Abstract
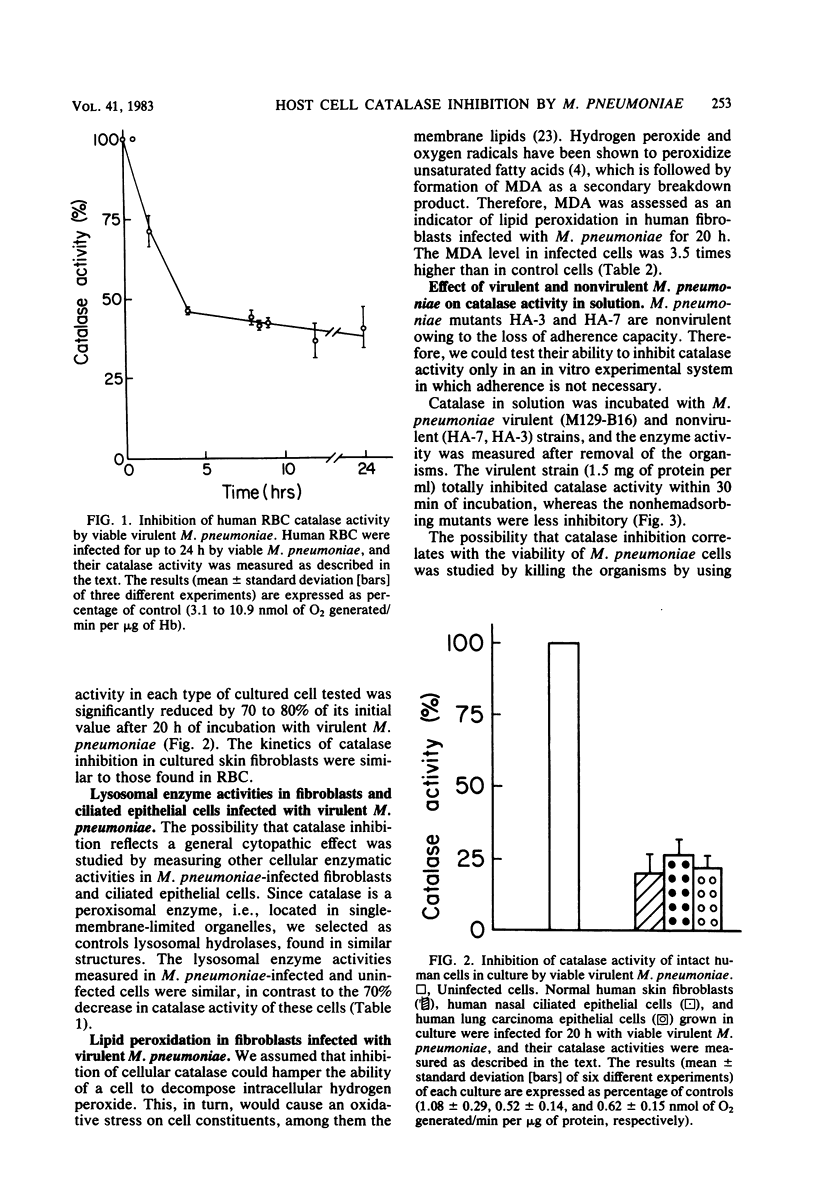
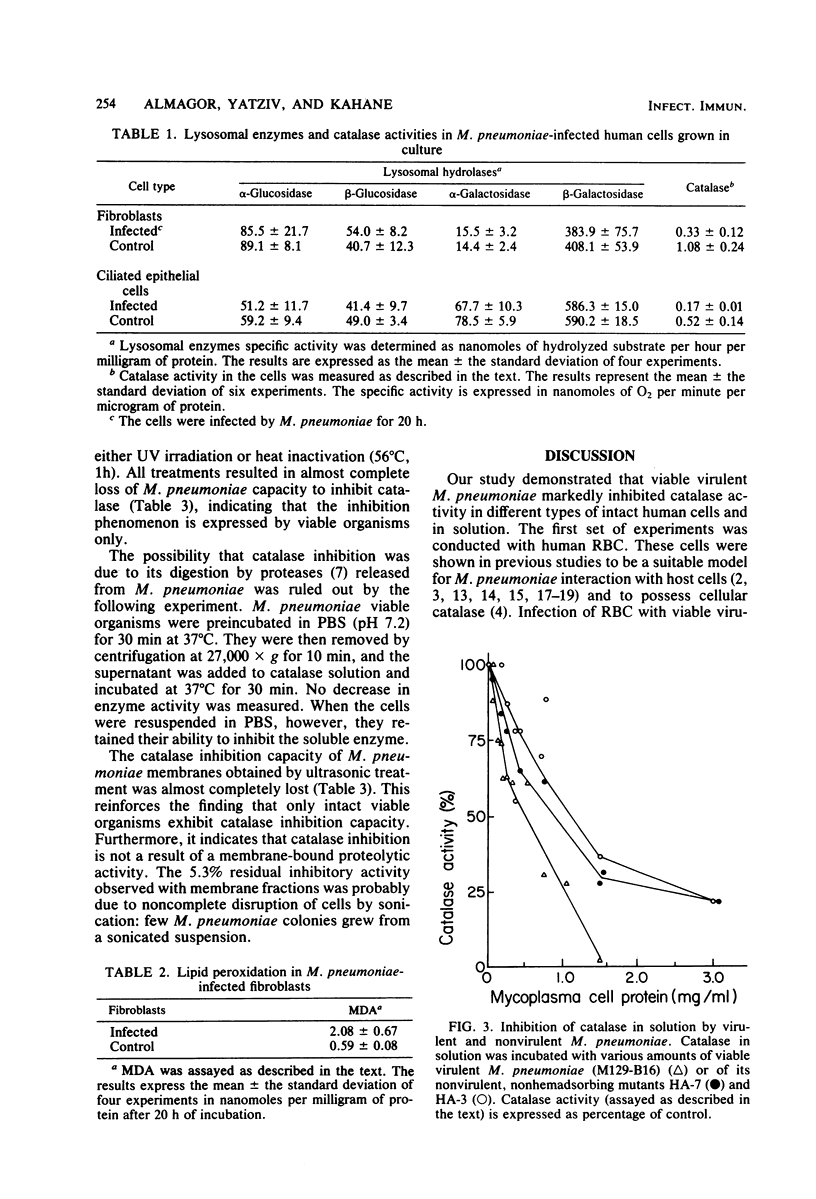
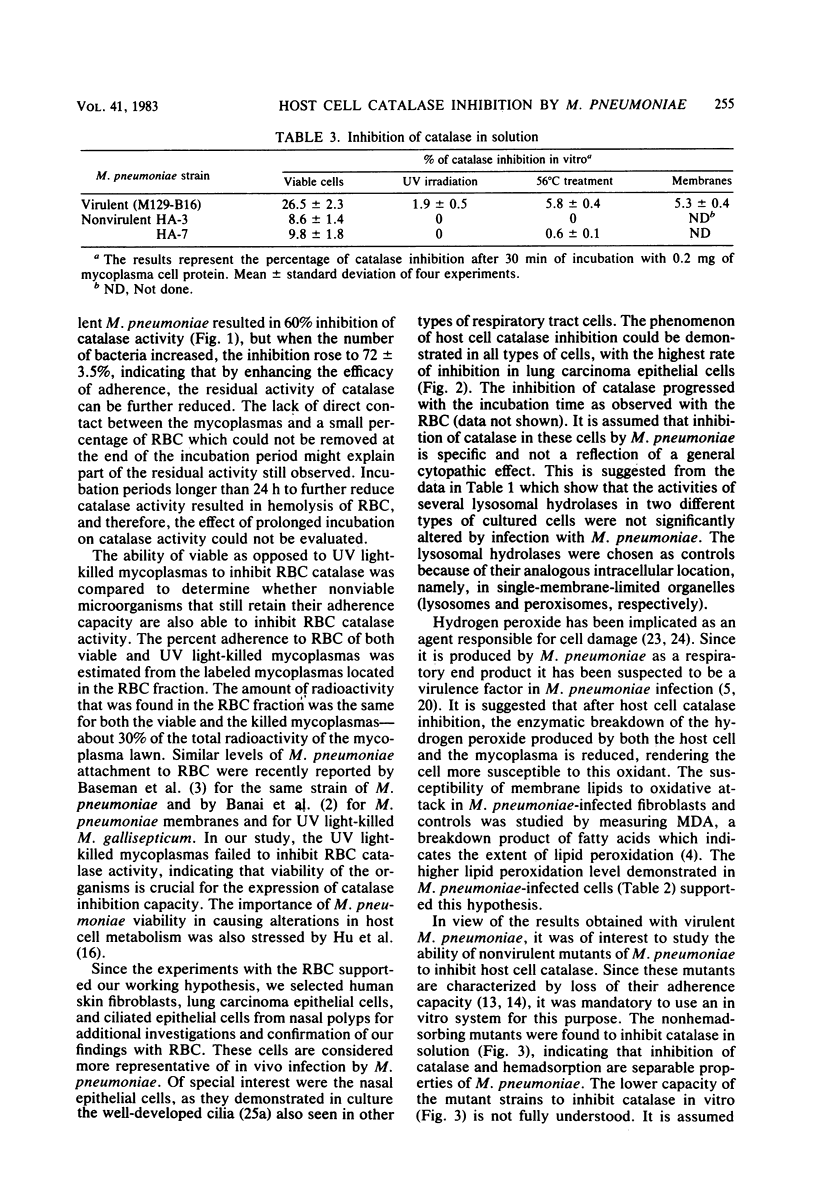
This study demonstrates that viable Mycoplasma pneumoniae cells inhibit catalase activity in several types of intact human cells as well as in solution. Human erythrocyte catalase was inhibited up to 72%, and the inhibition of catalase in human cultured skin fibroblasts, lung carcinoma epithelial cells, and ciliated epithelial cells from human nasal polyps ranged between 75 and 80%. UV light-killed mycoplasmas failed to inhibit catalase activity both in intact cells and in vitro. After M. pneumoniae infection of human cultured skin fibroblasts, the level of malonyldialdehyde, an indicator for membrane lipid peroxidation, was 3.5 times higher than in control fibroblasts. Virulent M. pneumoniae completely inhibited catalase activity in solution, whereas the nonvirulent strains had a lesser ability to inhibit catalase activity. These findings suggest that as a result of host cell catalase inhibition by M. pneumoniae, the toxicity of the hydrogen peroxide generated by the microorganism and the affected cell is enhanced, thereby inducing host cell damage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banai M., Kahane I., Feldner J., Razin S. Attachment of killed Mycoplasma gallisepticum cells and membranes to erythrocytes. Infect Immun. 1981 Nov;34(2):422–427. doi: 10.1128/iai.34.2.422-427.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai M., Razin S., Bredt W., Kahane I. Isolation of binding sites to glycophorin from Mycoplasma pneumoniae membranes. Infect Immun. 1980 Dec;30(3):628–634. doi: 10.1128/iai.30.3.628-634.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Banai M., Kahane I. Sialic acid residues mediate Mycoplasma pneumoniae attachment to human and sheep erythrocytes. Infect Immun. 1982 Oct;38(1):389–391. doi: 10.1128/iai.38.1.389-391.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- CHANOCK R. M., JAMES W. D., FOX H. H., TURNER H. C., MUFSON M. A., HAYFLICK L. Growth of Eaton PPLO in broth and preparation of complement fixing antigen. Proc Soc Exp Biol Med. 1962 Aug-Sep;110:884–889. doi: 10.3181/00379727-110-27681. [DOI] [PubMed] [Google Scholar]
- Cassell G. H., Cole B. C. Mycoplasmas as agents of human disease. N Engl J Med. 1981 Jan 8;304(2):80–89. doi: 10.1056/NEJM198101083040204. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chandler D. K., Barile M. F. Ciliostatic, hemagglutinating, and proteolytic activities in a cell extract of Mycoplasma pneumoniae. Infect Immun. 1980 Sep;29(3):1111–1116. doi: 10.1128/iai.29.3.1111-1116.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G., Somerson N. L. Mycoplasma pneumoniae: hydrogen peroxide secretion and its possible role in virulence. Ann N Y Acad Sci. 1967 Jul 28;143(1):85–87. doi: 10.1111/j.1749-6632.1967.tb27648.x. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A. Relationships Between Mycoplasma pneumoniae and Human Respiratory Epithelium. Infect Immun. 1971 May;3(5):694–701. doi: 10.1128/iai.3.5.694-701.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy H. M., Kenny G. E., Cooney M. K., Allan I. D. Long-term epidemiology of infections with Mycoplasma pneumoniae. J Infect Dis. 1979 Jun;139(6):681–687. doi: 10.1093/infdis/139.6.681. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Barden-Stahl Y. D., Polisky R. B., Engelhardt J. A. Differences in the attachment of Mycoplasma pneumoniae cells and membranes to tracheal epithelium. Infect Immun. 1977 Jun;16(3):766–772. doi: 10.1128/iai.16.3.766-772.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Wilson R. M., Baseman J. B. Two-dimensional gel electrophoretic comparison of proteins from virulent and avirulent strains of Mycoplasma pneumoniae. Infect Immun. 1979 May;24(2):468–475. doi: 10.1128/iai.24.2.468-475.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Wilson R. M., Clyde W. A., Jr, Baseman J. B. Characterization of hemadsorption-negative mutants of Mycoplasma pneumoniae. Infect Immun. 1981 Apr;32(1):127–136. doi: 10.1128/iai.32.1.127-136.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Interaction of virulent Mycoplasma pneumoniae with hamster tracheal organ cultures. Infect Immun. 1976 Jul;14(1):217–224. doi: 10.1128/iai.14.1.217-224.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane I., Pnini S., Banai M., Baseman J. B., Cassell G. H., Bredt W. Attachment of mycoplasmas to erythrocytes: a model to study mycoplasma attachment to the epithelium of the host respiratory tract. Isr J Med Sci. 1981 Jul;17(7):589–592. [PubMed] [Google Scholar]
- Krause D. C., Leith D. K., Wilson R. M., Baseman J. B. Identification of Mycoplasma pneumoniae proteins associated with hemadsorption and virulence. Infect Immun. 1982 Mar;35(3):809–817. doi: 10.1128/iai.35.3.809-817.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leith D. K., Hansen E. J., Wilson R. M., Krause D. C., Baseman J. B. Hemadsorption and virulence are separable properties of Mycoplasma pneumoniae. Infect Immun. 1983 Feb;39(2):844–850. doi: 10.1128/iai.39.2.844-850.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman R. P., Clyde W. A., Jr The interrelationship of virulence, cytadsorption, and peroxide formation in Mycoplasma pneumoniae. Proc Soc Exp Biol Med. 1969 Sep;131(4):1163–1167. doi: 10.3181/00379727-131-34061. [DOI] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Haemadsorption and haemagglutination by mycoplasmas. J Gen Microbiol. 1968 Mar;50(3):465–478. doi: 10.1099/00221287-50-3-465. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz E. A., Shifter A., Kahane I. Vitamin E deficiency in beta-thalassemia major: changes in hematological and biochemical parameters after a therapeutic trial with alpha-tocopherol. Am J Clin Nutr. 1979 Sep;32(9):1850–1858. doi: 10.1093/ajcn/32.9.1850. [DOI] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., Chanock R. M. Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence. J Bacteriol. 1968 Sep;96(3):695–705. doi: 10.1128/jb.96.3.695-705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel J. M., Gamiel H., Vlodavsky I., Gay I., Ben-Bassat H. Cell attachment, growth characteristics and surface morphology of human upper-respiratory tract epithelium cultured on extracellular matrix. Eur J Clin Invest. 1983 Feb;13(1):57–63. doi: 10.1111/j.1365-2362.1983.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Yatziv S., Flowers H. M. Action of -galactosidase on glycoprotein from human B-erythrocytes. Biochem Biophys Res Commun. 1971 Oct 15;45(2):514–518. doi: 10.1016/0006-291x(71)90849-7. [DOI] [PubMed] [Google Scholar]
- Yatziv S., Kahane I., Abeliuk P., Cividalli G., Rachmilewitz E. A. "Lysosomal" enzyme activities in red blood cells of normal individuals and patients with homozygous beta-thalassaemia. Clin Chim Acta. 1979 Aug 15;96(1-2):67–72. doi: 10.1016/0009-8981(79)90053-6. [DOI] [PubMed] [Google Scholar]


