Abstract
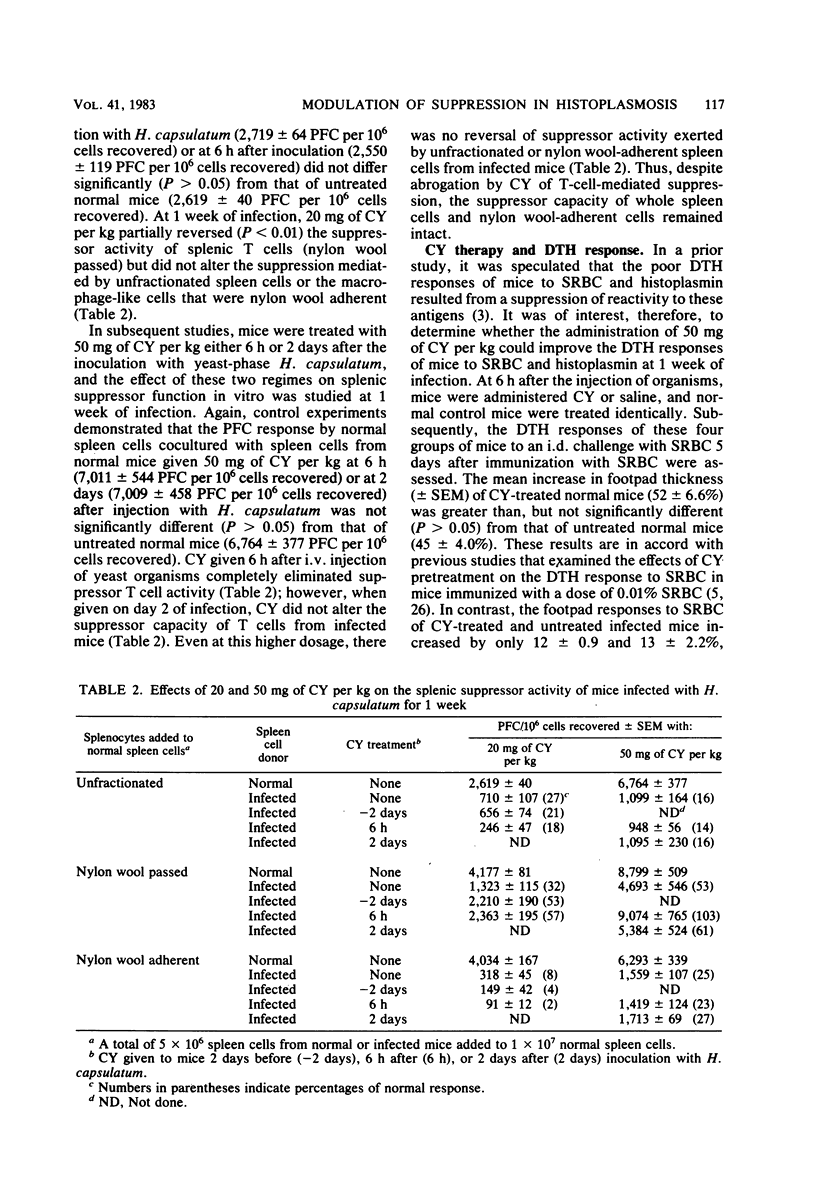
Indomethacin and cyclophosphamide (CY) were used in an attempt to modify the suppressive effects of spleen cell populations from mice with disseminated histoplasmosis at 1 week of infection. In vitro addition of indomethacin did not alter the depressed plaque-forming cell response to sheep erythrocytes of normal spleen cells cocultured with unfractionated or nylon wool-fractionated spleen cells from infected mice. Likewise, indomethacin given intraperitoneally did not enhance the subnormal in vivo plaque-forming cell response of spleen cells from infected mice. Conversely, 20 mg of CY per kg given intraperitoneally 2 days before or 6 h after the inoculation with Histoplasma capsulatum partially reversed the suppression effected by splenic T cells (nylon wool passed) in vitro, whereas 50 mg of CY per kg given intraperitoneally 6 h after the injection of H. capsulatum ablated suppressor T cell activity in vitro; neither dosage of CY altered the suppression mediated by unseparated or nylon wool-adherent spleen cells. Furthermore, the administration of 50 mg of CY per kg failed to improve the depressed footpad responses of mice infected for 1 week to sheep erythrocytes in sheep erythrocyte-sensitized mice or to histoplasmin. These findings indicate that in experimental disseminated histoplasmosis, suppression effected by splenic T cells can be alleviated by CY; however, there is a persistent immunosuppressor mechanism(s) that cannot be counteracted by either indomethacin or CY.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artz R. P., Bullock W. E. Immunoregulatory responses in experimental disseminated histoplasmosis: depression of T-cell-dependent and T-effectory responses by activation of splenic suppressor cells. Infect Immun. 1979 Mar;23(3):893–902. doi: 10.1128/iai.23.3.893-902.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artz R. P., Bullock W. E. Immunoregulatory responses in experimental disseminated histoplasmosis: lymphoid organ histopathology and serological studies. Infect Immun. 1979 Mar;23(3):884–892. doi: 10.1128/iai.23.3.884-892.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artz R. P., Jacobson R. R., Bullock W. E. Decreased suppressor cell activity in disseminated granulomatous infections. Clin Exp Immunol. 1980 Aug;41(2):343–352. [PMC free article] [PubMed] [Google Scholar]
- Askenase P. W., Hayden B. J., Gershon R. K. Augmentation of delayed-type hypersensitivity by doses of cyclophosphamide which do not affect antibody responses. J Exp Med. 1975 Mar 1;141(3):697–702. doi: 10.1084/jem.141.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bash J. A., Singer A. M., Waksman B. H. The suppressive effect of immunization on the proliferative responses of rat T cells in vitro. II. Abrogation of antigen-induced suppression by selective cytotoxic agents. J Immunol. 1976 May;116(5):1350–1353. [PubMed] [Google Scholar]
- Chiorazzi N., Fox D. A., Katz D. H. Hapten-specific IgE antibody responses in mice. VII. Conversion of IgE "non-responder" strains to IgE "responders" by elimination of suppressor T cell activity. J Immunol. 1977 Jan;118(1):48–54. [PubMed] [Google Scholar]
- Cozad G. C., Lindsey T. J. Effect of cyclophosphamide on Histoplasma capsulatum infections in mice. Infect Immun. 1974 Feb;9(2):261–265. doi: 10.1128/iai.9.2.261-265.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Deepe G. S., Jr, Watson S. R., Bullock W. E. Generation of disparate immunoregulatory factors in two inbred strains of mice with disseminated histoplasmosis. J Immunol. 1982 Nov;129(5):2186–2191. [PubMed] [Google Scholar]
- Ellner J. J. Suppressor adherent cells in human tuberculosis. J Immunol. 1978 Dec;121(6):2573–2579. [PubMed] [Google Scholar]
- Finerty J. F., Krehl E. P. Cyclophosphamide pretreatment and protection against malaria. Infect Immun. 1976 Oct;14(4):1103–1105. doi: 10.1128/iai.14.4.1103-1105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. I., Bostick-Bruton F. Depressed T cell proliferative responses in Hodgkin's disease: role of monocyte-mediated suppression via prostaglandins and hydrogen peroxide. J Immunol. 1982 Oct;129(4):1770–1774. [PubMed] [Google Scholar]
- Fulton A. M., Levy J. G. The possible role of prostaglandins in mediating immune suppression by nonspecific T suppressor cells. Cell Immunol. 1980 Jun;52(1):29–37. doi: 10.1016/0008-8749(80)90397-4. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Webb D. R. Regulation of the immune response by prostaglandins. Clin Immunol Immunopathol. 1980 Jan;15(1):106–122. doi: 10.1016/0090-1229(80)90024-0. [DOI] [PubMed] [Google Scholar]
- Gordon D., Bray M. A., Morley J. Control of lymphokine secretion by prostaglandins. Nature. 1976 Jul 29;262(5567):401–402. doi: 10.1038/262401a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H., Diamantstein T. Relative susceptibilities of T cell subsets involved in delayed-type hypersensitivity to sheep red blood cells to the in vitro action of 4-hydroperoxycyclophosphamide. J Immunol. 1980 Sep;125(3):1104–1108. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Melmon K. L., Bourne H. R., Weinstein Y., Shearer G. M., Kram J., Bauminger S. Hemolytic plaque formation by leukocytes in vitro. Control by vasoactive hormones. J Clin Invest. 1974 Jan;53(1):13–21. doi: 10.1172/JCI107530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger Z., Hoffeld J. T., Oppenheim J. J. Macrophage-mediated suppression. I. Evidence for participation of both hdyrogen peroxide and prostaglandins in suppression of murine lymphocyte proliferation. J Immunol. 1980 Feb;124(2):983–988. [PubMed] [Google Scholar]
- Mitsuoka A., Baba M., Morikawa S. Enhancement of delayed hypersensitivity by depletion of suppressor T cells with cyclophosphamide in mice. Nature. 1976 Jul 1;262(5563):77–78. doi: 10.1038/262077a0. [DOI] [PubMed] [Google Scholar]
- Nickerson D. A., Havens R. A., Bullock W. E. Immunoregulation in disseminated histoplasmosis: characterization of splenic suppressor cell populations. Cell Immunol. 1981 May 15;60(2):287–297. doi: 10.1016/0008-8749(81)90270-7. [DOI] [PubMed] [Google Scholar]
- Petersen E. A., Neva F. A., Oster C. N., Bogaert Diaz H. Specific inhibition of lymphocyte-proliferation responses by adherent suppressor cells in diffuse cutaneous leishmaniasis. N Engl J Med. 1982 Feb 18;306(7):387–392. doi: 10.1056/NEJM198202183060702. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Askenase P. W., Gershon R. K. Regulation of delayed-type hypersensitivity reactions by cyclophosphamide-sensitive T cells. J Immunol. 1978 Oct;121(4):1573–1577. [PubMed] [Google Scholar]
- Singer S. H., Noguchi P., Kirschstein R. L. Respiratory diseases in cyclophosphamide-treated mice. II. Decreased virulence of PR8 influenza virus. Infect Immun. 1972 Jun;5(6):957–960. doi: 10.1128/iai.5.6.957-960.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Utz J. P. Progressive disseminated histoplasmosis. A prospective study of 26 patients. Ann Intern Med. 1972 Apr;76(4):557–565. doi: 10.7326/0003-4819-76-4-557. [DOI] [PubMed] [Google Scholar]
- Stobo J. D. Immunosuppression in man: suppression by macrophages can be mediated by interactions with regulatory T cells. J Immunol. 1977 Sep;119(3):918–924. [PubMed] [Google Scholar]
- Stobo J. D., Paul S., Van Scoy R. E., Hermans P. E. Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest. 1976 Feb;57(2):319–328. doi: 10.1172/JCI108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Watanabe N., Kobayashi A. Nonspecific suppression of primary antibody responses and presence of plastic-adherent suppressor cells in Toxoplasma gondii-infected mice. Infect Immun. 1981 Oct;34(1):30–35. doi: 10.1128/iai.34.1.30-35.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D. R., Jr, Jamieson T. Control of mitogen-induced transformation: characterization of a splenic suppressor cell and its mode of action. Cell Immunol. 1976 Jun 1;24(1):45–57. doi: 10.1016/0008-8749(76)90130-1. [DOI] [PubMed] [Google Scholar]
- Webb D. R., Nowowiejski I. Control of suppressor cell activation via endogenous prostaglandin synthesis: the role of T cells and macrophages. Cell Immunol. 1981 Sep 15;63(2):321–328. doi: 10.1016/0008-8749(81)90011-3. [DOI] [PubMed] [Google Scholar]
- Wellhausen S. R., Mansfield J. M. Characteristics of the splenic suppressor cell--target cell interaction in experimental African trypanosomiasis. Cell Immunol. 1980 Sep 1;54(2):414–424. doi: 10.1016/0008-8749(80)90221-x. [DOI] [PubMed] [Google Scholar]


