Abstract
Confocal laser-scanning microscopy was used to carry out a comparative study of the immunostaining for three families of neuropeptides, viz., allatostatin-A (AS-A), allatostatin-C (AS-C) and allatotropin (AT), in adult female mosquitoes of Aedes aegypti and Anopheles albimanus. The specific patterns of immunostaining for each of the three peptides were similar in both species. The antisera raised against AT, AS-A, and AS-C revealed intense immunoreactivity in the cells of each protocerebral lobe of the brain and stained cells in each of the ventral ganglia and neuronal projections innervating various thoracic and abdominal tissues. Only the AS-A antiserum labeled immunoreactive endocrine cells in the midgut. The distribution of the peptides supports the concept that they play multiple regulatory roles in both species.
Keywords: Immunocytochemistry, Neuropeptides, Confocal laser-scanning microscopy, Mosquitoes Aedes aegypti, Anopheles albimanus (Insecta)
Introduction
Allatostatins (AS) and allatotropins (AT) are structurally diverse peptides that were first described as modulators of juvenile hormone (JH) biosynthesis in the corpora allata (CA) of a number of insect species (Kataoka et al. 1989; Woodhead et al. 1989; Kramer et al. 1991; Lorenz and Hoffmann 1995; Gilbert et al. 2000). AS and AT have subsequently been recognized as having multiple physiological effects, controlling processes such as heart rate and gut motility, nutrient absorption, migratory preparedness, and modulation of the circadian cycle (Bendena et al. 1999; Nässel 2002; Petri et al. 2002; Elekonich and Horodyski 2003).
Three families of AS have been identified in insects: YXFGL-amide-AS (cockroach or type-A; AS-A), W2W9-AS (cricket or type-B; AS-B), and PISCF-AS (Manduca or type-C; AS-C); (Stay et al. 1994; Tobe et al. 1995; Duve et al. 1998; Weaver et al. 1998; Bendena et al. 1999). In contrast, only one type of AT has been isolated and functionally characterized; this AT was originally identified from the heads of pharate adult Manduca sexta (Mas-AT), with analogs being isolated from brain of Spodoptera frugiperda (Kataoka et al. 1989; Oeh et al. 2000), from the male accessory glands of Locusta migratoria (Paemen et al. 1991), and from the mosquito Aedes aegypti (Veenstra and Costes 1999; Li et al. 2003).
The ability of these peptides to modulate JH synthesis in A. aegypti has recently been evaluated. Anopheles gambiae AS-C (homolog to M. sexta AS-C) significantly inhibits JH synthesis, whereas A. aegypti AS-A (homolog to cockroach AS-A) does not affect CA activity (Li et al. 2004). A. aegypti AT (Ae-AT) stimulates JH synthesis in a strong and dose-dependent manner (Li et al. 2003).
The aim in this work was to compare immunoreactivity for AT, AS-A, and AS-C in adult females of two important species of disease vector mosquitoes, viz., A. aegypti and Anopheles albimanus, by using confocal laser-scanning microscopy. The specific patterns of immunostaining for each of the three peptides were similar in both mosquito species. The AT, AS-A and AS-C antibodies stained brain and ventral ganglia cells and projections innervating various thoracic and abdominal tissues. Only AS-A antiserum showed immunoreactivity in midgut endocrine cells.
Materials and methods
Mosquitoes
A colony of A. albimanus white-striped pupa phenotype was established with insects collected in the state of Chiapas, Mexico (Chan et al. 1994). A colony of A. aegypti was established with insects collected in the state of Morelos, Mexico. Adults of both species were reared under a photoperiod cycle of 12 h light: 12 h dark, at 28°C and 70%–80% relative humidity, and were fed ad libitum with 5% sugar solution. All mosquitoes used in this study were 2–3 day-old mated females.
Tissue preparation
Mosquitoes were immobilized by brief exposure to ice, washed with 70% ethanol, and air-dried. Tissues were dissected in a drop of phosphate-buffered saline (PBS: 140 mM NaCl, 2.6 mM KCl, 1.5 mM KH2PO4, 20.4 mM Na2HPO4, pH 7.2) containing a cocktail of protease inhibitors (2 mM phenylmethylsulfonyl fluoride, 0.1 mM Nα-p-tosyl-L-lysine chloromethyl ketone, 1 mM EDTA, and 0.1 mg/ml leupeptin; Sigma, St. Louis, Mo., USA). The head and thorax were separated from the abdomen by making a small tear on both lateral pleural membranes. The abdomen was pulled off, and the gut was removed. Abdomens without gut were cut along the pleural membrane by using a needle. The abdominal body wall containing the fat body, epidermal sub-tegumental cells (mainly in pleural membranes), the dorsal vessel, the tracheal system, and the ventral abdominal ganglia will be referred to here as “abdomens”. The brain and subesophageal ganglion were removed with the corpus cardiacum–corpus allatum complex attached (Br–CC–CA). The three pairs of thoracic ganglia (prothoracic, mesothoracic, and methathoracic) were obtained by removing the coxae and dorsal thoracic area. Excess protease inhibitors in all tissues was removed by washes in PBS.
Primary antibodies against neuropeptides
Rabbit polyclonal antisera against A. gambiae AS-C and A. aegypti AT were produced against synthetic peptides conjugated to keyhole limpet hemocyanin by Genemed Synthesis (San Francisco, Calif., USA). Antisera titers were established, by enzyme-linked immunosorbent assay, to be adequate at dilutions of 1/10,000. The specificity of the AT and AS-C antisera was tested by liquid-phase preabsorption with the parent antigen at a concentration of 5.5 nmol/ml diluted antiserum (1/500), overnight at 4°C. Immunostaining was abolished following this treatment. The rabbit polyclonal antiserum against AS-A was a gift of Dr. Rene Feyereisen (Reichwald et al. 1994).
Immunocytochemistry
Midguts, Br–CC–CA complexes, thoracic ganglia, and abdomens were fixed for 4 h at room temperature with 4% formaldehyde in PBS. After fixation, tissues were rinsed in PBS (5×10 min each), permeated with 1% Triton X-100 in PBS at 4°C overnight, washed in PBS (3×20 min each) at room temperature, incubated for 2 h at 37°C in a solution of 2% bovine serum albumin in PBS containing 0.1% sodium azide (PBS-A; blocking solution), incubated at 4°C overnight with the primary antisera (anti-AT, anti-AS-A, or anti-AS-C) diluted 1:500 in PBS-A, and washed (5×10 min each) with 0.1% Tween-20 in PBS (PBS-T). All subsequent procedures were carried out with the samples being kept in the dark. Tissues were incubated for 2 h at 37°C with the secondary antiserum, a fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin (Oncogene Researchc Products, Boston, Mass., USA), diluted 1:100 in PBS-A. Tissues were washed in PBS-T (5×10 min each) and mounted on glass slides in a fluorescence-preserving medium (Vectashield, Vector, Burlinghame, Calif., USA). Twenty females were analyzed in each of the studies.
Confocal microscopy
Images of the immunostained preparations were obtained with an Ultra View laser-scanning confocal system (model CSU10, Perkin-Elmer, Wellesley, Mass., USA) with an air-cooled argon-krypton laser and a 488 single-filter block (excitation 488 nm, emission 510 nm). The confocal system was used on an epi-fluorescent microscope (Nikon E-600, Japan). Images were processed by using Imaging Suite (Spatial Module) version 3.0 from Perkin-Elmer and by Power Point programs. Three-dimensional (3D) reconstructions were obtained from Z-stack data sets. Results are presented as 3D views in a single projection.
Results
The patterns of immunostaining for each of the three peptides were similar in both mosquito species. No staining was observed in the controls in the absence of the three primary antisera.
AT immunoreactivity
Seven to ten neurons were intensively stained in each protocerebral lobe, whereas others were weakly stained (Figs. 1a, 2a). A small group of stained cells was always observed in a posterior location, projecting processes toward the frontal region of the brain (Figs. 1a, 2a, arrowheads). We did not observe labeled varicosities along the nerve extending to the CC–CA complexes or inside the CC–CA complex.
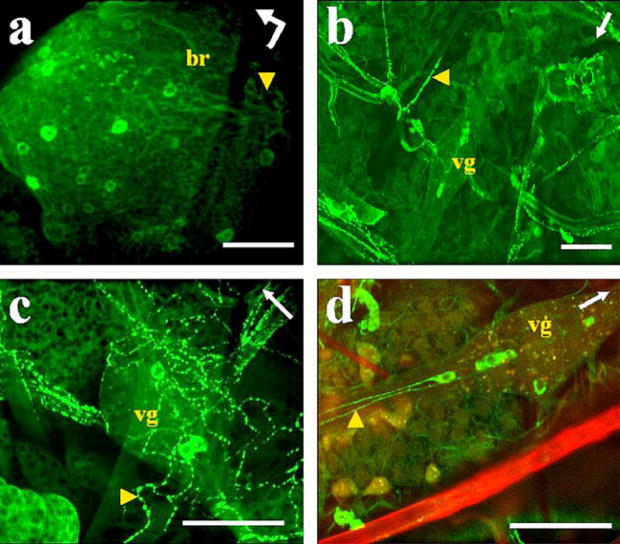
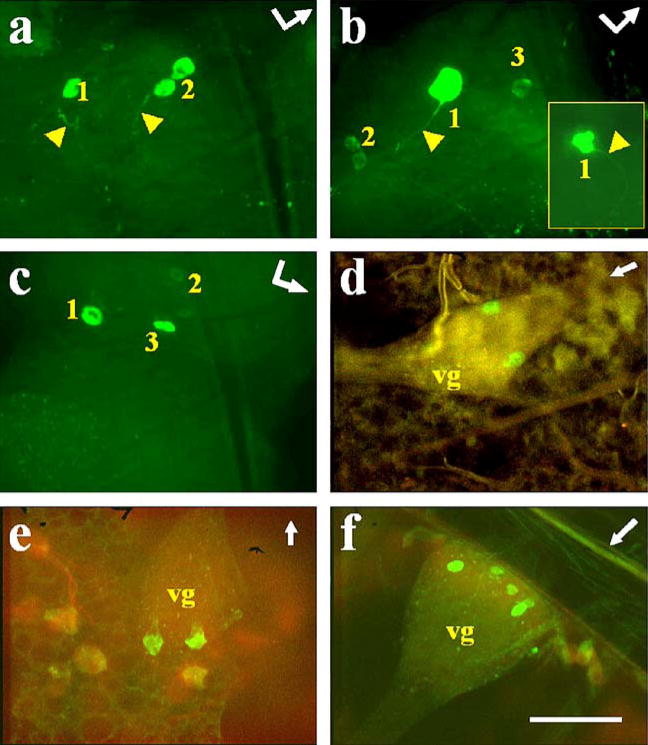
Fig. 1.
Immunostaining of AT in A. aegypti tissues. a Left protocerebral lobe of the brain (br) with a posterior group of labeled cells (arrowhead). Bent arrow Front (arrowhead) and left side (arrow end) of the brain. b–d Abdominal preparations with three cells labeled in ventral ganglia (vg) and varicosities labeled in ventral processes (arrowhead). Arrows Anterior part of the mosquito. d Combined images from confocal fluorescence (green) and light (red) microscopy. Bars 50 μm
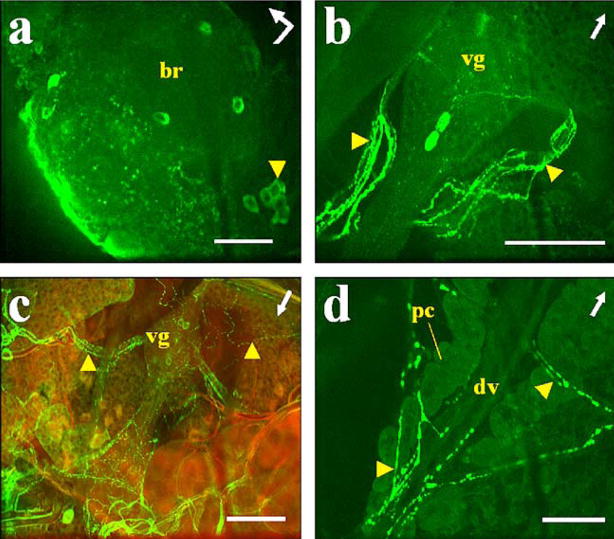
Fig. 2.
Immunostaining of AT in A. albimanus tissues. a Left protocerebral lobe of the brain (br) with a posterior group of labeled cells (arrowhead). Bent arrow Front (arrowhead) and left side (arrow end) of the brain. b, c Abdominal preparations with three cells labeled in ventral ganglia (vg) and varicosities labeled in ventral processes (arrowheads). c Combined images from confocal fluorescence (green) and light (red) microscopy. d Heart (arrowheads processes directly connected to the dorsal vessel containing immunostained varicosities, dv dorsal vessel of heart, pc pericardial cells). Arrows Anterior part of the mosquito. Bars 50 μm
All the abdominal ganglia of the ventral nerve cord showed AT immunoreactivity. Three cells were labeled in each ganglion, two in the posterior region and one in the anterior region (Figs. 1b–d, 2b, c). Immunostained projections from these cells were prominent in the ventral nerve cord (Fig. 1d, arrowhead) and emerging laterally (Figs. 1b, 2b, c, arrowheads). These varicose processes were observed innervating abdominal tissues (hindgut, heart, and oviducts; Figs. 1b, c, 2b, c, arrowheads). Strong staining was observed in many processes projecting from the nerve cord directly to the dorsal vessel; in addition, labeled varicosities were seen along the wall of the dorsal vessel (Fig. 2d). Immunoreactivity was not found in neurons of the thoracic ganglia or in midgut endocrine cells of either of the two species.
AS-A immunoreactivity
Six neurons were recognized in each protocerebral lobe (Figs. 3a, 4a). These cells had long processes (Figs. 3a, 4a, arrowheads) projecting toward the stomatogastric nervous system and leaving the brain most probably via the nervi corporis cardiaci. Only A. albimanus exhibited labeled processes innervating the CC–CA complex (Fig. 4b, arrowhead).
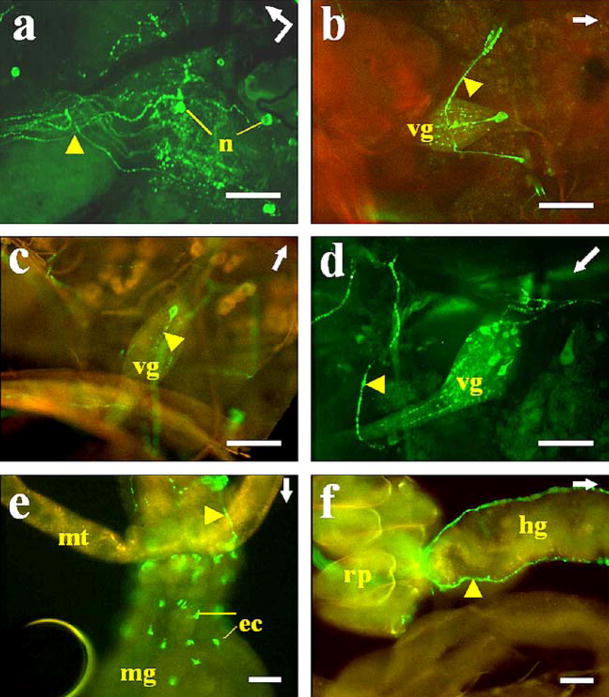
Fig. 3.
Immunostaining of AS-A in A. aegypti tissues. a Left protocerebral lobe of the brain. Neurons (n) with labeled processes (arrowhead) innervating the stomatogastric nervous system. Bent arrow indicates the front (arrowhead) and left side (arrow end) of the brain. b–d Abdominal ventral ganglia. Only one cell was stained in each ventral ganglion (vg), except in the last ganglion (d) in which eight cells were labeled. Note the immunostained varicosities in ventral processes (arrowheads). e, f Midgut. Endocrine cells (ec) showing immunoreactivity in the posterior midgut (mg) (mt Malpighian tubule). Two processes (arrowhead) can be seen running along the hindgut (hg) and ending in the rectal papillae (rp). Arrows Anterior part of the mosquito. b,c,e, f Combined images from confocal fluorescence (green) and light (yellow-red) microscopy. Bars 50 μm
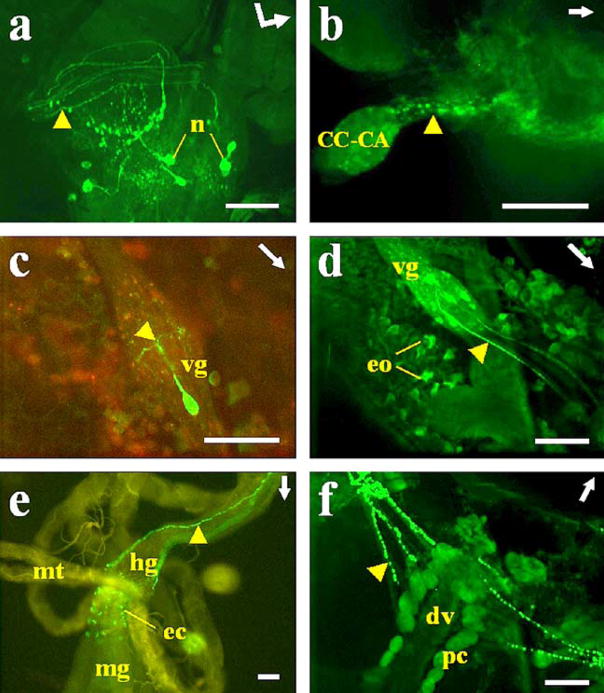
Fig. 4.
Immunostaining of AS-A in A. albimanus tissues. a Right protocerebral lobe of the brain. Neurons (n) with labeled processes (arrowhead) innervating the stomatogastric nervous system. Bent arrow Front (arrowhead) and left side (arrow end) of the brain. b Processes (arrowhead) with varicosities projecting into the corpus cardiacum–corpus allatum (CC–CA) complex. c, d Abdominal ventral ganglia: only one cell was stained in each ventral ganglion (vg), except in the last ganglion (d) in which eight cells were labeled. Note the immunostained varicosities in ventral processes (arrowhead). d Oenocytes (eo) ventrally located in abdominal fat body showing immunoreactivity. e Midgut (mt Malpighian tubule). Endocrine cells (ec) showing immunoreactivity in the posterior midgut (mg). Note the two processes (arrowhead) running along the hindgut (hg) and ending in the rectal papillae. f Heart. Inmmunolabeled varicosities in processes (arrowhead) ending in the proximity of pericardial cells (pc) around the dorsal vessel (dv). Arrows Anterior part of the mosquito. c, e Combined images from confocal fluorescence (green) and light (yellow-red) microscopy. Bars 50 μm
In the abdominal nerve cord (Figs. 3b–d, 4c, d), only one cell was stained per ganglion, except in the last ganglion (neuromeres 7+8) in which eight cells were stained (Figs. 3d, 4d). Immunoreactive processes projecting from the labeled cells of the last abdominal ganglion (Figs. 3d, 4d, arrowheads) innervated oviducts, bursa, and spermatheca (data not shown). In the other ganglia, only one process from a single cell bifurcated within the ganglion and projected laterally to the abdomen (Figs. 3b, 4c, arrowheads).
Oenocytes in the fat body were stained by anti-AS-A only in A. albimanus (Fig. 4d). The antiserum to AS-A intensely labeled processes projecting toward the pericardial cells but showed no staining that reached the wall of the dorsal vessel (Fig. 4f). About 50 endocrine cells located in the posterior midgut were strongly stained (Figs. 3e, 4e). Two prominent processes were labeled that extended along the hindgut and ended in the rectal papilla (Figs. 3e, f, 4e, arrowheads). Immunoreactivity was not observed in thoracic ganglia cells of either of the two species (data not shown).
AS-C immunoreactivity
Four cells were labeled in each protocerebral lobe (Fig. 5a–c). They had large nuclei (Fig. 5a, 2; Fig. 5c, 1) and large labeled vacuoles in their cytoplasm (Fig. 5b, inset). These cells projected toward the stomatogastric nervous system (Fig. 5a, b, arrowheads). We did not observe immunostaining in the CC–CA of either species (data not shown).
Fig. 5.
Immunostaining of AS-C in A. aegypti and A. albimanus tissues. Immunoreactivity in brain of A. aegypti (a, b) and A. albimanus (c). Bent arrows Front (arrowhead) and left side (arrow end) of the brain (arrowheads labeled processes, numbers location of the cells as depicted in Fig. 6a). Inset: Cell with large labeled vacuoles in the cytoplasm. d–f Abdominal ventral ganglia. Two cells were stained in each ventral ganglia (vg), except in the last ganglion (f) in which four cells were labeled. Arrows Anterior part of the mosquito. Bar 50 μm
In the ventral cord nerve, two cells were labeled in each of the first six abdominal ganglia (Fig. 5d, e), whereas in the last ganglion (neuromeres 7+8), four cells could be recognized (Fig. 5f). In general, we did not observe immunostaining in processes extending from the ganglia, although weakly labeled varicosities were seen on a few occasions (data not shown). Immunoreactivity was not observed in neurons of the thoracic ganglia or in midgut endocrine cells of either of the two species (data not shown).
Discussion
This study provides evidence of major similarities in the expression of three neuropeptides in A. aegypti and A. albimanus, representing the first comparative study of regulatory peptides in members of two mosquito subfamilies with more than 100 million years of separate evolution (Knudson et al. 2002). A. aegypti has been an excellent model for the study of mosquito physiology for more than 50 years, and ample information thus exists regarding the neuroendocrine regulation of its reproductive physiology. On the other hand, knowledge of these aspects of species of Anopheles is much more limited.
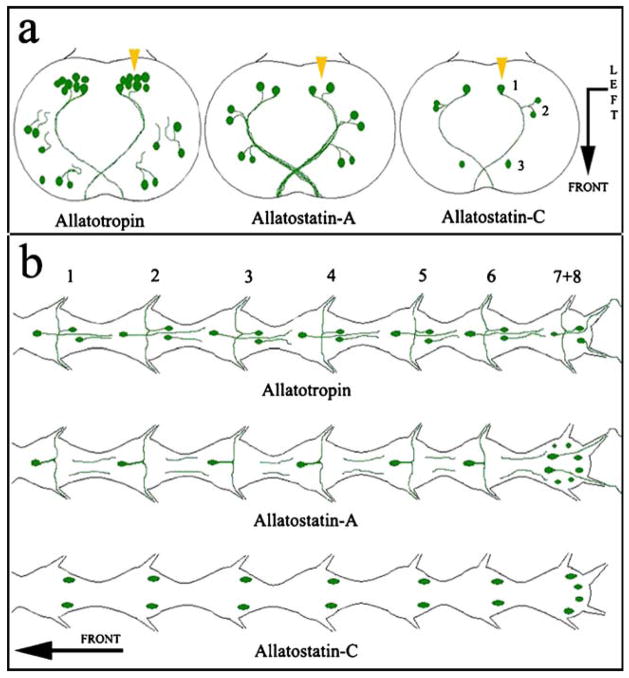
In the brains of A. aegypti and A. albimanus, each of the tested antisera reveals strong patterns of immunoreactivity exclusively in a small specific number of neurosecretory-like cells in the protocerebral lobes (summarized in Fig. 6a). Five paired groups of neurosecretory cells have been described in species of Aedes, Culex, and Culiseta (Clements 1992); these cells have processes extending from the brain in the nervi corporis cardiaci I and II and projecting into the stomatogastric nervous and the CC–CA complex. The labeled cells in our studies are difficult to categorize into the five groups described by Clements (1992). However, the three antisera recognize some of the medial neurosecretory cells located in the posterior pars intercerebralis (Fig. 6a, arrowheads). Differences are discernible in the labeled processes extending from the immunostained neurons. The processes from all the labeled neurons are well stained with anti-AS-A, but only processes projecting from the posterior neurosecretory cells are well stained with anti-AT. The processes are weakly stained with anti-AS-C.
Fig. 6.
Representation of neuropeptide distribution in the central nervous system of A. aegypti and A. albimanus. The pattern of immunostaining for each of the three peptides is similar in both mosquito species. a Brains showing neurons and processes stained in the protocerebral lobes. Bent arrow Front (arrowhead) and left side (arrow end) of the brain (numbers relative location of cells). b Abdominal ganglia in the ventral nerve cord showing neurons and processes stained with the three different antibodies (numbers position of the ganglia). Arrow Anterior part of the mosquito
Immunoreactivity of peptides from these three families has been described in the CC–CA complex of Lepidoptera (Zitnan et al. 1995; Duve and Thorpe 2003). In Diploptera punctata and Gryllus bimaculatus, protocerebral neurosecretory cells show AS-A or AS-B immunoreactivity, respectively, and immunoreactivity in nerves projecting into the CA (Stay et al. 1992; Neuhäuser et al. 1994). AS-A and AS-C immunoreactivities have been demonstrated in a large number of neurons in the adult brain of Drosophila melanogaster (Zitnan et al. 1993; Yoon and Stay 1995), whereas nerves to the CA are not stained by the AS-A antiserum (Yoon and Stay 1995). In contrast, protocerebral neurosecretory cells in D. melanogaster larvae send AS-C-immunoreactive processes to the ring gland, reinforcing the idea that this peptide is an endocrine regulator in Diptera. Although an AT precursor has not been identified in the Drosophila genome, M. sexta-AT immunoreactivity has been detected in small median neurosecretory cells and numerous interneurons of the brain of adult D. melanogaster (Zitnan et al. 1993).
We have only found AS-A immunoreactive processes innervating the CC–CA complex of A. albimanus. In contrast, processes innervating the CC–CA complex in A. aegypti are not immunostained for AS-A. Moreover, the CC–CA complex is not stained with antisera against AT or AS-C in either mosquito. This absence of immunoreactivity may be the result of the presence of only small amounts of the peptides because of the minute size of mosquito CC–CA and the physiological state of the females evaluated.
The three antisera recognize neurons in the seven abdominal ganglia of the ventral nerve cord, with differences in the numbers and types of neurons and their projections (Fig. 6b). Anti-AT normally recognizes three cells per ganglion, whereas anti-AS-A stains only one neuron from the first to the sixth ganglion and eight cells in the last one (7+8). The AS-C antiserum recognizes two neurons from the first to the sixth ganglion and four in the last one (7+8). The locations of the stained neurons in the ganglia suggest that different cells are labeled by each antiserum (Fig. 6b). If immunoreactivity against AT and AS-A was detected in the perikarya of a particular neuron, it was also observed in the projecting processes; in contrast, AS-C antiserum stained the ganglion neurons but not their projections (Fig. 6b).
Immunoreactivity against AS-A has been previously described in a specific group of endocrine midgut cells in female A. aegypti (Veenstra et al. 1995). We have found a similar result in A. albimanus. In addition, in situ hybridization has shown the expression of AS-A mRNA in the same group of midgut cells in A. aegypti (F.G. Noriega et al., unpublished). In adult D. melanogaster, AS-A immunoreactivity has also been described in endocrine cells of the midgut and in the hindgut axons (Yoon and Stay 1995). Veenstra and Costes (1999) have described the absence of AT immunoreactivity in midgut endocrine cells of A. aegypti. We have confirmed this observation in A. albimanus and the absence of immunoreactivity to AS-C in the midguts of both mosquitoes.
What does the distribution of the three peptides tell us about their functions? AT was first isolated from M. sexta as a factor that stimulates the synthesis of JH in the CA (Kataoka et al. 1989). Previously, we have reported that the CA of a newly emerged A. aegypti female needs to be exposed to AT before it is capable of synthesizing JH (Li et al. 2003). We have also reported that only females that emerge with large amount of nutrients have CA capable of synthesizing enough JH to activate reproductive maturation (Caroci et al. 2004). Factors from the head are essential for CA activation and reproductive maturation in mosquitoes; decapitation of A. aegypti females within 1 h after adult ecdysis prevents normal development of the previtellogenic follicles (Gwadz and Spielman 1973). A similar effect has been described when the brain medial neurosecretory cells are removed (Lea 1967). Cells in the brain of some species of Aedes and Anopheles are immunostained by the anti-AT antiserum, and thus AT can be proposed as being one of these critical factors from the head playing a role in the nutritional activation of JH biosynthesis.
AT-immunoreactive cells in the first six abdominal ganglia of the nerve cord have processes that innervate the dorsal vessel, indicating that the dorsal vessel is a target organ of this peptide. AT is a cardioacceleratory peptide in M. sexta (Veenstra et al. 1994) and Pseudaletia unipuncta (Koladich et al. 2002). Rudwall et al. (2000) have reported that AT increases the heart rate in Leucophaea maderae and Periplaneta americana and have described AT immunostaining in processes projecting to the heart in these two species of cockroaches.
Intense labeled has also been observed with anti-AT in varicosities in processes innervating abdominal tissues, such as the hindgut, dorsal vessel, and oviducts. Veenstra and Costes (1999) have reported that Aedes aegypti-AT stimulates oviduct contractions in the blowfly Phormia sp. Mas-AT stimulates hindgut contractions in L. maderae (Rudwall et al. 2000) and gut motility in Heliothis virescens (Oeh et al. 2003).
The reason for the specific staining of oenocytes associated with the fat body by the anti-AT antibody remains to be explained. Oenocytes are large abdominal cells that are rich in smooth endoplasmic reticulum and mitochondria and have been implicated in hydrocarbon and lipid synthesis (Chapman 1998; Fan et al. 2002). Cuticular hydrocarbons and pheromones are produced by abdominal oenocytes and are shuttled through the hemolymph by high-density lipophorin (Gu et al. 1995; Schal et al. 1998; Sevala et al. 1999). In mosquitoes, oenocytes might produce AT or might be a target for the peptide. It should be noted however that oenocytes were stained in A. albimanus but not in A. aegypti.
AS-A was initially identified by its inhibitory activity on JH production in the cockroach D. punctata (Pratt et al. 1989; Woodhead et al. 1989). We have previously reported that Aedes AS-A have no effect on JH biosynthesis in female A. aegypti (Li et al. 2004).
AS-A has been described as an inhibitory regulator of visceral muscle contraction in several parts of the digestive tract in a number of insects (Stay 2000; Nässel 2002). Immunostaining of AS-A has been found in various tissues of several insects, including endocrine midgut cells of A. aegypti (Veenstra et al. 1995). These AS-A endocrine cells are “open type” cells. They have a bottle shape and extend from the basal lamina to the midgut lumen. The apical part of the cell is in contact with the lumen and might be able to sense the midgut contents (Brown et al. 1985; Stracker et al. 2002). AS-A-immunoreactive endocrine cells are located in the most posterior part of the midgut, close to the muscles of the pyloric sphincter; these cells might directly or indirectly affect the activity of midgut muscles. Caroci and Noriega (2003) have proposed that free amino acids in the midgut lumen might be sensed by these midgut endocrine cells and might play a key role as a signal used by the midgut to control the retention of its lumen contents.
The AS-A antiserum has not been seen to stain varicosities along the wall of the dorsal vessel, although stained processes seem to innervate the pericardial cells in both species. Putative roles for pericardial cells (nephrocytes) are the filtration, clearance, and regulation of the composition of hemolymph (Wigglesworth 1970; Dallai et al. 1994). Nephrocytes have both secretory and degradative functions; in M. sexta, pericardial cells remove JH esterase from the hemolymph (Bonning et al. 1997). In A. gambiae, scavenging nephrocytes, which are present alongside the dorsal vessel, harbor numerous peroxisomes and catalase-rich organelles that are active in the detoxification and neutralization of reactive oxygen species (Kumar et al. 2003). Yeast particles are aggregated by A. albimanus pericardial cells and induce a strong increase in lysosomal activity, indicating that this tissue could be important in cell-mediated immune responses (S. Hernández-Martinez, unpublished). The strongly AS-A-immunoreactive varicosities in processes innervating the nephrocytes suggest either that this peptide plays a role in the regulation of pericardial cell physiology or that these cells are involved in the clearance of the peptide.
AS-C was originally identified in M. sexta (Kramer et al. 1991). In this species and other moths, it inhibits JH biosynthesis (Kramer et al. 1991; McNeil and Tobe 2001). In D. melanogaster, an AS-C homolog slows heart contraction (Price et al. 2002). We have previously described that the biosynthetic activity of the A. aegypti CA in vitro is inhibited by factors present in the head and by physiological concentrations of synthetic A. gambiae AS-C (Li et al. 2004). The presence of AS-C immunostaining in the brain strengthens the hypothesis that AS-C is the brain factor involved in the regulation of JH biosynthesis in mosquitoes. Using a combination of high performance liquid chromatography and mass spectrometry, we have isolated and characterized a putative AS-C peptide from the brain of A. aegypti (Y. Li et al., unpublished).
In summary, we have performed a comparative study of the distribution of AT, AS-A, and AS-C and provided evidence of major similarities in the expression of these three neuropeptides in two species of mosquitoes. Our results support the idea that AT and AS-C are factors from the brain playing a role in the regulation of JH synthesis and oogenesis in mosquitoes, whereas AS-A is an important modulator of the contraction of visceral muscle.
Acknowledgments
This work was supported by NIH grant AI 45545 to F.G.N. and CONACyT G-37186-M to M.H.R.
The authors thank Dr. Dick Nässel, Dr. Rafael Cantera, and Dr. Jorge Ronderos for critical reading of the manuscript, Dr. Rene Feyereisen and Dr. Norman Davis for the AS-A antiserum, and Dr. Norman Davis for his advice and support during the early stages of the project.
References
- Bendena WG, Donly BC, Tobe SS. Allatostatins: a growing family of neuropeptides with structural and functional diversity. Ann N Y Acad Sci. 1999;897:311–329. doi: 10.1111/j.1749-6632.1999.tb07902.x. [DOI] [PubMed] [Google Scholar]
- Bonning BC, Ward VK, van Meer MM, Booth TF, Hammock BD. Disruption of lysosomal targeting is associated with insecticidal potency of juvenile hormone esterase. Proc Natl Acad Sci USA. 1997;94:6007–6012. doi: 10.1073/pnas.94.12.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Raikhel AS, Lea AO. Ultrastructure of midgut endocrine cells in the adult mosquito, Aedes aegypti. Tissue Cell. 1985;17:709–721. doi: 10.1016/0040-8166(85)90006-0. [DOI] [PubMed] [Google Scholar]
- Caroci A, Noriega FG. Free amino acids are important for the retention of protein and non-protein meals by the midgut of Aedes aegypti females. J Insect Physiol. 2003;49:839–844. doi: 10.1016/S0022-1910(03)00134-3. [DOI] [PubMed] [Google Scholar]
- Caroci A, Li Y, Noriega FG. Reduced juvenile hormone synthesis in mosquitoes with low teneral reserves reduces ovarian previtellogenic development in Aedes aegypti. J Exp Biol. 2004;207:2685–2690. doi: 10.1242/jeb.01093. [DOI] [PubMed] [Google Scholar]
- Chan AS, Rodriguez MH, Torres JA, Rodriguez MD, Villarreal C. Susceptibility of 3 laboratory strains of Anopheles albimanus (Diptera, Culicidae) to coindigenous Plasmodium vivax in Southern Mexico. J Med Entomol. 1994;31:400–403. doi: 10.1093/jmedent/31.3.400. [DOI] [PubMed] [Google Scholar]
- Chapman RF. The insects. Structure and function. Cambridge University Press; Cambridge: 1998. [Google Scholar]
- Clements AN. The biology of mosquitoes. Chapman and Hall; London: 1992. [Google Scholar]
- Dallai R, Riparbelli MG, Callaini G. The cytoskeleton of the ventral nephrocytes of Ceratitis capitata larva. Cell Tissue Res. 1994;273:529–536. [Google Scholar]
- Duve H, Thorpe A. Neuropeptide co-localization in the lepidopteran frontal ganglion studied by confocal laser scanning microscopy. Cell Tissue Res. 2003;311:79–89. doi: 10.1007/s00441-002-0648-2. [DOI] [PubMed] [Google Scholar]
- Duve H, Thorpe A, Johnsen AH, Maestro JL, Scott AG, East PD. The dipteran Leu-callatostatins: structural and functional diversity in an insect neuroendocrine peptide family. In: Coast GM, Webster SG, editors. Recent advances in arthropod endocrinology. Society for Experimental Biology: Seminar Series 65. Cambridge University Press; Cambridge: 1998. pp. 229–247. [Google Scholar]
- Elekonich MM, Horodyski FM. Insect allatotropins belong to a family of structurally-related myoactive peptides present in several invertebrate phyla. Peptides. 2003;24:1623–1632. doi: 10.1016/j.peptides.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Fan Y, Chase J, Sevala VL, Schal C. Lipophorin-facilitated hydrocarbon uptake by oocytes in the German cockroach Blattella germanica (L.) J Exp Biol. 2002;205:781–790. doi: 10.1242/jeb.205.6.781. [DOI] [PubMed] [Google Scholar]
- Gilbert LI, Granger NA, Roe RM. The juvenile hormones: historical facts and speculations on future research directions. Insect Biochem Mol Biol. 2000;30:617–644. doi: 10.1016/s0965-1748(00)00034-5. [DOI] [PubMed] [Google Scholar]
- Gu X, Quilici D, Juarez P, Blomquist GJ, Schal C. Biosynthesis of hydrocarbons and contact sex pheromone and their transport by lipophorin in females of the German cockroach Blattella germanica. J Insect Physiol. 1995;41:257–267. [Google Scholar]
- Gwadz RW, Spielman A. Corpus allatum control of ovarian development in Aedes aegypti. J Insect Physiol. 1973;19:1441–1448. doi: 10.1016/0022-1910(73)90174-1. [DOI] [PubMed] [Google Scholar]
- Kataoka H, Toschi A, Li JP, Carney RL, Schooley DA, Kramer SJ. Identification of an allatotropin from adult Manduca sexta. Science. 1989;243:1481–1483. doi: 10.1126/science.243.4897.1481. [DOI] [PubMed] [Google Scholar]
- Knudson DL, Brown SE, Severson DW. Culicine genomics. Insect Biochem Mol Biol. 2002;32:1193–1197. doi: 10.1016/s0965-1748(02)00082-6. [DOI] [PubMed] [Google Scholar]
- Koladich PM, Cusson M, Bendena WG, Tobe SS, McNeil JN. Cardioacceleratory effects of Manduca sexta allatotropin in the true armyworm moth, Pseudaletia unipuncta. Peptides. 2002;23:645–651. doi: 10.1016/s0196-9781(01)00658-1. [DOI] [PubMed] [Google Scholar]
- Kramer SJ, Toschi A, Miller CA, Kataoka H, Quistad GB, Li JP, Carney RL, Schooley DA. Identification of an allatostatin from the tobacco hornworm Manduca sexta. Proc Natl Acad Sci USA. 1991;88:9458–9462. doi: 10.1073/pnas.88.21.9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Christophides GK, Cantera R, Charles B, Han YS, Meister S, Dimpoulos G, Kafatos FC, Barillas-Mury C. The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc Natl Acad Sci USA. 2003;100:14139–14144. doi: 10.1073/pnas.2036262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea AO. The medial neurosecretory cells and egg maturation in mosquitoes. J Insect Physiol. 1967;13:419–429. doi: 10.1016/0022-1910(67)90082-0. [DOI] [PubMed] [Google Scholar]
- Li Y, Unnithan GC, Veenstra JA, Feyereisen R, Noriega FG. Stimulation of JH biosynthesis by the corpora allata of adult female Aedes aegypti in vitro: effect of farnesoic acid and Aedes allatotropin. J Exp Biol. 2003;206:1825–1832. doi: 10.1242/jeb.00371. [DOI] [PubMed] [Google Scholar]
- Li Y, Hernandez-Martinez S, Noriega FG. Inhibition of juvenile hormone biosynthesis in mosquitoes: effect of allatostatic head factors, PISCF- and YXFGL-amide-allatostatins. Reg Peptides. 2004;118:175–182. doi: 10.1016/j.regpep.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Lorenz MW, Hoffmann KH. Allatotropic activity in the suboesophageal ganglia of crickets, Gryllus bimaculatus and Achaeta domesticus (Ensifera, Grullidae) J Insect Physiol. 1995;41:191–196. [Google Scholar]
- McNeil JN, Tobe SS. Flights of fancy: possible roles of allatostatin and allatotropin in migration and reproductive success of Pseudaletia unipuncta. Peptides. 2001;22:271–277. doi: 10.1016/s0196-9781(00)00379-x. [DOI] [PubMed] [Google Scholar]
- Nässel DR. Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Prog Neurobiol. 2002;68:1–84. doi: 10.1016/s0301-0082(02)00057-6. [DOI] [PubMed] [Google Scholar]
- Neuhäuser T, Sorge D, Stay B, Hoffmann KH. Responsiveness of the adult cricket (Gryllus bimaculatus and Acheta domesticus) retrocerebral complex to allatostatin-1 from a cockroach, Diploptera punctata. J Comp Physiol [B] 1994;164:23–31. doi: 10.1007/BF00714567. [DOI] [PubMed] [Google Scholar]
- Oeh U, Lorenz MW, Dyker H, Lösel P, Hoffmann KH. Interaction between Manduca sexta allatotropin and Manduca sexta allatostatin in the fall armyworm Spodoptera frugiperda. Insect Biochem Mol Biol. 2000;30:719–727. doi: 10.1016/s0965-1748(00)00043-6. [DOI] [PubMed] [Google Scholar]
- Oeh U, Antonicek H, Nauen R. Myotropic effect of helicokinins, tachykinin-related peptides and Manduca sexta allatotropin on the gut of Heliothis virescens (Lepidoptera: Noctuidae) J Insect Physiol. 2003;49:323–337. doi: 10.1016/s0022-1910(03)00017-9. [DOI] [PubMed] [Google Scholar]
- Paemen L, Tips A, Schoofs L, Proost P, Van Damme J, De Loof A. Lom-AG-myotropin: a novel myotropic peptide from the male accessory glands of Locusta migratoria. Peptides. 1991;12:7–10. doi: 10.1016/0196-9781(91)90158-l. [DOI] [PubMed] [Google Scholar]
- Petri B, Homberg U, Loesel R, Stengl M. Evidence for a role of GABA and Mas-allatotropin in photic entrainment of the circadian clock of the cockroach Leucophaea maderae. J Exp Biol. 2002;205:1459–1469. doi: 10.1242/jeb.205.10.1459. [DOI] [PubMed] [Google Scholar]
- Pratt GE, Farnsworth DE, Siegel NR, Fok KF, Feyereisen R. Identification of an allatostatin from adult Diploptera punctata. Biochem Biophys Res Commun. 1989;163:1243–1247. doi: 10.1016/0006-291x(89)91111-x. [DOI] [PubMed] [Google Scholar]
- Price MD, Merte J, Nichols R, Koladich PM, Tobe SS, Bendena WG. Drosophila melanogaster flatline encodes a myotropin orthologue to Manduca sexta allatostatin. Peptides. 2002;23:787–794. doi: 10.1016/s0196-9781(01)00649-0. [DOI] [PubMed] [Google Scholar]
- Reichwald K, Unnithan GC, Davis NT, Agricola H, Feyereisen R. Expression of the allatostatin gene in endocrine cells of the cockroach midgut. Proc Natl Acad Sci USA. 1994;91:11894–11898. doi: 10.1073/pnas.91.25.11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudwall AJ, Sliwowska J, Nässel DR. Allatotropin-like neuropeptide in the cockroach abdominal nervous system: myotropic actions, sexually dimorphic distribution and colocalization with serotonin. J Comp Neurol. 2000;428:159–173. doi: 10.1002/1096-9861(20001204)428:1<159::aid-cne11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Schal C, Sevala VL, Young HP, Bachmann JAS. Site of synthesis and transport pathways of insect hydrocarbons: cuticle and ovary as target tissues. Am Zool. 1998;38:382–393. [Google Scholar]
- Sevala V, Shu S, Ramaswamy SB, Schal C. Lipophorin of female Blattella germanica: characterization and relation to hemolymph titers of juvenile hormone and hydrocarbons. J Insect Physiol. 1999;45:431–441. doi: 10.1016/s0022-1910(98)00142-5. [DOI] [PubMed] [Google Scholar]
- Stay B. A review of the role of neurosecretion in the control of juvenile hormone synthesis: a tribute to Berta Scharrer. Insect Biochem Mol Biol. 2000;30:653–662. doi: 10.1016/s0965-1748(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Stay B, Chan KK, Woodhead AP. Allatostatin-immunoreactive neurons projecting to the corpora allata of adult Diploptera punctata. Cell Tissue Res. 1992;270:15–23. doi: 10.1007/BF00381875. [DOI] [PubMed] [Google Scholar]
- Stay B, Tobe SS, Bendena WG. Allatostatins—identification, primary structures, functions and distribution. Adv Insect Physiol. 1994;25:267–337. [Google Scholar]
- Stracker TH, Thompson S, Grossman GL, Riehle MA, Brown MR. Characterization of the AeaHP gene and its expression in the mosquito Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2002;39:331–342. doi: 10.1603/0022-2585-39.2.331. [DOI] [PubMed] [Google Scholar]
- Tobe SS, Garside CS, Jansons IS, Price MD, Bendena WG. Allatostatins. In: Suzuki A, Kataoka H, Matsumoto S, editors. Molecular mechanisms of insect metamorphosis and diapause. Industrial Publishing and Consulting; Tokyo: 1995. pp. 13–24. [Google Scholar]
- Veenstra JA, Costes L. Isolation and identification of a peptide and its cDNA from the mosquito Aedes aegypti related to Manduca sexta allatotropin. Peptides. 1999;20:1145–1151. doi: 10.1016/s0196-9781(99)00117-5. [DOI] [PubMed] [Google Scholar]
- Veenstra JA, Lehman HK, Davis NT. Allatotropin is a cardioacceleratory peptide in Manduca sexta. J Exp Biol. 1994;188:347–354. doi: 10.1242/jeb.188.1.347. [DOI] [PubMed] [Google Scholar]
- Veenstra JA, Lau GW, Agricola HJ, Petzel DH. Immunohistological localization of regulatory peptides in the midgut of the female mosquito Aedes aegypti. Histochem Cell Biol. 1995;104:337–347. doi: 10.1007/BF01458127. [DOI] [PubMed] [Google Scholar]
- Weaver RJ, Edwards JP, Bendena WG, Tobe SS. Structures, functions and occurrences of insect allatostatic peptides. In: Coast GM, Webster SG, editors. Recent advances in arthropod endocrinology. Society for Experimental Biology: Seminar Series 65. Cambridge University Press; Cambridge: 1998. pp. 3–32. [Google Scholar]
- Wigglesworth VB. The pericardial cells of insects: analogue of the reticuloendothelial system. J Reticuloendothel Soc. 1970;7:208–216. [PubMed] [Google Scholar]
- Woodhead AP, Stay B, Seidel SL, Khan MA, Tobe SS. Primary structure of four allatostatins: neuropeptide inhibitors of juvenile hormone synthesis. Proc Natl Acad Sci USA. 1989;86:5997–6001. doi: 10.1073/pnas.86.15.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JG, Stay B. Immunocytochemical localization of Diploptera punctata allatostatin-like peptide in Drosophila melanogaster. J Comp Neurol. 1995;363:475–488. doi: 10.1002/cne.903630310. [DOI] [PubMed] [Google Scholar]
- Zitnan D, Sehnal F, Bryant PJ. Neurons producing specific neuropeptides in the central nervous system of normal and pupariation-delayed Drosophila. Dev Biol. 1993;156:117–135. doi: 10.1006/dbio.1993.1063. [DOI] [PubMed] [Google Scholar]
- Zitnan D, Kingan TG, Kramer SJ, Beckage NE. Accumulation of neuropeptides in the cerebral neurosecretory system of Manduca sexta larvae parasitized by the braconid wasp Cotesia congregata. J Comp Neurol. 1995;356:83–100. doi: 10.1002/cne.903560106. [DOI] [PubMed] [Google Scholar]