Abstract
Background: Folate is postulated to protect against cell injury and long-term risk of cancer. Folate deficiency has been shown to be associated with inflammatory bowel disease (IBD). However, folate concentrations are poorly delineated in children with IBD.
Objective: The objective was to compare folate concentrations between children with newly diagnosed IBD and healthy controls.
Design: Red blood cell folate (RBCF) and whole-blood folate (WBF) concentrations were measured in 78 children (mean age: 12.8 ± 2.7 y): 22 patients with newly diagnosed untreated Crohn disease, 11 patients with ulcerative colitis, 4 patients with indeterminate colitis, and 41 controls. Vitamin supplementation and dietary intakes determined by food-frequency questionnaire were recorded for 20 IBD patients and 28 controls.
Results: RBCF concentrations were 19.4% lower in controls (587.0 ± 148.6 ng/mL) than in patients (728.7 ± 185.8 ng/mL; P = 0.0004), and WBF concentrations were 11.1% lower in controls (218.2 ± 49.7 ng/mL) than in patients (245.3 ± 59.1 ng/mL; P = 0.031). Total folate intake was 18.8% higher in controls (444.7 ± 266.7 μg/d) than in IBD patients (361.1 ± 230.6 μg/d), but this difference was not statistically significant (P = 0.264). Folate intakes were below the Recommended Dietary Allowance (200–400 μg/d), adjusted for age and sex, in 35.4% of study subjects.
Conclusions: In contrast with previous evidence of folate deficiency in adult IBD patients, our data indicate higher folate concentrations in children with newly diagnosed untreated IBD than in controls. This finding was unexpected, especially in light of the higher dietary folate intakes and hematocrit values in children without IBD. The influence of IBD therapy on folate metabolism and the long-term clinical implications of high RBCF and WBF concentrations at the time of IBD diagnosis should be explored further.
INTRODUCTION
Inflammatory bowel disease (IBD) occurs in children of all ages (1). Pediatric patients with IBD are a distinctly different group compared with adult subjects with IBD, allowing for investigation of initial host immune responses and characterization of genotype-phenotype relations, environmental influences, and natural history of the disease (2). Folate, a water-soluble B vitamin, has been shown to prevent DNA damage and cell injury, which can lead to different types of cancer, including those associated with IBD (3–6).
Folate deficiency may be also associated with tumor growth in IBD (7). Folate concentrations in adult patients with IBD have been reported to be normal or low compared with those in non-IBD controls (8, 9). Evidence of folate deficiency in IBD may have multiple causes, including low dietary intake, malabsorption, and medication interactions (10, 3). Some studies have indicated that adult patients with Crohn Disease (CD) may have abnormal folate absorption (11). Other studies indicate a high prevalence of inadequate nutrient intakes (9). Sulfasalazine and other IBD medications can decrease folate concentrations (12, 13). Folate supplementation, therefore, is generally recommended for patients with IBD. However, recent studies suggest that folate supplementation may actually predispose to the development of certain tumors, including colorectal cancer—a major concern for patients with IBD (14–16).
Folate concentrations are poorly delineated in children with IBD. A few studies have reported that children with IBD may have equivalent or slightly higher red blood cell folate (RBCF) concentrations (17, 18). However, it remains unclear whether children with newly diagnosed IBD typically have a folate deficiency or elevated concentrations of folate. Furthermore, whether folate concentrations are associated with dietary intake, host factors (eg, age, ethnicity, and sex), or clinical characteristics (eg, hematocrit level, location of disease, and disease activity index) is similarly unclear. We describe our observations of RBCF and whole-blood folate (WBCF) concentrations in pediatric patients with untreated, recently diagnosed IBD compared with observations in non-IBD controls.
SUBJECTS AND METHODS
Subjects
Children aged 5–17 y were recruited from May 2005 to November 2006 from 8 major clinical centers: UCSF Children's Hospital, University of California, San Francisco; The University of Chicago Children's Hospital, Chicago, IL; Children's Center for Digestive Health Care and Emory University School of Medicine, Atlanta, GA; Texas Children's Hospital, Baylor College of Medicine, Houston, TX; Children's Hospital of Philadelphia, Philadelphia, PA; MassGeneral Hospital for Children, Boston, MA; and Children's Hospital, Oakland, CA. Blood samples were sent to the Holland Laboratory at the University of California, Berkeley (UCB) for processing, analysis, and storage. Patients were enrolled at the time of initial IBD diagnoses and included children with ulcerative colitis (UC), CD, and indeterminate colitis (IC). The diagnoses were confirmed by clinical, histologic, and/or radiologic features. Patients were excluded if they were taking any medications for IBD treatment (including 5-aminosalicyclic acids, antibiotics, steroids, and immunomodulators).
Controls were similarly recruited from children referred to the gastroenterology clinics for evaluation of abdominal pain, constipation, and/or diarrhea and with diagnoses (eg, chronic functional constipation, chronic recurrent abdominal pain, and irritable bowel syndrome) that excluded inflammatory conditions (ie, eosinophilic esophagitis and celiac disease).
Each center obtained institutional ethical review board approval for the study, and all procedures complied with the approved protocol and the Health Insurance Portability and Accountability Act. Patients and parents provided assents and consents before entry into the study.
Measurements
Blood samples were collected from 78 pediatric patients for the measurement of hematocrit, RBCF, and WBF concentrations by electrochemiluminescence immunoassay (ECLIA) at a central laboratory (ARUP Laboratories, Salt Lake City, UT). RBCF concentrations were calculated from WBF concentrations [RBCF = (WBF × 100)/hematocrit]. Clinical information, including body mass index (BMI; in kg/m2), history of recent illness, medication use, family history of IBD, and medical history, was collected. Dietary intake was determined for 20 IBD patients and 28 controls at the California sites by using a detailed food-recall questionnaire (Block questionnaire) and reported vitamin supplement use (19, 20).
Statistical analysis
For the initial analysis, 2-tailed t tests and chi-square tests were used to compare IBD patients with controls by demographic factors (ie, age, sex, and ethnicity) and clinical measurements (ie, hematocrit, WBF, and RBCF). Because WBF concentrations did not differ significantly between the 3 IBD subtypes, the UC, CD, and IC patients were combined into one group for subsequent analyses. Multiple linear regression models were used to assess the effect of IBD on folate concentrations after adjustment for potential confounders, including age and ethnicity. We used several indicator variables for each ethnic group (African Americans, Asians, and others) in the model and compared them with those for whites (reference group). To identify which clinical characteristics may be predictive of folate concentrations in IBD patients, we used a stepwise regression procedure (SAS PROC Reg; SAS Institute Inc, Cary, NC). The full model included dummy variables for disease location, Pediatric Crohn's Disease Activity Index (PCDAI; 21), time to diagnosis, and demographic variables. A P value of 0.15 was specified for entry and removal in the model as a preliminary screening technique to determine potential predictors. All statistical analyses were performed in STATA 9.0 (Statacorp, College Station, TX) and SAS version 9.1 (SAS Institute).
RESULTS
Patient characteristics
Folate concentrations were measured in 78 children: 37 with newly diagnosed, untreated IBD (22 with CD, 11 with UC, and 4 with IC) and 41 healthy controls (Table 1). These 2 groups differed slightly by age (P = 0.042) and ethnicity (P = 0.048), but not by sex (P = 0.370). Thus, subsequent multiple linear regression models adjusted for both age and ethnicity. BMI ranged from 12.4 to 41.9 in controls and from 13.9 to 26.3 in IBD patients. The mean values were not significantly different between cases and controls (20.1 and 18.7, respectively; P = 0.189). The controls enrolled were most often referred for evaluation of abdominal pain (55.8%), constipation (34.9%), and/or diarrhea (14%) but had no diagnosed underlying inflammatory conditions. The IBD patients had a variable presentation at diagnosis (Table 2). There were no statistically significant differences in age, hematocrit, or erythrocyte sedimentation rate (ESR) in patients with different subtypes of IBD. The most common presenting symptom in the CD group was abdominal pain (50%). In UC and IC patients, the most common presenting symptom was blood in the stool (63.6% and 100%, respectively). Other symptoms and disease locations varied between the 3 subtypes as described in detail in Table 2.
TABLE 1.
Characteristics of patients with inflammatory bowel disease (IBD) and controls
| Patients | Controls (n = 41) | IBD (n = 37) | P value1 |
| Age (y) | 11.4 ± 3.22 | 12.8 ± 2.7 | 0.042 |
| Female | 18 (43.9)3 | 20 (54.0) | 0.37 |
| Male | 23 (56.1) | 17 (46.0) | — |
| Hispanic | 8 (19.5) | 4 (11.8) | — |
| White | 37 (90.2) | 27 (72.9) | 0.048 |
| African American | 0 (0.0) | 6 (16.2) | — |
| Asian | 2 (4.9) | 3 (8.1) | — |
| Other4 | 2 (4.9) | 1 (2.7) | — |
| BMI (kg/m2) | 20.1 ± 5.2 | 18.7 ± 3.4 | 0.19 |
Two-tailed t test or chi-square test.
Mean ± SD (all such values).
Number with percentage in parentheses (all such values).
Pacific Islander, Native American, or unknown.
TABLE 2.
Characteristics of the patients with inflammatory bowel disease (IBD), by subtype1
| Crohn disease (n = 22) | Ulcerative colitis (n = 11) | Indeterminate colitis (n = 4) | |
| Age at diagnosis (y) | 12.8 ± 2.02 | 12.5 ± 3.6 | 13.5 ± 4.0 |
| ESR (mm/h) | 36.2 ± 19.5 | 34 ± 19.4 | 18.5 ± 17.7 |
| Hematocrit (%) | 35.1 ± 3.4 | 32.4 ± 4.4 | 36.8 ± 3.0 |
| Symptoms | Abdominal pain: 11 (50%) | Blood in stool: 7 (63.6%) | Blood in stool: 4 (100%) |
| Weight loss: 7 (3.18%) | Abdominal pain: 5 (45.5%) | Abdominal pain: 2 (50%) | |
| Diarrhea: 8 (13.6%) | Diarrhea: 4 (36.4%) | Diarrhea: 1 (25%) | |
| Blood in stool: 2 (9.1%) | Weight loss: 2 (18.2%) | ||
| Other (eg, poor growth, fever, fatigue, anemia, perianal problems): 4 (18.2%) | Other (eg, anemia, vomiting, fatigue, constipation, perianal problems): 1 (9.1%) | ||
| Disease location | Ileal involvement: 4 | Proctitis: 4 | Colonic involvement: 2 |
| Colonic involvement: 8 (4 with perianal disease) | Left-sided ulcerative colitis: 1 | Ileocolonic involvement: 2 | |
| Ileocolonic involvement: 8 (4 with upper disease and 1 with perianal disease) | Pancolitis: 6 | ||
| Two with isolated upper disease |
There were no significant differences in age (P = 0.81), erythrocyte sedimentation rate (ESR; P = 0.25), or hematocrit (P = 0.11) between the IBD subtypes as determined by ANOVA.
Mean ± SD (all such values).
Folate concentrations
Mean RBCF concentrations in pediatric patients with newly diagnosed, untreated UC and CD were similar (776.6 ± 202.4 and 735.3 ± 167.1 ng/mL, respectively; P = 0.537) and noticeably higher than in IC patients (n = 4) (560.5 ± 189.1 ng/mL; P = 0.084). Thus, the comparison of RBCF concentrations between cases and controls only included UC and CD patients in the “case” group. None of our patients or controls was folate deficient on the basis of blood measurements, and 6 subjects (2 controls, 4 IBD patients) had elevated concentrations on the basis of minimum and maximum values provided by ARUP Laboratories (280–903ng/mL). Higher WBF and RBCF concentrations were found independent of hematocrit concentrations, which were slightly lower in IBD patients (P = 0.030) than in controls (Table 3).
TABLE 3.
Hematocrit and whole-blood folate (WBF) and red blood cell folate (RBCF) concentrations in patients with inflammatory bowel disease (IBD) and controls
| Controls (n = 41) | IBD (n = 37) | P value1 | |
| Hematocrit (%) | 35.9 ± 4.9 (23.5–48.1)2 | 33.6 ± 3.9 (26.9–43.0) | 0.032 |
| WBF (ng/mL) | 218.2 ± 49.7 (136.0–335.8) | 245.3 ± 59.1 (131.1–396.0) | 0.031 |
| RBCF (ng/mL) | 587.0 ± 148.6 (314.0–982.0) | 728.7 ± 185.8 (405.0–1211.0) | 0.0005 |
Two tailed t test.
Mean ± SD; range in parentheses (all such values).
In the multiple linear regression model (Table 4), mean folate concentrations remained significantly associated with disease status after adjustment for age and ethnicity (P = 0.001 and P < 0.0005 for WBF and RBCF, respectively). WBF concentrations on average were 41.8 ng/mL higher in IBD patients (95% CI: 17.0, 66.7 ng/mL) than in controls after adjusting for age and ethnicity. Similar results were found for RBCF (P < 0.001). We identified a trend of lower folate RBCF and WBF concentrations in older children, but it was not statistically significant (P = 0.13 and P = 0.08, respectively). In addition, higher folate concentrations were observed in whites than in all other ethnic groups. However, only the difference from African American IBD patients was significant (P = 0.046 for RBCF). No significant differences in folate concentrations were found by sex in either IBD patients or controls (P = 0.469 and P = 0.776 for RBCF and WBF, respectively).
TABLE 4.
Multiple linear regression analysis of the differences in red blood cell folate (RBCF) concentrations between patients with inflammatory bowel disease (IBD) and controls (n = 80)1
| RBCF |
|||
| Variable | β Coefficient (95% CI) | P value | R2 |
| Intercept | 710.5 | — | 0.29 |
| Controls2 | — | — | — |
| IBD patients | 181.0 (102.8, 259.1) | <0.001 | — |
| Age | −9.8 (−22.4, 2.8) | 0.126 | — |
| White2 | — | — | — |
| African American | −147.1 (−291.4, −2.9) | 0.046 | — |
| Asian | −133.3 (−283.6, 17.1) | 0.081 | — |
| Other | −117.6 (−307.6, 72.4) | 0.221 | — |
Multiple linear regression analysis was used to determine the effects of disease status, age, and ethnicity on RBCF. RBCF concentrations were significantly greater in patients with IBD than in controls (P < 0.001) after adjustment for age and ethnicity and were significantly lower in African American than in white patients.
Reference group.
Dietary intake
Total dietary folate intake was 18.8% higher in controls (445 ± 267 μg/d; n = 28) than in IBD patients (361 ± 231 μg/d for IBD; n = 20), but this difference was not statistically significant (P = 0.264) (Table 5). RBCF and WBF concentrations in patients with dietary folate intake data were not significantly different from those in subjects lacking these data (P > 0.05). The Recommended Dietary Allowance (RDA) of folic acid is 200 μg/d for 4–8-y-olds, 300 μg/d for 9–13-y-olds, and 400 μg/d for 14–18-y-olds. Of 48 subjects with dietary intake data, 17 (35.4%) had intakes below the RDA (10 IBD and 7 control), adjusted for age and sex. Although dietary intake below the RDA was more frequent in patients than in controls, the difference was not statistically significant (P = 0.074). Dietary folate intake did not correlate with either RBCF or WBF concentrations. Despite having a slightly lower dietary folate intake, IBD patients had higher WBF and RBCF concentrations than did controls. Using a multiple linear regression model, we found an association between lower ratios of dietary folate intake to blood folate concentrations (RBCF and WBF) in cases but not in controls (P = 0.041 and P = 0.045, respectively) after adjustment for BMI and age. We also saw a suggestive but nonsignificant trend of increased ratios of dietary folate to blood folate concentrations for RBCF and WBF with increasing BMI after adjustment for age and disease status (P = 0.11 and P = 0.18, respectively). The interaction between disease status and BMI was not statistically significant. Folate concentrations did not correlate significantly with multivitamin use (P = 0.206 and P = 0.071 for RBCF and WBF, respectively). In addition, folate supplementation, measured by dietary questionnaire, was not associated with RBCF or WBF (P = 0.478 and P = 0.658, respectively).
TABLE 5.
Folate intakes in patients with inflammatory bowel disease (IBD) and controls
| Patients | Controls (n = 28) | IBD (n = 20) | P value1 |
| μg/d | μg/d | ||
| Folic acid, fortified | 142.4 ± 63.12 | 119.2 ± 57.8 | 0.20 |
| Folate, dietary | 142.7 ± 57.6 | 135.0 ± 76.0 | 0.69 |
| Folic acid, supplemental | 159.6 ± 241.2 | 106.87 ± 186.2 | 0.42 |
| Folate intake, total (dietary + supplemental) | 444.7 ± 266.7 | 361.1 ± 230.6 | 0.26 |
Two-tailed t test.
Mean ± SD (all such values).
Clinical factors
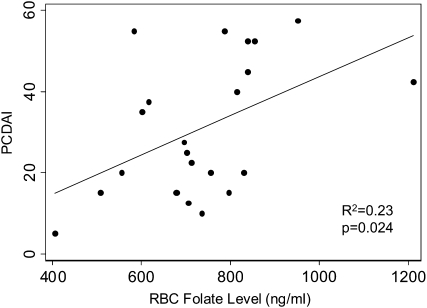
The mean PCDAI score in CD patients (30.9 ± 16.8) ranged from 5 to 57.5. Elevated PCDAI scores were associated with higher RBCF concentrations (Figure 1). The mean PCDAI score was 4.84 higher (95% CI: 0.72, 8.96; P = 0.024) for every 100-ng/mL increase in RBCF. The results for WBF were similar (P = 0.047). Length of time from onset of symptoms to date of diagnosis did not correlate with blood folate concentrations. Symptoms at presentation, specifically abdominal pain, bloody stool, or diarrhea, were also not related to folate concentrations (P = 0.484, 0.606, and 0.287, respectively). Stepwise regression analyses identified several potential predictors of folate concentrations in IBD patients, including location of disease, age, and ethnicity (Table 6). Upper gastrointestinal tract involvement was associated with lower WBF concentrations (P = 0.013), and disease in the colon was associated with higher WBF concentrations (P = 0.037) after adjustment for age and ethnicity. Similar associations were observed with RBCF (P = 0.021 and P = 0.019, respectively). We also analyzed the disease locations according to the Montreal classification system. However, this did not affect any of our conclusions (data not shown).
FIGURE 1.
Relation between mean (±SD) red blood cell (RBC) folate concentrations and the Pediatric Crohn's Disease Activity Index (PCDAI; 22), determined by linear regression analysis. The PCDAI score increased 4.84 (95% CI: 0.72, 8.96) for every 100-ng/mL increase in RBCF concentration (P = 0.024). The PCDAI score for all patients with Crohn disease (n = 22) was 30.91 ± 16.84 (range: 5–57.5).
TABLE 6.
Multiple linear regression analysis of red blood cell folate (RBCF) concentrations in patients with inflammatory bowel disease (IBD) (n = 37)1
| RBCF |
|||
| Variable | β Coefficient (95% CI) | P value | R2 |
| Intercept | 886.8 | — | 0.42 |
| Location: not upper GI2 | — | — | — |
| Location: upper GI | −147.1 (−269.8, −24.3) | 0.021 | — |
| Location: not colon2 | — | — | — |
| Location: colon | 136.5 (24.1, 248.9) | 0.019 | — |
| Age | −4.8 (−25.8, 16.1) | 0.642 | — |
| White2 | — | — | — |
| African American | −277.9 (−443.2, −112.6) | 0.002 | — |
| Asian | −162.4 (−357.4, 32.6) | 0.099 | — |
| Other | −227.0 (−570.6, 116.7) | 0.187 | — |
Multiple linear regression analysis was used to determine the effects of disease location, age, and ethnicity on RBCF in patients with IBD. After adjustment for age and ethnicity, upper gastrointestinal (GI) tract involvement was associated with decreased RBCF concentrations (P = 0.021), and the location of disease in the colon was associated with increased RBCF concentrations (P = 0.019).
Reference group.
DISCUSSION
In contrast with previous studies reporting folate deficiencies in adult IBD patients (8, 10), we observed higher WBF and RBCF concentrations in pediatric patients with newly diagnosed IBD than in controls. This is particularly interesting because we observed lower hematocrit levels and folate intakes in IBD patients. This substantiates our previous report of higher blood folate concentrations in pediatric IBD patients (18). Furthermore, RBCF was positively correlated with PCDAI in CD patients. Additionally, several other factors were associated with blood folate concentrations, including age, location of disease, and ethnicity.
The clinical impact of these findings may be important to the therapeutic management of patients with IBD. Our data suggest that anemia in patients with IBD at presentation is independent of folate status. However, consideration of additional nutrients (eg, iron, vitamin B-6, vitamin B-12, zinc, selenium, and copper) in the early development of anemia in children with IBD should also be addressed. Furthermore, differences in metabolic pathways or intestinal flora in patients with IBD can inherently affect folate absorption and intestinal metabolism, which can alter folate concentrations in patients with IBD.
To date, many clinicians have recommend folate supplementation for all IBD patients. However, recent studies have reported a potential increased risk of colon cancer and other cancers in patients with an underlying predisposition to these tumors who are supplemented with folate (14, 15, 22, 16). Because we found normal folate concentrations in pediatric patients with newly diagnosed IBD, further evaluation of current folate supplementation recommendations is necessary. Additionally, the effects of subsequent IBD treatments on folate concentrations also warrant further investigation because it is known that sulfasalazine and other IBD medications can decrease folate concentrations (12, 13).
We found that folate concentrations did not differ significantly between UC and CD patients. However, given our relatively small sample size, this observation still remains to be verified in larger studies. Because CD patients are more likely to experience small intestinal malabsorption, anorexia, and weight loss than are UC patients, we expected to observe lower folate concentrations in these patients. However, it is possible that small intestinal bacterial overgrowth in patients with CD compared with UC contribute to higher folate concentrations in this group of patients.
We also found that younger children with IBD tended to have higher folate concentrations than did older patients. Higher concentrations of RBCF and WBF concentrations in whites than in other ethnic groups, particularly African Americans, were also observed in our study. These differences may be related to some unknown nutrient intake factor or possibly to the length of time that the underlying disease may be developing undiagnosed. African American children often experience a delayed diagnosis and present at an older age than do white children (1). Genetic background may further explain some of the variation in folate concentrations in children with comparable dietary folate intakes. Further studies are underway to investigate functionally significant polymorphisms, including those in the 5,10-methylenetetrahydrofolate reductase gene, which may help to explain why children with similar dietary folate intakes have significantly different RBCF and WBF concentrations (23).
Limitations in our study include the lack of precise dietary intake data, because retrospective food recall is sometimes inaccurate when assessing nutritional status. However, the Block food-frequency questionnaire used in our study was previously thoroughly validated for adults (19) and children (20). Another limitation was the slight difference in age and race-ethnicity composition between the 2 groups. However, multiple regression techniques enabled us to adjust for these differences in statistical models. Despite these limitations, we identified significantly higher blood folate concentrations in pediatric patients with newly diagnosed IBD than in controls, even though the latter group appeared to have higher dietary folate intakes.
The findings of the present study suggest that folate deficiency is uncommon in pediatric patients with newly diagnosed IBD. The effects of genetic polymorphisms and the ultimate influences of IBD therapies on folate status and metabolism remain to be determined. The long-term implications of folate supplementation on growth and development, the natural history of disease, and the eventual potential for development of intestinal cancers remain to be systematically investigated.
Acknowledgments
The study coordinators at each clinical center were essential to the collection of the data, including, but not limited to, Erin DeMicco and Ann L Clark.
The authors' responsibilities were as follows—MBH, EAG, NS, KH, FAJ, and NH: worked directly to recruit patients, process the samples, analyze and interpret the data, and draft the final manuscript; and PH, HSW, RNB, SAC, BDG, BSK, GDF, and ES: worked as a collaborative group to recruit the patients, provide the samples and clinical data, and complete the final manuscript. All authors had a significant role in this project and approved the final version of the manuscript. None of the authors had a conflict of interest to report.
REFERENCES
- 1.Heyman MB, Kirschner BS, Gold BD, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr 2005;146:35–40 [DOI] [PubMed] [Google Scholar]
- 2.Kugathasan S, Judd RH, Hoffmann RG, et al. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population based study. J Pediatr 2003;143:525–31 [DOI] [PubMed] [Google Scholar]
- 3.Fenech M, Aitken C, Rinaldi J. Folate, vitamin B12, homocysteine status and DNA damage in young Australian adults. Carcinogenesis 1998;19:1163–71 [DOI] [PubMed] [Google Scholar]
- 4.Kamen B. Folate and antifolate pharmacology. Semin Oncol 1997;24:S18-30–S18-39 [PubMed] [Google Scholar]
- 5.Kim YI. Role of folate in colon cancer development and progression. J Nutr 2003;133(suppl 1):3731S–9S [DOI] [PubMed] [Google Scholar]
- 6.Mouzas IA, Papavassiliou E, Koutroubakis I. Chemoprevention of colorectal cancer in inflammatory bowel disease? A potential role for folate. Ital J Gastroenterol Hepatol 1998;30:421–5 [PubMed] [Google Scholar]
- 7.Phelip JM, Ducros V, Faucheron JL, Flourie B, Roblin X. Association of hyperhomocysteinemia and folate deficiency with colon tumors in patients with inflammatory bowel disease. Inflamm Bowel Dis 2008;14:242–8 [DOI] [PubMed] [Google Scholar]
- 8.Chowers Y, Sela BA, Holland R, Fidder H, Simoni FB, Bar-Meir S. Increased levels of homocysteine in patients with Crohn's disease are related to folate levels. Am J Gastroenterol 2000;95:3498–502 [DOI] [PubMed] [Google Scholar]
- 9.Vagianos K, Bector S, McConnell J, Bernstein CN. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr 2007;31:311–9 [DOI] [PubMed] [Google Scholar]
- 10.Elsborg L, Larsen L. Folate deficiency in chronic inflammatory bowel diseases. Scand J Gastroenterol 1979;14:1019–24 [PubMed] [Google Scholar]
- 11.Steger GG, Mader RM, Vogelsang H, Schöfl R, Lochs H, Ferenci P. Folate absorption in Crohn's disease. Digestion 1994;55:234–8 [DOI] [PubMed] [Google Scholar]
- 12.Longstreth GF, Green R. Folate status in patients receiving maintenance doses of sulfasalazine. Arch Intern Med 1983;143:902–4 [PubMed] [Google Scholar]
- 13.Pironi L, Cornia GL, Ursitti MA, et al. Evaluation of oral administration of folic and folinic acid to prevent folate deficiency in patients with inflammatory bowel disease treated with salicylazosulfapyridine. Int J Clin Pharmacol Res 1988;8:143–8 [PubMed] [Google Scholar]
- 14.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA 2007;297:2351–9 [DOI] [PubMed] [Google Scholar]
- 15.Kim YI. Folate and colorectal cancer: an evidence based critical review. Mol Nutr Food Res 2007;51:267–92 [DOI] [PubMed] [Google Scholar]
- 16.Solomons NW. Food fortification with folic acid: has the other shoe dropped?. Nutr Rev 2007;65:512–5 [DOI] [PubMed] [Google Scholar]
- 17.Nakano E, Taylor CJ, Chada L, McGaw J, Powers HJ. Hyperhomocystinemia in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2003;37:586–90 [DOI] [PubMed] [Google Scholar]
- 18.Holland N, Harmatz P, Golden D, et al. Cytogenetic damage in blood lymphocytes and exfoliated epithelial cells of children with inflammatory bowel disease. Pediatr Res 2007;61:209–14 [DOI] [PubMed] [Google Scholar]
- 19.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124:453–69 [DOI] [PubMed] [Google Scholar]
- 20.Cullen KW, Watson K, Zakeri I. Relative reliability and validity of the Block Kids Questionnaire among youth aged 10 to 17 years. J Am Diet Assoc 2008;108:862–6 [DOI] [PubMed] [Google Scholar]
- 21.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr 1991;12:439–47 [PubMed] [Google Scholar]
- 22.Kim YI. Folic acid fortification and supplementation—good for some but not so good for others. Nutr Rev 2007;65:504–11 [DOI] [PubMed] [Google Scholar]
- 23.Nishio K, Goto Y, Kondo T, et al. Serum folate and methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism adjusted for folate intake. J Epidemiol 2008;18:125–31 [DOI] [PMC free article] [PubMed] [Google Scholar]