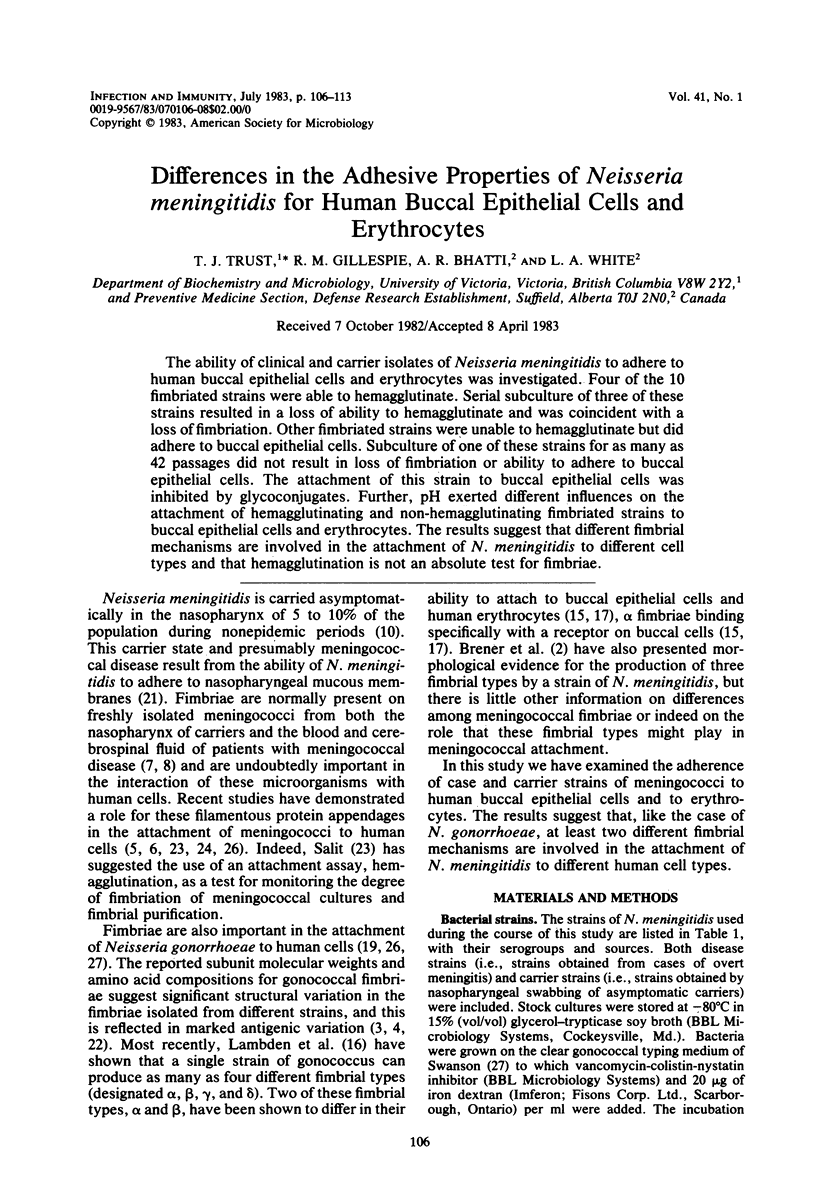






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson H. M., Trust T. J. Hemagglutination properties and adherence ability of Aeromonas hydrophila. Infect Immun. 1980 Mar;27(3):938–946. doi: 10.1128/iai.27.3.938-946.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M. Antigenic heterogeneity of gonococcal pili. J Exp Med. 1975 Jun 1;141(6):1470–1475. doi: 10.1084/jem.141.6.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven D. E., Peppler M. S., Frasch C. E., Mocca L. F., McGrath P. P., Washington G. Adherence of isolates of Neisseria meningitidis from patients and carriers to human buccal epithelial cells. J Infect Dis. 1980 Oct;142(4):556–568. doi: 10.1093/infdis/142.4.556. [DOI] [PubMed] [Google Scholar]
- DeVoe I. W., Gilchrist J. E. Pili on meningococci from primary cultures of nasopharyngeal carriers and cerebrospinal fluid of patients with acute disease. J Exp Med. 1975 Feb 1;141(2):297–305. doi: 10.1084/jem.141.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W., Gilchrist J. E. Piliation and colonial morphology among laboratory strains of meningococci. J Clin Microbiol. 1978 Apr;7(4):379–384. doi: 10.1128/jcm.7.4.379-384.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W. The meningococcus and mechanisms of pathogenicity. Microbiol Rev. 1982 Jun;46(2):162–190. doi: 10.1128/mr.46.2.162-190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Mocca L. F. Heat-modifiable outer membrane proteins of Neisseria meningitidis and their organization within the membrane. J Bacteriol. 1978 Dec;136(3):1127–1134. doi: 10.1128/jb.136.3.1127-1134.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield S., Sheehe P. R., Feldman H. A. Meningococcal carriage in a population of "normal" families. J Infect Dis. 1971 Jan;123(1):67–73. doi: 10.1093/infdis/123.1.67. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Chen K. C., Buchanan T. M. Neisseria pili proteins: amino-terminal amino acid sequences and identification of an unusual amino acid. Biochemistry. 1978 Feb 7;17(3):442–445. doi: 10.1021/bi00596a010. [DOI] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Robertson J. N., Watt P. J. Biological properties of two distinct pilus types produced by isogenic variants of Neisseria gonorrhoeae P9. J Bacteriol. 1980 Jan;141(1):393–396. doi: 10.1128/jb.141.1.393-396.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Robertson J. N., Watt P. J. The preparation and properties of alpha and beta pili from variants of Neisseria gonorrhoeae P9. J Gen Microbiol. 1981 May;124(1):109–117. doi: 10.1099/00221287-124-1-109. [DOI] [PubMed] [Google Scholar]
- Lindahl M., Faris A., Wadström T., Hjertén S. A new test based on 'salting out' to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta. 1981 Nov 5;677(3-4):471–476. doi: 10.1016/0304-4165(81)90261-0. [DOI] [PubMed] [Google Scholar]
- McGee Z. A., Street C. H., Chappell C. L., Cousar E. S., Morris F., Horn R. G. Pili of Neisseria meningitidis: effect of media on maintenance of piliation, characteristics of Pili, and colonial morphology. Infect Immun. 1979 Apr;24(1):194–201. doi: 10.1128/iai.24.1.194-201.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. N., Vincent P., Ward M. E. The preparation and properties of gonococcal pili. J Gen Microbiol. 1977 Sep;102(1):169–177. doi: 10.1099/00221287-102-1-169. [DOI] [PubMed] [Google Scholar]
- Salit I. E. Hemagglutination by Neisseria meningitidis. Can J Microbiol. 1981 Jun;27(6):586–593. doi: 10.1139/m81-089. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Morton G. Adherence of Neisseria meningitidis to human epithelial cells. Infect Immun. 1981 Jan;31(1):430–435. doi: 10.1128/iai.31.1.430-435.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Jonsson P., Olsson E., Soderlind O., Rosengren J., Hjertén S., Wadström T. Differences in hydrophobic surface characteristics of porcine enteropathogenic Escherichia coli with or without K88 antigen as revealed by hydrophobic interaction chromatography. Infect Immun. 1978 Nov;22(2):462–472. doi: 10.1128/iai.22.2.462-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S., McGee Z. A. Attachment of Neisseria meningitidis to human mucosal surfaces: influence of pili and type of receptor cell. J Infect Dis. 1981 Apr;143(4):525–532. doi: 10.1093/infdis/143.4.525. [DOI] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trust T. J., Lambden P. R., Watt P. J. The cohesive properties of variants of Neisseria gonorrhoeae strain P9: specific pilus-mediated and non-specific interactions. J Gen Microbiol. 1980 Jul;119(1):179–187. doi: 10.1099/00221287-119-1-179. [DOI] [PubMed] [Google Scholar]


