Abstract
Chemotherapy aims to limit proliferation and induce apoptotic cell death in tumor cells. Owing to blockade of signaling pathways involved in cell survival and proliferation, nuclear factor κB (NF-κB) inhibitors can induce apoptosis in a number of hematological malignancies. The efficacy of conventional chemotherapeutic drugs, such as vincristine (VCR) and doxorubicine (DOX), may be enhanced with combined therapy based on NF-κB modulation. In this study, we evaluated the effect of caffeic acid phenylethyl ester (CAPE) and MG-132, two nonspecific NF-κB inhibitors, and conventional chemotherapeutics drugs DOX and VCR on cell proliferation and apoptosis induction on a lymphoblastoid B-cell line, PL104, established and characterized in our laboratory. CAPE and MG-132 treatment showed a strong antiproliferative effect accompanied by clear cell cycle deregulation and apoptosis induction. Doxorubicine and VCR showed antiproliferative effects similar to those of CAPE and MG-132, although the latter drugs showed an apoptotic rate two-fold higher than DOX and VCR. None of the four compounds showed cytotoxic effect on peripheral mononuclear cells from healthy volunteers. CAPE- and MG-132-treated bone marrow cells from patients with myeloid and lymphoid leukemias showed 69% (P < .001) and 25% decrease (P < .01) in cell proliferation and 42% and 34% (P < .01) apoptosis induction, respectively. Overall, our results indicate that CAPE and MG-132 had a strong and selective apoptotic effect on tumor cells that may be useful in future treatment of hematological neoplasias.
Introduction
Cancer is a multifactorial disease that generally requires multimodal therapy owing to the complexity and diversity of factors that control its progression and dissemination. The accepted modality of treatment may involve surgery, radiation, and drugs, singly or in combination. Chemotherapeutic agents can often provide temporary relief from symptoms, prolongation of life span, and occasionally, a complete cure. However, drug and radiation treatments have two major problems, namely, a time-dependent development of tumor resistance to therapy and a nonspecific toxicity toward normal cells, which are in part responsible for the impediment to achieve an effective cancer treatment [1].
Previous data have shown that apoptosis, the most common and well-defined form of programmed cell death, is the main cause of tumor cell death after radiotherapy and chemotherapy treatment of hematological malignancies, representing an efficient strategy for cancer chemotherapy [2]. However, abnormal control of cell death pathways has also been related to treatment failure, which may in turn lead to multidrug resistance (MDR) emergence [3]. Despite this, apoptosis induction may be considered a reliable marker for the evaluation of potential anticancer agents [4], and its modulation may be a useful weapon in the oncologist's armamentarium for the design and management of suitable therapeutic protocols for tumor malignancies.
Nuclear factor κB (NF-κB) is a transcription factor that operates in virtually all mammalian cells [5]. Because NF-κB regulates the expression of a wide variety of proteins that inhibit apoptosis and promote cell survival and proliferation, it is strongly implicated in the promotion, progression, and metastasis [6,7] of solid cancers [8], sarcomas [9], and several hematological malignancies, as well as acute and chronic myeloid leukemias [10], lymphoblastic leukemias [11], myelodysplastic syndrome [12], multiple myeloma [13], and lymphomas [14]. The antiapoptotic role of the NF-κB cascade has made it an attractive target for the development of specific anticancer drugs [15]. Over the years, several conventional drugs have shown to be effective anti-NF-κB compounds. Some of these agents are currently used in cancer therapy, whereas others are being tested in clinical trials [16,17]. Agents that inhibit the 26S proteasome physiological function [18], such as peptide aldehydes (MG-132, ALLN), may indirectly interfere with NF-κB pathway [19]. Many dietary agents of vegetable origin have shown to inhibit NF-κB and induce apoptosis of tumor cells [20,21]. Caffeic acid phenylethyl ester (CAPE), a biologically active polyphenolic compound of propolis from honeybee hives, has demonstrated to be a potent inhibitor of NF-κB activation [20]. In addition, CAPE has several interesting biological properties including antioxidant [22], anti-inflammatory [23], antiviral [24], antibacterial [25], antifungal [26], immunostimulatory, antiangiogenic, anti-invasive, antitumoral, and antimetastatic activities [27].
The aim of this study was to assess the potential usefulness of two nonspecific NF-κB inhibitors, CAPE and MG-132, as chemotherapeutic agents or chemosensitizers of tumor cells in the treatment of hematological malignancies.
Our results showed that CAPE and MG-132 exerted a potent and highly selective cytotoxic effect mediated by apoptosis induction, cell cycle deregulation, and reduction of the proliferation rate on a novel lymphoblastoid B-cell line as well as on primary human leukemic cells. The combined treatment with CAPE or MG-132 plus conventional chemotherapeutic agents showed an increase in apoptotic cell death, which deserves further studies.
Materials and Methods
Materials
CAPE and MG-132 were obtained from Calbiochem (San Diego, CA), and stock solutions were prepared in dimethylsulfoxide (DMSO) at 350 and 5 mM stock concentration, respectively. Vincristine (VCR) was kindly provided by Filaxis Pharmaceuticals (Buenos Aires, Argentina) and doxorubicine (DOX) by Gador Pharmaceuticals (Buenos Aires, Pharmaceuticals). RPMI-1640, penicillin, and streptomycin reagents were purchased from Invitrogen (Buenos Aires, Argentina). Antibodies against p65, IκB-α, p-IκB-α, actin, horseradish peroxidase-labeled antirabbit, antimouse, antigoat secondary antibodies and Western blot analysis chemiluminiscence reagent were bought from Santa Cruz Biotechnology, Inc. (Buenos Aires, Argentina).
Cell Lines and Culture Conditions
The cell line PL104 was established in our laboratory from bone marrow aspirate from a 14-year-old patient with an atypical acute myeloid leukemia according to the French-American-British classification. A written informed consent was previously obtained from the patient in accordance with the Declaration of Helsinki. After Ficoll-Hypaque (Sigma-Aldrich, St. Louis, MO) density gradient separation, cells were recovered and grown in a suspension culture at 37°C in a 5% CO2 atmosphere using RPMI-1640 medium supplemented with 15% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, 20 mM HEPES buffer, 100 mg/ml penicillin, 150 mg/ml streptomycin, and 50 mM 2-mercaptoethanol. Cells were subcultured at 1:2 to 1:4 ratios every 2 to 3 days with a viability greater than 90% (assessed by trypan blue) and finally showed definite proliferation in vitro after 30 passages. The established cell line grew in suspension culture either as single units or as loose clumps with a doubling time of 25.09 ± 4.41 hours, as determined by the MTS assay. Aliquots of the cells were frozen in liquid nitrogen, and thawed cells proliferated under standard culture conditions.
Collection and Processing of Primary Cells
Patient-derived leukemic (PDL) cells were obtained, previous informed consent, from bone marrow aspirates in sterile syringes containing EDTA as anticoagulant. Samples from 20 nontreated patients at diagnosis, immunophenotypically characterized as acute myeloid leukemia [8], acute lymphoblastic leukemia [7], chronic lymphoblastic leukemia [3], and chronic myeloid leukemia [2], were analyzed. The group of patients comprised 16 males and 4 females, and the mean age at presentation was 61.6 ± 23.1 years (range, 3–63 years). Patients who were under treatment or relapse phase were excluded. Normal peripheral blood samples were obtained after informed consent from 10 healthy volunteers. The male-to-female ratio was 5:5 with a mean age of 35 years (range, 23–60 years). Accordingly, bone marrow or blood cells were subjected to Ficoll-Hypaque density gradient separation to isolate leukemic cells or peripheral blood mononuclear cells (PBMCs). In both cases, primary cells were cultured in RPMI-1640 containing 20% FCS at 37°C in a humidified atmosphere with 5% CO2. Cell viability was greater than 95%, as assessed by trypan blue exclusion. These studies have been approved by The Scientific Review Board of FUNDALEU (Buenos Aires, Argentina) and were in accordance with the Declaration of Helsinki.
Immunophenotyping
Three-color immunofluorescence analysis of surface and intracellular antigens was performed by using combinations of phycoerythrin, fluorescein isothiocyanate (FITC), and/or peridinin chlorophyll protein-conjugated monoclonal antibodies (MoAbs), as previously described [28]. The following MoAbs were used: CD3, CD19, CD34, CD45 (peridinin chlorophyll protein); CD8, CD10, CD15, CD16, CD20, CD41a, CD64, HLADR, myeloperoxidase (MPO), kappa chain (FITC); CD4, CD11b, CD13, CD14, CD22, CD33, CD38, CD56, CD79a, CD117, and lambda chain (phycoerythrin), all purchased from BD Biosciences (BD PharMingen, San Diego, CA). Analysis was carried out on a FACScan Becton Dickinson (Los Angeles, CA) flow cytometer using Cell Quest (Becton Dickinson Immunocytometry Systems) software. Staining patterns for all antibodies used were compared with their respective isotype control. Positive results were expressed as a percentage with cutoff values of 20% over the corresponding isotypic control.
Cell Clot Block Preparation
Cells (2 x 107/ml) from a cell culture suspension were spun down at 900g, and the supernatant was finally discarded. Two volumes of a 90% ethanol/40% formalin/acetic acid (80:15:5) solution were added and gently mixed by inversion. After 24 hours, the clot was pulled out onto a filter paper, and slides were prepared for histologic analysis.
Detection of Epstein-Barr Virus Antigens by Immunohistochemistry
Formalin-fixed paraffin-embedded sections of a cell clot block were assayed for EBNA1 (clone 1H4) and LMP2A (clone 4E11) expression with MoAbs kindly provided by Dr. E. Kremmer (Forschungszentrum für Umwelt und Gesundheit GmbH, Institut für Immulogie,Munchen, Germany). Antigen unmasking was carried out in 0.01 M sodium citrate (pH 6) for 5 minutes at 500 W in a microwave oven. Immunohistochemical detection of MoAbs was performed using an antirat polyclonal antibody peroxidase-conjugated. Slides were stained in freshly prepared substrate solution (10 mg of diaminobenzidine in 15 ml of Tris-buffered saline, 30 µl of H2O2, pH 7.6). Hematoxylin counterstaining was applied. As negative controls, the pertinent antibodies were omitted. As a positive control, a well-characterized Epstein-Barr virus (EBV)-positive Hodgkin lymphoma biopsy was used.
Polymerase Chain Reaction Amplification
To confirm EBV presence, a polymerase chain reaction (PCR) was carried out to amplify a 122-bp fragment of EBV DNA sequence encoding BamH1 W(IR1) as described by Saito et al. [29]. In a separate reaction tube, a PCR was carried out to amplify a beta-globin gene as a control to monitor the amplification ability of a single-copy gene. Amplified DNA was subjected to electrophoresis on a 2% agarose gel with ethidium bromide (EtBr).
Epstein-Barr Encoded RNA In Situ Hybridization
Epstein-Barr Encoded RNA in situ hybridization was performed on paraffin sections according to the manufacturer's instructions using an EBV Probe ISH Kit, NCL-EBV-K (Novocastra Laboratories, Ltd, Newcastle, United Kingdom).
Cell Growth Inhibition Assay
Sensitivity of the cell line to increased doses of DOX (0–2 µM), VCR (0–10 µM), CAPE (0–100 µg/ml), or MG-132 (0–4 µM) was determined by culturing 5 x 105 cells/ml at 37°C in a 5% CO2 atmosphere for 24 and 48 hours, pulsed with 1 µCi [3H]TdR (DuPont, Nen Products, Boston, MA) for the last 18 hours and harvested as described previously [30]. Cells were counted in a liquid scintillation beta counter (Beckman, MD). Results were calculated from the mean counts per minute of [3H]TdR incorporated in triplicate cultures. Percentage of cell proliferation inhibition for each treatment was calculated as follows:
%Cell survival = [(cpm cells plus drug treatment/cpm cells alone)] x100
%Cell proliferation inhibition = 100 - % cell survival
Each experiment was performed at least three times. Nontreated cells represented 100% cell survival. Cell viability at the beginning of the experiment was higher than 95%, as assessed by trypan blue exclusion.
Preparation of Nuclear and Cytoplasmic Extracts
Nuclear extracts were prepared as previously described [31]. Briefly, cells were incubated in 400 µl of hypotonic buffer (10 mM HEPES pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 0.5 mM PMSF, 0.1% Nonidet P-40) for 15 minutes on ice and centrifuged at 11,000g for 10 minutes. Nuclear pellets were resuspended in 60 µl of nuclear hypotonic buffer (20 mM HEPES, 1.5 mM MgCl2, 420 mM NaCl, 0.5 mM DTT, 0.5 mM PMSF, 0.2 mM EDTA, 25% glycerol) and incubated at 4°C for 15 minutes followed by centrifugation at 13,000g for 15 minutes.
For cytoplasmic extracts preparation, cells were lysed with a hypotonic buffer (20 mM Tris pH 8.0, 150 mM NaCl, 100 mM NaF, 10% glycerol, 1% Nonidet P-40, 1 mM PMSF, 40 µg/ml leupeptin, and 20 µg/ml aprotinin) for 30 minutes at 4°C.
The supernatants were stored at -70°C until further use, and protein concentration was determined by the Bradford assay.
Western Blot Analysis
Equal amounts of protein extracts (30 µg/lane) were resolved by SDS-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane (Sigma-Aldrich). The membrane was blocked in Tris-buffered saline, containing 2% glycine and 3% nonfat dried milk overnight at 4°C. The membrane was then incubated with specific antibodies to IκB-α, pIκ-≠, p65, and actin for 2 hours at 37°C followed by horseradish peroxidase-labeled secondary antibody for 1.5 hours at 37°C. The reaction was developed using a chemiluminiscence detection system. Densitometric analysis was performed using Scion Image software (Scion Corporation, NIH, Bethesda, MD). Relative index (RI) was calculated as follows:
RI: OD treatment=OD control.
MTS Assay
Patient-derived leukemic cells were exposed to increased concentrations of DOX (0–2 µM), VCR (0–10 µM), CAPE (0–100 µg/ml), or MG-132 (0–4 µM) to assess their effect on cell proliferation using the CellTiter 96-AQueous One Solution Cell Proliferation Assay (Promega Corporation, Madison, WI) according to the manufacturer's instructions. Briefly, 5 x 105 cells per well were cultured in RPMI-1640 supplemented with 15% of FCS for 24 hours, added with 20 µl of CellTiter 96-Aqueous One Solution Reagent containing a tetrazolium compound, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS), and an electron-coupling reagent (phenazine ethosulfate). The plate was incubated at 37°C for 2.5 hours in a humidified atmosphere with 5% CO2. Absorbance at 492 nm corresponding to a reduction of MTS was measured using a microtiter plate reader (Multiscan EX; Thermo-Electron Corporation, Waltham, MA) [32].
Cell Cycle Analysis
The different cell cycle stages in the DOX-, VCR-, CAPE-, and MG-132-treated groups were measured by flow cytometry. Cells (1 x 106/ml) were harvested, washed once with ice-cold PBS, and fixed with 1 ml of ice-cold 70% ethanol at 4°C for 30 minutes. Next, PL104 cells were incubated with 1 µg/ml of 4′-6-diamidino-2-phenylindole (DAPI; Roche S.A., Buenos Aires, Argentina)-0.1% Triton X-100 for 30 minutes at room temperature [33], and cellular DNA content was measured using a PASS III flow cytometer (Partec, Görlitz, Germany). Fifteen thousand events were acquired for each sample. Results were analyzed using Cylchred 1.0.2 software (Cardiff University, United Kingdom).
Apoptosis Detection
Morphological features associated with apoptosis were analyzed by acridine orange and EtBr staining after treatment of PL104 (6, 15, and 24 hours), PDL cells (24 hours) and PBMCs (24 hours) with DOX (0–2 µM), VCR (0–5 µM), CAPE (0–100 µg/ml), or MG-132 (0–4 µM) [34]. Briefly, cell pellets were resuspended in dye mix (100 µg/ml acridine orange plus 100 µg/ml EtBr in PBS) and visualized by fluorescence microscopy (Carl Zeiss, Germany). A minimum number of 200 cells were counted, and the number of cells with fragmented nuclei, enlarged cytoplasm, and condensed chromatin were determined. The percentage of apoptotic cells was calculated as follows:
%Apoptotic cells = (number of cells with apoptotic nuclei/total number of cells counted) x 100
%Apoptotic index = (%apoptotic treated cells - %apoptotic control cells)
Apoptosis was also determined by staining of exposed phosphatidylserine with Annexin V-FITC/propidium iodide (PI; Apoptosis Detection Kit; BioVision, Inc, Mountain View, CA) assay. PL104 cells were treated with DOX (1.5 and 2 µM), VCR (1 and 5 µM), CAPE (50 and 100 µg/ml), or MG-132 (2 and 4 µM) for 24 hours. Single-cell suspensions were analyzed by FACScan (Becton Dickinson, San Jose, CA) and data acquired using WinMDI 2.8 software (Scripps Institute, La Jolla, CA). Apoptotic cells were scored as Annexin V+, PI- and Annexin V+, PI+.
Statistical Analysis
All data are presented as the mean ± SD for a series of three independent experiments. Statistical analyses were performed with Prism3 software (GraphPad, San Diego, CA) using one-way analysis of variance followed by Tukey's post hoc comparison test. Group differences, treated versus untreated, were considered statistically significant when P < .05.
Results
The Established Cell Line, PL104, Features a Mature B-cell Phenotype
The immunophenotypic profile assessed by flow cytometry was performed on PL104 to determine cell origin and degree of cell differentiation (Table W1). Most PL104 cells expressed CD19, CD20, CD22, CD79a, CD38, HLA-DR, and CD45 antigens plus µ/λ immunoglobulins on their surface (Figure W1). Myelomonocytic lineage-related markers, such asMPO, CD33, CD117, CD11b, CD13, CD14, and CD15, were not expressed. CD34 and CD10 antigens, which indicate the degree of differentiation and maturation, commonly expressed in progenitors and immature cells, were also negative. Besides, an aberrant expression of the CD71 marker was determined, in coincidence with the fact that this is a common feature in tumor cell lines of different origins with high proliferation activity. The immunophenotyping studies provided evidence that PL104 cell line expresses phenotypic characteristics of B mature monoclonal cells.
The cell line presents a modal number of 46 chromosomes, evaluated in 50 cells. Karyotype analysis, performed in 20 metaphases, showed that 30% presented a chromosome marker: der(13) t(12;13)(q13; p11) confirmed by FISH analysis (data not shown). No other numerical or structural chromosome alterations were detected, being the definite karyotype: 46,XX,der(13)t(12;13)(q13;p11)[6]/46,XX[14].
Epstein-Barr Virus Is Detected in PL104 Cells
Because the established cell line had a B phenotype, and the original sample was typified as atypical myeloid lineage, and considering that EBV has the ability to transform B cells into immortalized lymphoblastoid cell lines (LCLs), we decided to investigate the expression of EBV-related antigens on the PL104 cell line.
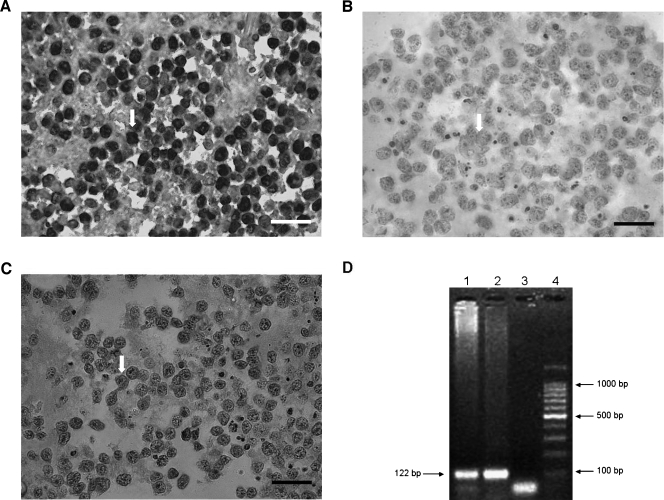
EBERs in situ hybridization, the criterion standard technique to assess virus presence, revealed a nuclear localization of the signal with a characteristic labeling of the inner nuclear membrane and around the nucleolus of nearly all lymphoid cells (Figure 1A). To assess viral protein expression, different MoAbs were used. Anti-EBNA1 staining displayed a perinuclear pattern, whereas anti-LMP2A showed cytoplasmic and membrane labeling (Figure 1, B and C). Viral antigens were expressed in almost every lymphoid cell. Finally, by means of PCR a 122-bp amplification fragment of EBV DNA sequence encoding BamH1 W was detected (Figure 1D).
Figure 1.
EBV is detected in PL104 cells. (A) EBERs in situ hybridization showing a nuclear localization of the signal in PL104 cells (original magnification, x400; scale bar, 30 µm). (B) Perinuclear pattern after EBNA1 antigen labeling (original magnification, x400; scale bar, 30 µm). (C) LMP-2A detection by immunohistochemistry showing a cytoplasmic and membrane labeling (original magnification, x400; scale bar, 30 µm). Arrows indicate a positive cell. (D) Polymerase chain reaction amplification of EBV DNA sequence, BamH1 W. Lane 1: PL104 cells. Lane 2: A positive control of a known, positive cell line (EBV-positive LCL P3HR1). Lane 3: A negative control distilled H2O replacing DNA. Lane 4: A 100-bp molecular weight size marker.
DOX and VCR Treatment Significantly Reduce PL104 Proliferation Rate
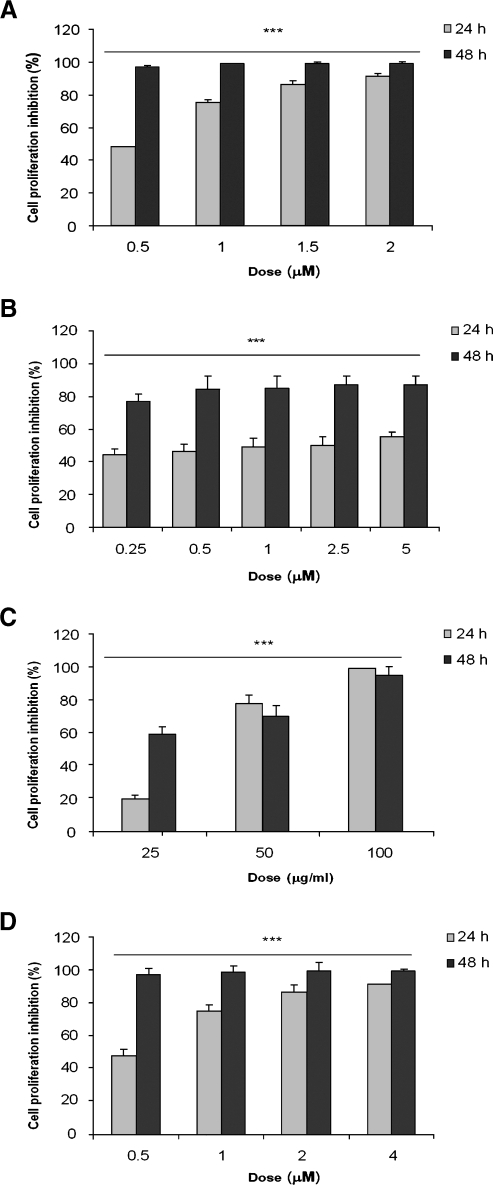
As DOX and VCR are two antineoplastic agents commonly included in most leukemia and lymphoma induction protocols, we decided to test whether treatment of PL104 cells resulted in a reduction of cell proliferation as assessed by [3H]TdR incorporation. All doses tested exhibited an antiproliferative effect after 24 hours with maximal values of 50% and 90% of growth inhibition for VCR and DOX, respectively (Figure 2, A and B). However, highest inhibitions were reached after 48 hours of treatment either with 1.5 µM DOX (99.45 ± 0.11% growth inhibition, P < .001) or 10 µM VCR (88.10 ± 5.08% growth inhibition, P < .001). These results reveal that both agents were able to interfere with PL104 proliferation rate through significant inhibition of this process. In addition, DOX showed a comparatively better response than VCR in the tested conditions.
Figure 2.
DOX, VCR, CAPE, and MG-132 significantly reduced PL104 cell growth. Cell growth inhibition assessed by [3H]TdR incorporation at 24 and 48 hours after exposure to different concentrations of DOX (A VCR (B), CAPE (C), and MG-132 (D). Results are expressed as percentage of cell proliferation inhibition for each treatment (for additional details, see Materials and Methods). Bars, mean ± SD of at least three independent experiments (***P < .001).
CAPE and MG-132 Exert an Antiproliferative Effect on PL104 Cells and Modulate NF-κB Expression
The antiproliferative effect of CAPE and MG-132 was investigated on PL104 cell line. Figure 2, C and D, shows that PL104 proliferation was abrogated after 24 and 48 hours of exposure to CAPE and MG-132 compared with DMSO control cultures. Maximal cell growth inhibition was 99.29 ± 0.30% and 99.27 ± 0.19% (P < .001), for 100 µg/ml CAPE and 4 µM MG-132, respectively. Lowest doses of MG-132 (0.125 and 0.25 µM) induced only 9% of inhibition after 24 hours of treatment.
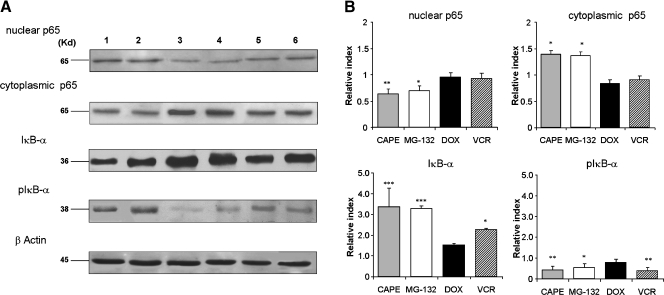
NF-κB is known to promote cell survival and proliferation of normal B lymphoid cells as well as of many tumor-related malignancies where it is constitutively activated [35]. Thus, assuming that, among other effects, both CAPE and MG-132 can affect NF-κB signaling pathway, we evaluated the cytoplasmic and nuclear expressions of p65 protein by Western blot. Figure 3 shows that treatment of PL104 cells with both CAPE (50 µg/ml) and MG-132 (2 µM) for 2 hours resulted in a significant increase in the cytoplasmic level of p65 and a further decrease in the nuclear expression level. In addition, we investigated the expression level of NF-κB inhibitory subunit IκB-α and its phosphorylated form pIκB-α after treatment with CAPE and MG-132. We found that both compounds upregulated IκB-α and downregulated pIκB-α expression (Figure 3). In contrast, DOX (1.5 µM) and VCR (1 µM) did not modulate the expression level of either cytoplasmic or nuclear p65. Doxorubicine treatment of PL104 cells was not able to modify IκB-α and pIκB-α levels and VCR slightly increased IκB-α and decreased pIκB-α (Figure 3). Thus, results indicate that CAPE and MG-132 have a strong antiproliferative effect because only 0.7% of cells still proliferate after treatment and that both compounds upregulated NF-κB inhibitor while decreasing nuclear NF-κB levels, suggesting an overall inhibitory effect.
Figure 3.
CAPE and MG-132 treatment of PL104 cells modulate NF-κB expression. Nuclear or cytoplasmic protein extracts were subjected to SDS-PAGE and Western blot analysis by using antibodies against p65, IκB-α, pIκB-α, and β-actin. (A) Representative results are shown. Lane 1: RPMI-1640. Lane 2: DMSO 0.1% v/v 2 hours. Lane 3: CAPE 50 µg/ml 2 hours. Lane 4: MG-132 2 µM 2 hours. Lane 5: DOX 1.5 µM 2 hours. Lane 6: VCR 1 µM 2 hours. (B) Bar graph showing the RI for each treatment. Upper bars, mean ± SD of at least three independent experiments (*P < .05, **P < .01, ***P < .001).
DOX, VCR, CAPE, and MG-132 Differentially Affect Cell Cycle Progression of PL104 Cell Line
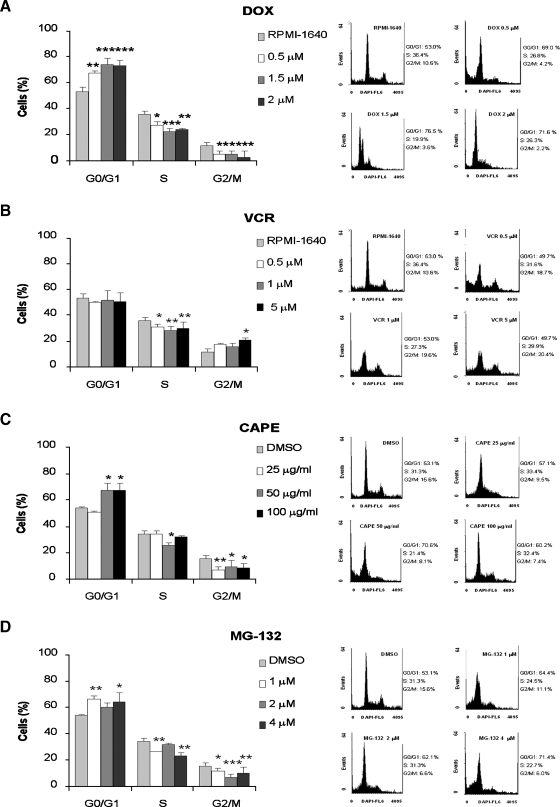
As both chemotherapeutic agents and CAPE and MG-132 displayed an antiproliferative effect on in vitro treatment of PL104 cells, we further examined their effects on cell mitotic cycle and DNA content of cells stained with DAPI by flow cytometry analysis. Figure 4A shows that 53% of PL104 cells were in G0/G1 phase, 35% in S phase, and only 12% were in G2/M phase. Treatment with 1.5 and 2 µM DOX caused an increase in the proportion of cells in G0/G1 (74%-73%) and a substantial decrease in both cells in G2/M phase (3%–5%) and S phase (23%–24%) (Figure 4A). Vincristine treatment failed to modify the percentage of cells in G0/G1 at the concentrations tested, whereas the G2/M population distribution was only altered at 5 µM (9% increase). A reduction of the percentage of cells in S phase was observed at all doses tested (5%, 7%, and 6% for 0.5, 1, and 5 µM VCR, respectively; Figure 4B). Assessment of cell cycle distribution after 25 µg/ml CAPE showed no significant differences in the percentage of cells in each cell cycle phase compared with DMSO control. In contrast, a higher percentage of cells in G0/G1 (67%) and a lower percentage of cells in G2/M phase (8.5%) were recorded after treatment with 50 or 100 µg/ml CAPE. In addition, a substantial reduction of cells in S phase was observed at 50 µg/ml CAPE (Figure 4C). MG-132 treatment increased the proportion of cells in G0/G1 phase and decreased both the percentages of cells in G2/M and S phase (Figure 4D). Thus, PL104 distribution in the different phases of the cell cycle varies with the compound used: DOX significantly retards G1 → S cell cycle transition, VCR mainly affects cells at S phase, CAPE mainly affects cells at G0/G1, and MG-132 shows a partial effect on all stages of cell cycle progression.
Figure 4.
PL104 cell cycle distribution is affected after treatment with both chemotherapeutic agents, CAPE and MG-132. Cell cycle analysis of PL104 cells exposed to a range of concentrations of DOX (A), VCR (B), CAPE (C), and MG-132 (D). The percentage of cells in G0/G1, S, and G2/M phases is indicated in the bar graph (left of each panel). Upper bars, mean ± SD of at least three independent experiments (*P < .05, **P < .01, ***P < .001). A representative DNA histogram of each treatment is shown on the right side of each panel. Control cells were treated with RPMI-1640 medium or 0.1% v/v DMSO.
CAPE and MG-132 Treatments Show Greater Efficacy in Apoptosis Induction Than DOX and VCR
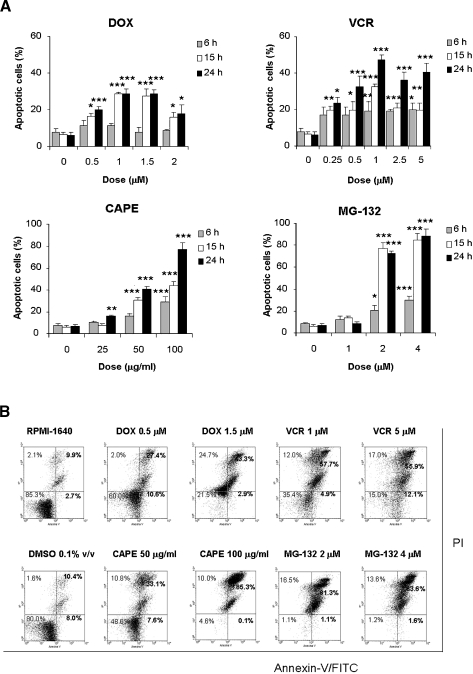
Because treatment of PL104 cells with the above drugs resulted in significant cell growth inhibition, we decided to evaluate whether this biological effect caused cell death through apoptosis. Apoptosis hallmarks were analyzed in PL104-treated cells after acridine orange and EtBr staining. After 6 hours of treatment with increasing doses of DOX, no significant cell death was observed (Figure 5A). A maximal rate of 28.65 ± 2.68% of apoptosis (P < .001) was recorded after 24 hours. Treatment with VCR showed earlier apoptosis induction beginning at 6 hours after 1-µM dose exposure (Figure 5A). The maximal apoptotic rate attained was of 47.34 ± 2.66% of apoptosis (P < .001). CAPE and MG-132 showed a remarkable apoptosis induction in PL104 cells. Both treatments with 100 µg/ml CAPE and 2 to 4 µM MG-132 showed statistically significant apoptosis values in 6-hour exposure assays. Increased values were observed in a time/dose-course-dependent manner, both with CAPE and MG-132. Maximal apoptotic rates were 77.10 ± 5.17% and 88.57 ± 6.09% (P < .001) after CAPE (100 µg/ml) and MG-132 (4 µM) 24-hour exposure, respectively (Figure 5A). The induction of apoptosis was further confirmed by phosphatidylserine exposure on plasma membrane by Annexin V-FITC/PI staining of PL104 cells and evaluated by flow cytometry (Figure 5B). Results obtained for apoptotic rates were in agreement with those obtained from morphological assessment. Overall, these results indicate that CAPE and MG-132 induced higher apoptotic rates than DOX and VCR in PL104 cells, suggesting that these compounds might affect critical aspects of death/survival balance in this cell line having worth chemotherapeutic potential.
Figure 5.
CAPE and MG-132 are apoptosis inducers stronger than DOX and VCR on treatment of PL104 cells. Apoptosis induction after treatment of PL104 cells with the four drugs. (A) Morphological evaluation of apoptosis of cells exposed to different concentrations of DOX, VCR, CAPE, or MG-132 for 6, 15, and 24 hours. Results are expressed as the percentage of cells stained with acridine orange and EtBr having morphological hallmarks of apoptosis. Bars, mean ± SD of at least three independent experiments (*P < .05, **P < .01, ***P < .001). Control cells were treated with RPMI-1640 or 0.1% v/v DMSO. (B) Evaluation of apoptosis through Annexin V-FITC/IP staining and flow cytometry of PL104 cells exposed to different concentrations of DOX, VCR, CAPE, or MG-132 for 24 hours.
CAPE and MG-132 Are Potent Inducers of Apoptotic Death on PDL Cells
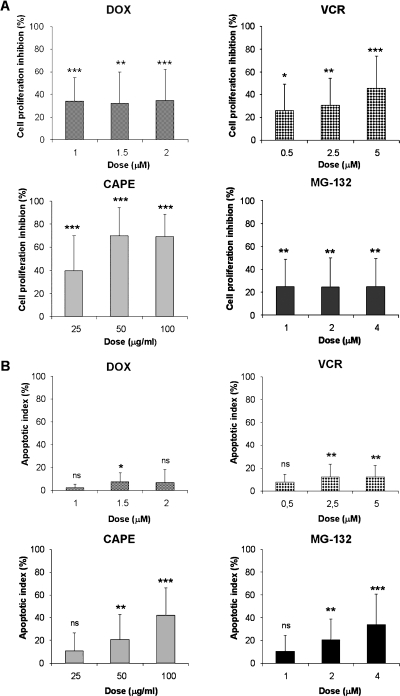
To verify whether the effect of NF-κB inhibitors on PL104 cell proliferation and apoptosis induction can be extended to different leukemia cell types, similar studies were performed in bone marrow samples obtained from patients with the characteristics described in Materials and Methods. Doxorubicine and VCR were also tested as control drugs. Figure 6A shows that treatment of PDL cells with CAPE resulted in a remarkable inhibition of proliferation at all concentrations tested (0–100 µg/ml). The highest inhibition (69.80 ± 24.43%, P < .001) was observed at 50 µg/ml CAPE. This value was considerably higher than the maximal inhibitory effect observed with MG-132 treatment (25.17 ± 23.54%, P < .01) at 1 µM dose and also higher than the percentages observed with DOX and VCR treatment.
Figure 6.
CAPE and MG-132 treatment showed an antiproliferative and cytotoxic effect on PDL cells. (A) PDL cells were cultured in the presence of increasing concentrations of DOX, VCR, CAPE, or MG-132 for 24 hours. Results are presented as percentage of cell proliferation inhibition of each treatment. (B) Apoptosis induction evaluated after acridine orange and EtBr staining. PDL cells were treated for 24 hours with DOX, VCR, CAPE, or MG-132. Bars, mean ± SD of at least three independent experiments (ns indicates not significant, *P < .05, **P < .01, ***P < .001).
The percentage of apoptotic PDL cells after treatment with 100 µg/ml CAPE was 42.04 ± 23.94% (P < .01) and 34.05 ± 26.30% (P < .01) with 4 µM MG-132. In contrast, DOX and VCR treatment did not induce significant apoptosis (Figure 6B). The percentage of apoptotic cells was of 7.63 ± 7.43% with DOX treatment and 12.38 ± 9.79% with VCR treatment. These results support a strong cytotoxic effect for CAPE and MG-132 in PDL cells, most probably related to an alteration of the cell survival/death balance, and confirm the predominance of cytostatic over cytotoxic effects of the conventional antiproliferative DOX and VCR compounds.
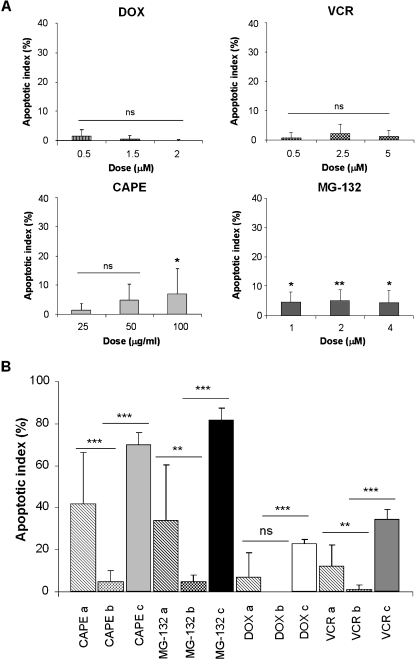
The Chemotherapeutic Agents, CAPE and MG-132, Have No Considerable Cytotoxic Effect on Normal Mononuclear Human Cells
The promising results obtained after treatment of PL104 cell line and PDL cells with CAPE and MG-132 prompted us to evaluate whether these compounds were able to exert a similar effect on normal cells. In 24-hour morphological assays, only low rates of apoptosis induction varying from 0.1% to 5% were observed in PBMC from healthy donors either when exposed to CAPE and MG-132 or after treatment with DOX and VCR (Figure 7A). However, the differences in the apoptosis rate between drug-treated PL104 cells and drug-treated normal PBMC showed statistically significant differences of 65.1%, 76.9%, 22.7%, and 32.9% (P < .001) for CAPE, MG-132, DOX, and VCR, respectively (Figure 7B). When analyzing the differences in the apoptosis rate between drug-treated PDL cells and normal PBMC, smaller but still significant values were observed with CAPE (37.2%, P < .001), MG-132 (29.5%, P < .01), and VCR (11.1%, P < .01; Figure 7B). These results suggest that selectivity toward malignant cells, in cytotoxic effects, is considerably higher for CAPE and MG-132 than for the conventional chemotherapeutic drugs DOX and VCR.
Figure 7.
DOX, VCR, and NF-κB inhibitors have no considerable cytotoxic effect on normal PBMCs. Cells were treated with DOX, VCR, CAPE, or MG-132 at different concentrations for 24 hours. (A) Acridine orange and EtBr staining was performed to determine the percentage of apoptotic cell death. Bars, mean ± SD of at least three independent experiments. (B) Apoptosis comparative analysis for 1.5 µM DOX, 5 µM VCR, 100 µg/ml CAPE, and 4 µM MG-132 treatments among different cell types: a, PDL cells; b, normal PBMCs; c, PL104 cells (ns indicates not significant, *P < .05, **P < .01, ***P < .001).
Simultaneous Exposure of PL104 Cells to DOX or VCR and CAPE or MG-132 Results in an Increase of Apoptotic Cell Death
To investigate whether CAPE or MG-132 can increase the susceptibility of PL104 cells to chemotherapeutic agents, we tested the combined effect of DOX or VCR plus either CAPE or MG-132 at concentrations associated with minimal lethality. Apoptotic rates were evaluated in PL104 cells simultaneously exposed to the different combinations of drugs for 24 and 48 hours (Table 1). After a 24-hour exposure, only VCR treatment combined with either CAPE or MG-132 showed a slight statistically significant increase in apoptosis percentage (8.6%, P < .05 and 11%, P < .001, respectively, vs the chemotherapeutic drug alone). However, after 48 hours, both DOX and VCR treatment combined with either CAPE or MG-132 resulted in a higher increase of 1.6- to 2-fold in apoptosis percentage (P < .001 vs the chemotherapeutic drug alone), reaching in all cases a maximal apoptotic rate of 54% to 57%. No significant effects were recorded after CAPE and MG-132 combined treatment on PL104 cells. Collectively, these findings indicate that the combination of chemotherapeutic drugs with CAPE or MG-132 enhances the cytotoxic effect of each drug alone.
Table 1.
Apoptosis Induction of CAPE or MG-132 + Chemotherapeutic Agents.
| Treatment | Apoptotic Index (%) | P | ||||
| 24 hours | ||||||
| CAPE + MG-132 | 1.00 ± 0.95 | >0.05 | ||||
| CAPE + DOX | 9.270 ± 0.88 | >0.05 | ||||
| CAPE + VCR | 22.75 ± 3.63 | <0.05 | * | |||
| MG-132 + DOX | 2.34 ± 1.47 | <0.05 | ||||
| MG-132 + VCR | 25.32 ± 3.52 | <0.001 | *** | |||
| DOX 0.5 µM | 0.47 ± 0.16 | |||||
| VCR 0.25 µM | 14.16 ± 3.28 | |||||
| CAPE 25 µg/ml | 4.56 ± 1.77 | |||||
| MG-132 1 µM | 1.16 ± 0.99 | |||||
| 48 hours | ||||||
| CAPE + MG-132 | 17.78 ± 3.18 | >0.05 | ||||
| CAPE + DOX | 57.13 ± 3.92 | <0.001 | *** | |||
| CAPE + VCR | 52.02 ± 5.08 | <0.001 | *** | |||
| MG-132 + DOX | 54.69 ± 2.31 | <0.001 | *** | |||
| MG-132 + VCR | 55.29 ± 4.21 | <0.001 | *** | |||
| DOX 0.5 µM | 33.98 ± 0.08 | <0.001 | ||||
| VCR 0.25 µM | 28.51 ± 3.38 | <0.001 | ||||
| CAPE 25 µg/ml | 13.09 ± 3.05 | <0.001 | ||||
| MG-132 1 µM | 0.30 ± 0.01 | <0.001 | ||||
DOX or VCR plus NF-κB inhibitor combined treatment increased apoptosis induction in PL104 cells. Cells were treated with the different combinations for 24 and 48 hours, and apoptosis induction was analyzed afterward by acridine orange and EtBr staining.
p < 0.05.
p < 0.001.
Discussion
The experimental use of continuous human cell lines represents a valuable tool for understanding the biology, pathogenesis, and treatment options for various tumor malignancies. In this study, we report the establishment of a novel cell line, PL104, from bone marrow aspirate of a patient with an atypical myeloid malignancy. PL104 cells correspond to mature B monoclonal lymphocytes, EBV-positive. In this type of cells, as well as in those infected by Kaposi sarcoma herpesvirus (KSHV), activated NF-κB is considered a critical protective mechanism against apoptotic stress [35].
The physiological functions of NF-κB and many upstream regulators have been intensively studied, revealing an essential role for this transcription factor in inflammation and lymphoid organ development and as a positive mediator of T and B lymphocyte maturation, activation, proliferation, and survival [36]. Its constitutive activity is also crucial for cell survival, proliferation, and differentiation; thus, its inhibition may facilitate apoptosis in different cell types [37,38].
Many NF-κB inhibitors have emerged over the recent years in an attempt to contribute to cancer treatment, although only one (bortezomib) is currently included in some chemotherapeutic protocols [39]. In this work, we evaluated the relevance of CAPE and MG-132, which, among their broad effects, can inhibit NF-κB, on PL104 cell line in terms of cell survival and proliferation. Both agents had a strong antiproliferative effect on PL104 cells, which was dose- and time-dependent and involved cell cycle deregulations. As suggested by Keller et al. [35] on KSHV- and EBV-lymphoma cells, inhibition of NF-κB may be related to the down-regulation of specific antiapoptototic and growth-related proteins involved in controlling cell survival, thus leading to a reduction of cell proliferation. In agreement with our results, the NF-κB inhibitor Bay 11-7082 has also been reported to downregulate a specific subset of genes and to induce apoptosis in IB4 cells, an EBV-infected cord blood-derived LCL [40].
We showed that CAPE treatment increased the percentage of cells in G0/G1 and decreased the percentage of cells in S and G2/M phase. These modifications in PL104 cell cycle distribution are in agreement with the ability of this compound to inhibit DNA, RNA, and protein synthesis [41], thus delaying cell cycle progression to G2/M phase. Cell cycle analysis of PL104 cells revealed that MG-132, similarly to CAPE, reduced the percentage of cells at S and G2/M phases and increased the percentage of G0/G1 cells. These findings may be related to the fact that inhibition of the 26S proteasome function blocks cellular transition from G1 to S phase and from late S to G2/M phase [42].
We further analyzed the antiproliferative activity of DOX and VCR, two cytostatic drugs used to suppress cell growth and proliferation of leukemia, lymphoma, and solid tumors cells. Both DOX and VCR were able to significantly inhibit cell proliferation, although DOX-treated cells were more sensitive to the antiproliferative effects than VCR-treated cells at 1 µM (76.6% and 48.7% growth inhibition, respectively). Cell cycle analysis by flow cytometry demonstrated that DOX increased the percentage of cells at G0/G1 with a concomitant reduction in the percentage of cells at S phase. Thus, the accumulation of PL104 cells at G0/G1 retarded S phase entry. Vincristine-treated PL104 cells showed a significantly reduced percentage of cells at S phase, suggesting that VCR might be able to influence the G2-to-M progression by retaining cells in a stage of the cell cycle where they are less sensitive to the cytotoxic effects [43].
The therapeutic goal of cancer treatment is to induce apoptotic death of cancer cells rather than necrosis due to the deleterious consequences of the latter, which include leakage of lysosomal enzymes to the extracellular media and spawning a substantial inflammatory reaction. CAPE and MG-132 maximal concentrations caused high levels of apoptotic cell death of 77.1% and 88.6%, respectively, with no signs of significant necrosis in PL104 cells, values considerably higher than the ones reached with DOX and VCR, suggesting that these two agents mainly exert antiproliferative effects with little or no cytotoxic effect on PL104 cells.
It has been previously found that proteasome inhibitors may cause cell death of tumor cell lines of different origins and preclinical studies have demonstrated antineoplastic activity in hematological malignancies and solid tumors [44,45]. CAPE has been reported to inhibit the growth of oral carcinoma [46] and to produce a cytotoxic effect against brain tumors [47], prostate and breast cancer cells [48,49], and human myeloid leukemia cells [50].
Beyond antiproliferative and cytotoxic effects on PL104 cells, our study showed that both CAPE and MG-132 exerted dissimilar effects on PDL cells. CAPE showed higher antiproliferative potential than MG-132. However, both drugs had a similar effect on apoptosis induction in PDL cells as opposed to our findings with PL104 cells, where MG-132 induced higher levels of apoptosis than CAPE.
The chemotherapeutic agents DOX and VCR displayed only antiproliferative activity, which was not associated to cell death, as evidenced by our apoptosis studies. Although DOX and VCR are known to have mainly an antiproliferative effect against tumor human malignancies, our study demonstrated that the percentage of cell proliferation inhibition reached with CAPE was 25% to 35% higher than that with DOX and VCR. This finding is most valuable because, to our knowledge, this is the first study to assess the effects of CAPE and MG-132 on PDL cells.
Drug selectivity contributes to minimize or eliminate undesirable and harmful side effects of cytotoxic drugs due to prolonged administration and is a crucial point when designing a suitable therapeutic protocol for patients with malignant neoplasias or when evaluating possible new treatment agents. Thus, we analyzed the effect of CAPE and MG-132, DOX and VCR on normal PBMCs. CAPE and MG-132 showed enhanced selectivity of apoptosis toward PDL cells and PL104 cells compared with normal PBMCs. These results are consistent with previous findings showing that, at concentrations inducing a high leukemic cell apoptosis rate, proteasome inhibitors lactacystin and MG-132 only marginally induce apoptosis in human CD34+ hematopoietic progenitor cells and moderately affect their outgrowth [45]. MG-132 cytotoxic selectivity may be because cancer cells have more defective proteins accumulating at much higher rates than normal cells, thus increasing their dependency on optimal proteasome function. The selectivity of CAPE cytotoxic effects observed in our study has also been shown in oral carcinoma cell lines compared with normal human oral fibroblasts [46] and in medulloblastoma cells compared with human fetal glial cells [51]. Although DOX and VCR treatment of normal PBMCs had no considerable cytotoxic effect, both drugs also failed to induce apoptosis in PDL cells.
Recent reports point out that safe and nontoxic cancer chemopreventive phytochemicals can function as sensitizer drugs, increasing the effectiveness of cancer treatment [47,52,53]. Similarly, inhibition of NF-κB signaling could potentially be effective as a single agent or in combination with radiotherapy or chemotherapy in pathologies that solely depend on this transcription factor for survival [54,55]. Our results in PL104 cell line demonstrate that subtoxic or sublethal concentrations of DOX, VCR, CAPE, or MG-132 administered individually had little effect on apoptosis induction, whereas combined treatment of cells with CAPE or MG-132 plus a conventional chemotherapeutic drug resulted in an enhancement of cell death. Drug combination/drug cocktails that modulate apoptosis induction may be considered a powerful weapon against different tumor malignancies and also a mean to enhance the cytotoxic effect and reduce the toxicity of current anticancer agents.
In this work, CAPE and MG-132 showed a considerable potential as apoptosis inductors on an LCL as well as on primary cells recovered from patients with leukemia. Their cytotoxic effect was mainly restricted to pathologic cells rather than normal ones, thus conferring a selective action to both compounds and a promising treatment option that will deserve further studies. Altering the thresholds of tumor cells to apoptosis induction and sensitizing them to establish therapy protocol is expected to contribute to progress in cancer therapy in the future.
Supplementary Material
Footnotes
This study was supported by grants from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) PIP no. 5273 and Secretaría de Ciencia y Tecnología Universidad de Buenos Aires B021, B007.
This article refers to supplementary materials, which are designated by Figure W1 and Table W1 and are available online at www.neoplasia.com.
References
- 1.Dalton WS. The tumor microenvironment: focus on myeloma. Cancer Treat Rev. 2003;29:11–19. doi: 10.1016/s0305-7372(03)00077-x. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Gong J, Feldman E, Seiter K, Traganos F, Darzynkiewicz Z. Apoptotic cell death during treatment of leukemias. Leukemia. 1993;7:659–670. doi: 10.3109/10428199409052678. [DOI] [PubMed] [Google Scholar]
- 3.Del Poeta G, Stasi R, Aronica G. Clinic relevance of P-glycoproteins expression in de novo acute myeloid leukemia. Blood. 1996;5:1997–2004. [PubMed] [Google Scholar]
- 4.Taraphdar AK, Roy M, Bhattacharya RK. Natural products as inducers of apoptosis: implication for cancer therapy and prevention. Curr Sci. 2001;80:387–1396. [Google Scholar]
- 5.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 6.Brummelkamp TR, Nijman SMB, Dirac AMG, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-κB. Nature. 2003;424:797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- 7.Pikarsky E, Porat RM, Stein I, Abramovitch SA, Kasem S, Gutkovich-pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 8.Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, Sonenshein GE. Aberrant nuclear factor NFκB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keller SA, Schattner EJ, Cesarman E. Inhibition of NF-κB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood. 2000;96:2537–2542. [PubMed] [Google Scholar]
- 10.Cilloni D, Martinelli G, Messa F, Baccarani M, Saglio G. Nuclear factor κB as a target for new drug development in myeloid malignancies. Haematologica. 2007;92:1224–1229. doi: 10.3324/haematol.11199. [DOI] [PubMed] [Google Scholar]
- 11.Witkowski JM, Zmuda-Trzebiatowska E, Swiercz JM, Cichorek M, Ciepluch H, Lewandowski K, Bryl E, Hellmann A. Modulation of the activity of calcium-activated neutral proteases (calpains) in chronic lymphocytic leukemia (B-CLL) cells. Blood. 2002;100:1802–1809. doi: 10.1182/blood-2001-11-0073. [DOI] [PubMed] [Google Scholar]
- 12.Kerbauy DM, Lesnikov V, Abbasi N, Seal S, Scott B, Deeg HJ. NF-κB and FLIP in arsenic trioxide (ATO)-induced apoptosis in myelodysplastic syndromes (MDSs) Blood. 2005;106:3917–3925. doi: 10.1182/blood-2005-04-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bharti AC, Shishodia S, Reuben JM, Weber D, Alexanian R, Raj-Vadhan S, Estrov Z, Talpaz M, Aggarwal BB. Nuclear factor κB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–3184. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- 14.Jost PJ, Ruland J. Aberrant NF-κB signaling in lymphoma: mechanisms, consequences and therapeutic implications. Blood. 2007;109:2700–2707. doi: 10.1182/blood-2006-07-025809. [DOI] [PubMed] [Google Scholar]
- 15.Cilloni D, Messa F, Arruga F, Defilippi I, Morotti A, Messa E, Carturan S, Giugliano E, Pautasso M, Bracco E, et al. The NF-κB pathway blockade by the IKK inhibitor PS1145 can overcome imatinib resistance. Leukemia. 2006;20:61–67. doi: 10.1038/sj.leu.2403998. [DOI] [PubMed] [Google Scholar]
- 16.Pande V, Ramos MJ. NF-kappaB in human disease: current inhibitors and prospects for de novo structure based design of inhibitors. Curr Med Chem. 2005;12:357–374. doi: 10.2174/0929867053363180. [DOI] [PubMed] [Google Scholar]
- 17.Celec P. Nuclear factor kappa B-molecular biomedicine: the next generation. Biomed Pharmacother. 2004;58:365–374. doi: 10.1016/j.biopha.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Rajkumar SV, Richardson PG, Hidheshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;20:630–639. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 19.Elliott PJ, Zollner TM, Boehncke WH. Proteasome inhibition: a new anti-inflammatory strategy. J Mol Med. 2003;81:397–403. doi: 10.1007/s00109-003-0422-2. [DOI] [PubMed] [Google Scholar]
- 20.Natarajan K, Singh S, Burke TR, Grumberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of the activation of nuclear transcription factor NF-κB. Proc Natl Acad Sci. 1996;USA 93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and Ikappa B alpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 22.Wua WM, Lu L, Long Y, Wang T, Liu L, Chen Q, Wang R. Free radical scavenging and antioxidative activities of caffeic acid phenethyl ester (CAPE) and its related compounds in solution and membranes: a structure-activity insight. Food Chem. 2007;105:107–115. [Google Scholar]
- 23.Michaluart P, Masferrer JL, Carothers AM, Subbaramaiah K, Zweifel BS, Koboldt C, Mestre JR, Grunberger B, Sacks PG, Tanabe T, et al. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxigenase-2. Cancer Res. 1999;59:2341–2352. [PubMed] [Google Scholar]
- 24.Fesen MR, Pommier Y, Leteurtre F, Hiroguchi S, Yung J, Kohn KW. Inhibition of HIV integrase by flavones, caffeic phenethyl acid and related compounds. Biochem Pharmacol. 1994;48:595–608. doi: 10.1016/0006-2952(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 25.Bankova V, Christov R, Kujumgiev A, Marcucci MC, Popov S. Chemical composition and antibacterial activity of Brazilian propolis. Z Naturforsch [C] 1995;50:167–172. doi: 10.1515/znc-1995-3-402. [DOI] [PubMed] [Google Scholar]
- 26.Breger J, Burgwyn FB, Aperis G, Moy TI, Ausubel FM, Mylonakis E. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007;3:168–178. doi: 10.1371/journal.ppat.0030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orsolic N, Sver L, Terzic S, Tadic Z, Basic L. Inhibitory effect of water-soluble derivatives of propolis and its polyphenolic compounds on tumor growth and metastasizing ability: a possible mode of antitumor action. Nut Cancer. 2003;47:156–163. doi: 10.1207/s15327914nc4702_8. [DOI] [PubMed] [Google Scholar]
- 28.Kiyoyuki O, Yoshifumi K, Chikako S, Hideto T, Kazuo D, Akio H. Diagnostic application of flow cytometric characteristics of CD34+ cells in low-grade myelodysplastic syndromes. Blood. 2006;108:1037–1044. doi: 10.1182/blood-2005-12-4916. [DOI] [PubMed] [Google Scholar]
- 29.Saito I, Servenius V, Compton T, Fox R. Detection of EBV DNA by polymerase chain reaction in blood and tissue biopsies from patients with Sjogren's syndrome. J Exp Med. 1998;169:2191–2198. doi: 10.1084/jem.169.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerdá Zolezzi P, Fernández T, Aulicino P, Cavaliere V, Greczanik S, Caldas Lopez E, Wagner M, Ricco R, Gurni A, Hajos S, et al. Ligaria cuneifolia fractions modulate cell growth of normal lymphocytes and tumor cells as well as multidrug resistant cells. Immunobiology. 2005;209:737–749. doi: 10.1016/j.imbio.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Alaniz L, García MG, Cabrera P, Arnaiz M, Cavaliere V, Blanco G, Álvarez E, Hajos S. Modulation of matrix metalloproteinase-9 activity by hyaluronan is dependent on NF-κB activity in lymphoma cell lines with dissimilar invasive behavior. Biochem Biophys Res Commun. 2004;324:736–743. doi: 10.1016/j.bbrc.2004.09.120. [DOI] [PubMed] [Google Scholar]
- 32.Ciccarelli R, D'Alimonte I, Ballerini P, D'Auro M, Nargi E, Buccella S, Di Iorio P, Bruno V, Nicoletti F, Caciagli F. Molecular signaling mediating the protective effect of A1 adenosine and mGlu3 metabotropic glutamate receptor activation against apoptosis by oxygen/glucose deprivation in cultured astrocytes. Mol Pharmacol. 2007;71:1369–1380. doi: 10.1124/mol.106.031617. [DOI] [PubMed] [Google Scholar]
- 33.Xingming D, Fengqin G, Stratford May WJ. Bcl2 retards G1/S cell cycle transition by regulating intracellular ROS. Blood. 2003;102:3179–3185. doi: 10.1182/blood-2003-04-1027. [DOI] [PubMed] [Google Scholar]
- 34.García MG, Alaniz L, Caldas Lopes E, Blanco G, Alvarez E, Hajos S. Inhibition of NF-κB activity by BAY 11-7082 increases apoptosis in multidrug resistant leukemic T-cell lines. Leukemia Res. 2005;29:1425–1434. doi: 10.1016/j.leukres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Keller SA, Hernandez-Hopkins D, Vider J, Ponomarev V, Hyjek E, Schattner EJ, Cesarman E. NF-κB is essential for progression of KSHV- and EBV-infected lymphomas in vivo. Blood. 2006;107:3295–3302. doi: 10.1182/blood-2005-07-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 37.Beg AA, Baltimore D. An essential role for NF-κB in preventing TNFα-induced cell death. Nature. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 38.Wu M, Lee H, Bellas RE, Schauer SL, Arsura M, Katz D, FitzGerald MJ, Rothstein TL, Sherr DH, Sonenshein GE. Inhibition of NFκB/Rel induces apoptosis of murine B cells. EMBO J. 1996;15:4682–4690. [PMC free article] [PubMed] [Google Scholar]
- 39.San-Miguel JF, Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, et al. Efficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 study. Leukemia. 2008;4:842–849. doi: 10.1038/sj.leu.2405087. [DOI] [PubMed] [Google Scholar]
- 40.Cahir-McFarland ED, Carter K, Rosenwald A, Giltnane JM, Henrickson SE, Staud LM, Kieff E. Role of NF-kappa B in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J Virol. 2004;78:4108–4119. doi: 10.1128/JVI.78.8.4108-4119.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan J, Yan R, Kramer A, Eckerdt F, Roller M, Kaufmann M, Strebhardt K. Cyclin B1 depletion inhibits proliferation and induces apoptosis in human tumor cells. Oncogene. 2004;23:5843–5852. doi: 10.1038/sj.onc.1207757. [DOI] [PubMed] [Google Scholar]
- 42.Machiels BM, Henfling ME, Gerards WL, Broers JL, Bloemendal H, Ramaekers FC, Schutte B. Detailed analysis of cell cycle kinetics upon proteasome inhibition. Cytometry. 1997;28:243–252. [PubMed] [Google Scholar]
- 43.Skladanowski A, Côme MG, Sabisz M, Escargueil AE, Larsen AK. Down-regulation of DNA topoisomerase IIα leads to prolonged cell cycle transit in G2 and early M phases and increased survival to microtubule-interacting agents. Mol Pharmcol. 2005;68:625–634. doi: 10.1124/mol.105.013995. [DOI] [PubMed] [Google Scholar]
- 44.Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5:417–421. doi: 10.1016/s1535-6108(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 45.Naujokat C, Sezer O, Zinke H, Leclere A, Hauptmann S, Possinger K. Proteasome inhibitors induce caspase-dependent apoptosis and accumulation of p21WAF1/Cip1 in human immature leukemic cells. Eur J Haematol. 2000;65:221–236. doi: 10.1034/j.1600-0609.2000.065004221.x. [DOI] [PubMed] [Google Scholar]
- 46.Leea YT, Donb MJ, Hungc PS, Shenb YC, Loc YS, Changc KW, Chenb CF, Hoa LK. Cytotoxicity of phenolic acid phenethyl esters on oral cancer cells. Cancer Lett. 2005;223:19–25. doi: 10.1016/j.canlet.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 47.Lin YH, Chiu JH, Tseng EWS, Wong TT, Chiou ESH, Yen ESH. Antiproliferation and radiosensitization of caffeic acid phenethyl ester on human medulloblastoma cells. Cancer Chemother Pharmacol. 2006;57:525–532. doi: 10.1007/s00280-005-0066-8. [DOI] [PubMed] [Google Scholar]
- 48.Mc Eleny K, Coffrey R, Morrissey C, Fitzapatrick JM, Watson WG. Caffeic acid phenethyl ester-induced PC-3 cell apoptosis is caspase-dependent and mediated through the loss of inhibitors of apoptosis proteins. BJU Int. 2004;94:402–406. doi: 10.1111/j.1464-410X.2004.04936.x. [DOI] [PubMed] [Google Scholar]
- 49.Watabe M, Hishikawa K, Takayanagi A, Shimizu N, Nakaki T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFκB and activation of Fas in human breast cancer MCF-7 cells. J Biol Chem. 2004;279:6017–6026. doi: 10.1074/jbc.M306040200. [DOI] [PubMed] [Google Scholar]
- 50.Chen YJ, Shiao MS, Hsu ML, Tsai TH, Wang SY. Effect of caffeic acid phenethyl ester, an antioxidant from propolis, on inducing apoptosis in human leukemic HL-60 cells. J Agric Food Chem. 2001;49:5615–5619. doi: 10.1021/jf0107252. [DOI] [PubMed] [Google Scholar]
- 51.Lin YH, Chiu JH, Tseng EWS, Wong TT, Chiou ESH, Yen ESH. Antiproliferation and radiosensitization of caffeic acid phenethyl ester on human medulloblastoma cells. Cancer Chemother Pharmacol. 2006;57:525–532. doi: 10.1007/s00280-005-0066-8. [DOI] [PubMed] [Google Scholar]
- 52.Guo J, Verma UN, Gaynor RB, Frenkel EP, Becerra CR. Enhanced chemosensitivity to irinotecan by RNA interference mediated down regulation of the NF-κB p65 subunit. Clin Cancer Res. 2004;10:3333–3334. doi: 10.1158/1078-0432.CCR-03-0366. [DOI] [PubMed] [Google Scholar]
- 53.Kuo HC, Kuo WH, Lee YJ, Wang CJ, Tseng TH. Enhancement of caffeic acid phenethyl ester on all-trans retinoic acid-induced differentiation in human leukemia HL-60 cells. Toxicol Appl Pharmacol. 2006;216:80–88. doi: 10.1016/j.taap.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Fabre C, Carvalho G, Tasdemir E, Braun T, Adès L, Grosjean J, Boehrèr S, Métivier D, Ouquère SS, Pierron G, et al. NF-κB inhibition sensitizes to starvation-induced cell death in high-risk myelodysplastic syndrome and acute myeloid leukemia. Oncogene. 2007;26:1–13. doi: 10.1038/sj.onc.1210187. [DOI] [PubMed] [Google Scholar]
- 55.Munshi A, Kurland JF, Nishikawa T, Chiao PJ, Andreeff M, Raymond EM. Inhibition of constitutively activated nuclear factor-kappaB radiosensitizes human melanoma cells. Mol Cancer Ther. 2004;3:985–992. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.