Abstract
During human latent tuberculosis (TB) infection, dormant bacilli putatively reside within the hypoxic environment of caseating lung granulomas. The anaerobic drug metronidazole has antituberculous activity under hypoxic conditions in vitro, but lacks activity against murine TB. In this study, we used the hypoxia marker pimonidazole to demonstrate the presence of tissue hypoxia in a novel in vivo granuloma model of M. tuberculosis latency. We also used a high-throughput, microarray-based technique to identify hypoxia-essential mycobacterial genes, and showed that this in vivo model correctly identified 51% of hypoxia-attenuated mutants, a significantly larger percentage than that identified by the mouse (29%) and guinea pig (29%) aerosol models of TB. Although isoniazid showed activity during the first 28 days of therapy, and rifampin was active against dormant bacilli after the establishment of tissue hypoxia, metronidazole showed no antituberculous activity in this in vivo hypoxic granuloma model of M. tuberculosis dormancy.
Keywords: Mycobacterium tuberculosis, hypoxia, metronidazole, dormancy, latency, persistence, hollow fiber, Wayne model, animal models
INTRODUCTION
Latent tuberculosis (TB) infection (LTBI) poses a major obstacle to TB eradication [1, 2]. Human LTBI is characterized by tuberculin test-positivity without clinical or radiographic findings [3]. LTBI apparently comprises a paucibacillary population of dormant organisms with reduced replication and metabolism [4] within host granulomas [5].
Hypoxia may be a primary microenvironmental condition responsible for M. tuberculosis dormancy within granulomas associated with human LTBI [6]. Unlike LTBI, most active TB infections occur in well-oxygenated body sites [6], and cavities open to airways generally harbor greater bacillary numbers [7]. Furthermore, disease reactivation usually involves the highly-oxygenated upper lung lobes [6].
Exposure of M. tuberculosis to progressive hypoxia in vitro induces a dormant state characterized by reduced replication and metabolism analogous to that postulated for bacilli in human LTBI. In the best characterized such model, Wayne et al. [8, 9] have shown that when the dissolved oxygen content drops below 1%, M. tuberculosis enters microaerophilic nonreplicating persistence (NRP) stage 1 [8], characterized by DNA synthesis termination and outer cell wall thickening. As the dissolved oxygen drops to ~0.06%, the bacilli enter NRP stage 2, demonstrating reduced susceptibility to standard antituberculous drugs but increased susceptibility to nitroimidazole drugs [8, 10].
Metronidazole becomes reductively activated by the pyruvate:ferredoxin oxidereductase system under anoxic conditions [11]. Despite activity against dormant bacilli under progressive hypoxia conditions in vitro, metronidazole appears inactive against M. tuberculosis in vivo. Specifically, metronidazole lacks activity against bacilli within infected bone marrow-derived macrophages, and does not kill bacilli in mouse lungs during the active or containment phases of disease [12]. Furthermore, metronidazole lacks sterilizing activity in the Cornell model of murine TB persistence [12, 13]. However, the poor antituberculous activity of metronidazole in the mouse model potentially may be explained by the absence of tissue hypoxia in mouse lungs during TB infection [14, 15].
We developed a novel in vivo granuloma model of M. tuberculosis dormancy (mouse hollow fiber model), in which bacilli achieve stationary-state colony forming unit (CFU) counts, decreased metabolic activity, and greater susceptibility to rifampin than isoniazid, which are key features of human LTBI [16]. In this model, host inflammatory cells are recruited around the subcutaneously-implanted, semi-diffusible fibers, resulting in progressive formation of peri-fiber granulomas. Dormant organisms in this model show significant upregulation of the DosR regulon, which is also induced upon hypoxic exposure of bacilli in vitro [17].
In this study, we characterized the oxygen content of the mouse hollow fiber model using the tissue hypoxia marker pimonidazole, as well as a high-throughput microarray-based technique to evaluate the survival of several different M. tuberculosis mutants containing mutations in hypoxia-induced genes. Having found evidence of tissue hypoxia using these immunohistochemical and mutant survival approaches, we tested the tuberculocidal activity of metronidazole in the mouse hollow fiber model.
MATERIALS AND METHODS
Strain selection criteria
Insertion mutagenesis of M. tuberculosis CDC 1551 using the Himar1 transposon (Tn) yielded a library of ~1500 unique mutants [18]. Tn insertion sites were identified by sequencing [18, 19]. Available mutants were cross-referenced with a list of hypoxia-induced genes compiled from published data [20–24], and 107 mutants were selected for this study (Supplemental Table 1).
Bacterial strains and growth conditions
Tn mutants were grown individually to mid-log phase at 37°C in Middlebrook 7H9 liquid broth supplemented with oleic acid-albumin-dextrose-catalase (Becton Dickinson), 0.05% Tween-80, and 0.1% glycerol. Equal volumes of same-density cultures were mixed, forming two pools (Supplemental Table 1). Glycerol was added to a final concentration of 10% and aliquots (OD600 = 0.4) were frozen at −80°C.
Progressive hypoxia model of infection
Each Tn mutant pool culture was diluted to ~104 CFU counts/ml as determined by OD600. A culture with a ratio of head-space air to medium volume (HSR) of 0.5 was grown in individual test tubes containing a magnetic stir bar and sealed with rubber stoppers. The cultures were incubated upright at 37°C with stir-bars spun at a speed sufficient for uniform suspension of organisms without agitating the culture surface. The reduction and decolorization of the methylene blue dye served as a visual indicator of oxygen levels corresponding to NRP stage 2 [8], which was defined as Day 0. Samples were collected at Day 0 (input pool) and at Day 28 (output pool) after color change. Sample collection, by piercing the rubber stoppers, did not introduce atmospheric oxygen into the tubes, as assessed by maintenance of the decolorized dye state.
Animals
Seven-eight week-old female SKH1 hairless, immunocompetent mice and female outbred Hartley guinea pigs (300–350 g) were purchased from Charles River Labs (Wilmington, MA). Animals were maintained under pathogen-free conditions and fed water and chow ad libitum. All procedures followed protocols approved by the Institutional Animal Care and Use Committee at the Johns Hopkins University.
Aerosol animal infections
Animals were aerosol-infected with the Tn mutant pools using a Glas-Col Inhalation Exposure System (Terre Haute, IN), calibrated to deliver ~500 CFU counts/lung on Day 1 after infection. Sacrifices were on Day 14 (input pool), and on Day 56 (output pool), and organs were removed aseptically. Day 14 was chosen as the input time point to allow for adequate representation of each individual Tn mutant prior to organized granuloma formation in animal lungs. Guinea pig organs were stored at −80 °C until the time of homogenization. Guinea pig lungs were homogenized in 5–10 ml PBS using a Kinematica Polytron Homogenizer with a 12 mm generator (Brinkman) within a BSL-III Glovebox Cabinet (Germfree). Mouse lungs were homogenized using glass homogenizers.
Hollow fiber animal infections
Approximately 104 organisms of two separate Tn mutant pools, from the same mixed culture as the aerosol infections, were inoculated into each individual hollow fiber (each mutant represented ~500-fold). Fibers were heat-sealed and implanted subcutaneously, as described [16, 25, 26]. Mice were sacrificed and fibers recovered on Day 14 (input pool) and Day 56 (output pool) after fiber implantation.
Mutant pool recovery and genomic preparations
Samples were plated on Middlebrook supplemented 7H10 plates (Fisher). Log-transformed CFU counts were used to calculate averages and standard deviations for graphing purposes. From 500 to 10,000 colonies per sample (as available) were scraped from plates and pooled, and genomic DNA was extracted as described [27]. DNA samples were processed according to the designer arrays for defined mutant analysis (DeADMAn) technique [28]. Samples generated from input and output pools for each experiment were labeled with different fluorochromes, combined, and hybridized to mutant-specific 60-mer microarrays. Control probes were printed onto the array as a mix of all 107 mutant-specific 60-mers and used to normalize the ratio of signal between the two channels. The variance of each ratio, between at least two technical replicates of two biological replicates, was analyzed using SAM software [29]. Probes with greater signal from the input pool compared to the output pool were determined to reflect attenuation of a particular Tn mutant if the corresponding q-value was less than the False Discovery Rate (FDR), selected such that the mean number of false discoveries was < 1 gene/array.
Metronidazole chemotherapy
Hollow fibers, containing ~1000 M. tuberculosis CDC1551 bacilli, were implanted into SKH1 mice. Antibiotic therapy was initiated 14 days after implantation. Groups of mice were treated daily (5/week) by esophageal cannulation (gavage) with isoniazid (25 mg/kg/day), rifampin (10 mg/kg/day), metronidazole (100 mg/kg/day), or pyrazinamide (150 mg/kg/day). A control group received deionized water daily by gavage. Mice were sacrificed and hollow fibers retrieved at Day -13, Day -7, Day 0, Day 7, Day 14, Day 28, and Day 56 relative to treatment onset. Hollow fiber contents were plated on 7H10 plates and bacterial colonies were enumerated after 3 weeks. Results are representative of two independent experiments.
Pimonidazole immunohistochemistry
Fibers containing ~3000 CFU bacilli were implanted into one group of 20 SKH1 mice, and groups of 5 animals were sacrificed at Days 1, 7, 14, and 28 after implantation. Three additional groups of 15 mice received fibers containing ~10 CFU, ~103 CFU, or ~104 CFU, and 5 mice from each group were sacrificed at Days 1, 14, and 28 after implantation. Mice received pimonidazole (Chemicon International; 60 mg/kg by intraperitoneal injection) 2 hrs before sacrifice. Hollow fiber contents from 3 mice per time point were used for CFU enumeration. The remaining hollow fibers were removed in situ and placed in 10% neutral buffered formalin. Paraffin-embedded tissue sections were visualized for pimonidazole staining following manufacturer recommendations.
RESULTS
Peri-fiber tissue staining with the hypoxia marker pimonidazole
To test directly for the presence of microaerophilic conditions in the mouse hollow fiber model, we used the tissue hypoxia marker pimonidazole. This 2-nitroimidazole becomes activated by mammalian nitroreductases and stably binds to cellular proteins at tissue pO2 < 10 mmHg [30]. This binding can be detected in tissue sections by immunostaining with a monoclonal antibody to pimonidazole adducts. Tissue pimonidazole adducts yield chromogenic production of a brown color through a horseradish peroxidase-linked reaction. Interestingly, the hollow fiber walls appeared brown in all samples, even in the absence of primary antibody.
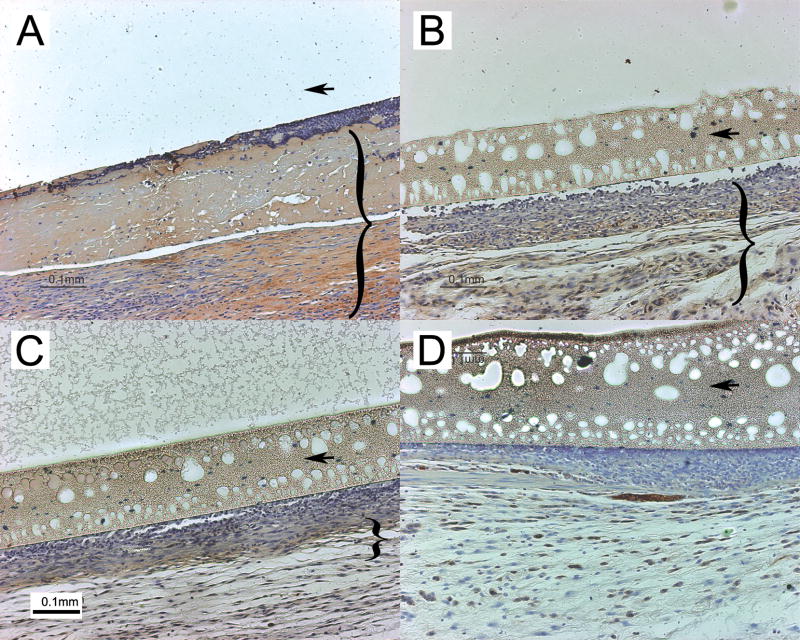
In a preliminary experiment, ~103 bacilli were encapsulated and implanted subcutaneously in mice. Although pimonidazole adducts were undetectable on Days 7 or 14 after fiber implantation (Fig. 1A, B), peri-fiber staining was observed on Day 28 in fibers containing live bacilli (Fig. 1C), but not those containing vehicle (Fig. 1F). Negative in situ fiber staining results are accompanied by corresponding positively-staining kidney sections (Fig 1D, E), containing hypoxic renal tubular cells [31].
Figure 1.
Pimonidazole staining of peri-fiber tissues. Hollow fibers were recovered on Day 7 (A), Day 14 (B), and Day 28 (C) after implantation. Positive controls comprising cross-sections of mouse kidney on Day (D) and Day 14 (E) after fiber implantation. Hollow fiber containing vehicle, recovered on Day 28 after implantation (F). Arrows indicate hollow fiber wall. Bracket in Panel C shows layer of greatest inflammation and pimonidazole staining.
To determine if tissue hypoxia in the mouse hollow fiber model is a dose-dependent phenomenon, hollow fibers containing ~10 CFU (low dose), ~103 CFU (mid-dose), or ~3.5 ×104 CFU (high dose) were implanted subcutaneously into separate mouse groups. Control mice received fibers containing ~3.5 ×104 heat-killed bacilli. As shown in Figure 2, the total number of CFU counts increased less than ten-fold over a 28-day period (high dose p=0.03, mid-dose p=0.09), except in the low-dose group, where the intra-fiber CFU count reached ~103 by Day 28 after fiber implantation (p<0.002). The degree of peri-fiber cellular inflammation and pimonidazole staining intensity on Day 28 appeared to correlate directly with the number of encapsulated live bacilli on Day 1 (Fig. 3).
Figure 2.
CFU counts from hollow fibers used for pimonidazole immunohistochemistry. Separate groups of mice received hollow fibers containing ~10 CFU (Low Dose), ~103 CFU (Mid Dose), and ~3.5 ×104 CFU (High Dose). Numbers are log10-converted CFU counts with standard deviations. Pimonidazole staining results for these experiments are presented in Figure 3.
Figure 3.
Peri-fiber tissue hypoxia is dependent on the number of intra-fiber bacilli. Cross-sections of hollow fibers containing Low Dose (A), Mid Dose (B), or High Dose (C) CFU, or ~3.5 ×104 heat-killed organisms (D). Arrow in Panel A indicates location of missing hollow fiber wall. Arrows in Panels B-D indicate hollow fiber walls. Brackets in Panels A-C indicate tissue layers containing pimonidazole staining.
M. tuberculosis genes required for survival in hypoxia and animal models
Genome-wide expression analysis using microarrays provides a powerful means to study adaptive bacterial survival strategies in response to various stress conditions [32]. M. tuberculosis gene regulation during hypoxia has been monitored in vitro [20–24], revealing induction of genes potentially involved in bacillary survival under these conditions. We hypothesized that M. tuberculosis genes induced under hypoxia may be important for mycobacterial hypoxic survival.
We screened an archived library of ~1500 unique M. tuberculosis CDC1551 Tn insertion mutants [18] for those containing mutations in hypoxia-induced genes [20–24], and selected a total of 107 different mutants, which were split into two pools (Supplemental Table 1). These mutants were tested for survival in a reference model of progressive hypoxia in vitro [8] using a high-throughput microarray-based approach termed DeADMAn [28]. These mutants also were tested for survival in the mouse hollow fiber model, and a subset of mutants was tested in the mouse and guinea pig aerosol models of infection. Mutant survival patterns in each model were compared with those in the reference model.
Of the 107 mutants tested in the reference model, 74 were significantly attenuated (mean false positives per array < 1) relative to wild-type by Day 28 after entry of the organisms into NRP-2 (Table 1). Fifteen of the 74 mutant strains were grown individually in this model to confirm the microarray survival data from the pooled groups. Thirteen of these 15 Tn mutants showed reduced CFU counts relative to the isogenic wild-type strain by Day 28 after entry into NRP-2 (Table 2). Of these 13 mutants, 11 were attenuated by two-fold or more and 3 mutants (containing Tn insertions in Rv3134c, Rv1894c, and Rv0020c) were at least ten-fold attenuated relative to the wild-type strain.
Table 1.
Tn mutant survival in four models, including the hypoxia reference model
| Model | Total # mutants attenuated in each model | # hypoxia-attenuated mutants in each model (percent) |
|---|---|---|
| Pool 1 | ||
| In vitro progressive hypoxia (reference model) | 21 | 21/21 (1.0) |
| Mouse Hollow Fiber | 43 | 14/21 (0.67) |
| Mouse aerosol | 14 | 6/21 (0.29) |
| GP aerosol | 10 | 6/21 (0.29) |
| Pools 1 and 2 | ||
| Reference model | 74 | 74/74 (1.0) |
| Mouse Hollow Fiber | 67 | 38/74 (0.51) |
Table 2.
Survival of individual Tn mutants in the hypoxia reference model
| MT# | Rv# | Name | Models attenuated1 | Fold attenuation2 |
|---|---|---|---|---|
| MT0023 | Rv0020c | TB39.8 | R,Mo HF,Mo Aero | 10.64 |
| MT0595 | Rv0569 | - | R,Mo HF,Mo Aero | 2.30 |
| MT0607 | Rv0578c | PE_PGRS7 | R | 1.87 |
| MT0845 | Rv0823c | - | R,Mo HF | 2.07 |
| MT0909 | Rv0886 | fprB | R,Mo HF,Mo Aero | 3.57 |
| MT1161 | Rv1129c | - | R,Mo HF | 2.00 |
| MT1283 | Rv1245c | - | R,Mo HF | 2.56 |
| MT1449 | Rv1405c | - | R | <1 |
| MT1944 | Rv1894c | - | R,Mo HF,GP Aero | 36.92 |
| MT2084 | Rv2025c | - | R,GP HF,GP Aero | <1 |
| MT2090 | Rv2031c | hspX/acr | R,Mo HF | 8.30 |
| MT2137 | Rv2077c | - | R,Mo HF,GP Aero | 4.25 |
| MT2940 | Rv2873 | mpt83 | R,Mo Aero,GP Aero | 3.06 |
| MT3220 | Rv3134c | - | R,Mo HF | 150.73 |
| MT3851 | Rv3743c | ctpJ | R,Mo Aero | 1.19 |
Models in which each particular mutant was found to be attenuated by DeADMAn (see Methods for details). R = in vitro progressive hypoxia (reference) model; HF = mouse implantable hollow fiber model; Mo Aero = mouse aerosol model; GP Aero = guinea pig aerosol model
Numbers represent fold attenuation in the reference model at 28 days relative to day 0, determined by enumeration of colony forming units from individually grown mutant strains.
The two Tn mutant pools (Supplemental Table 1) were next tested in the mouse hollow fiber model. Total intra-fiber CFU counts for both mutant pools 1 and 2 remained stable between Days 14 and 56 after hollow fiber implantation ((Fig. 4A); pool 1 (p=0.14) and pool 2 (p=0.18)). Of the 21 attenuated mutants in the reference model, 14 mutants (67%) were attenuated relative to the wild-type strain by Day 56 in the mouse hollow fiber model (Table 1). Of the 74 total mutants found to be defective for survival in the reference model, 38 mutants (51%) also were attenuated relative to the wild-type strain by Day 56 in the mouse hollow fiber model (Table 1). An additional 29 mutants among both pools showed reduced survival compared to the wild-type strain by Day 56 in the mouse hollow fiber model, but not in the reference model, presumably reflecting conditions present in the former, but absent in the latter model.
Figure 4.
Total mutant CFU counts in hollow fibers (A), and in guinea pig and mouse lungs (B) as a function of time. CFU counts were log10-converted and error bars represent standard deviations.
Recent studies have reported tissue hypoxia in guinea pig tuberculous lung lesions [33], but not in mouse lung lesions [14, 15]. We hypothesized that Tn mutants containing mutations in genes required for microaerophilic survival of M. tuberculosis would show an attenuated phenotype in guinea pig lungs, but not in mouse lungs. Guinea pigs and mice were aerosol-infected with Tn mutant pool 1, and lung samples were collected for CFU counts (Fig. 4B) and mutant survival analysis. Between Day 14 and Day 56, mouse lung CFU counts remained relatively stable (p=0.64), while guinea pig lung CFU counts increased slightly (p<0.05), consistent with bacillary growth containment following the onset of adaptive immunity [34]. Of the 21 Tn mutants found to be attenuated in the reference model, 4 mutants (19%) were attenuated in guinea pig lungs relative to the wild-type strain by Day 56 after infection. Six of the 21 hypoxia-attenuated mutants (29%) showed reduced survival in mouse lungs relative to wild-type by Day 56 after infection (Table 1). Only the mutant in Rv2873, which encodes the highly immunogenic cell surface lipoprotein mpt83 [35], was attenuated in both mouse and guinea pig lungs.
A Tn mutant in the narG gene, the product of which is involved in the alternate respiration nitrate reduction pathway, was attenuated only in the mouse hollow fiber model, but not in the other models tested. Tn mutants in Rv2031c, which encodes HspX (α-crystallin), and Rv3134c, the gene immediately upstream of dosR/devR, were attenuated only in the reference and mouse hollow fiber models, but not in the mouse or guinea pig aerosol models.
Metronidazole activity in the mouse hollow fiber model
Upon exposure to progressive hypoxia in vitro, M. tuberculosis becomes phenotypically tolerant to the first-line antituberculous agents isoniazid and rifampin, and increasingly susceptible to metronidazole [8, 10]. Given the positive tissue staining results with pimonidazole in the mouse hollow fiber model, as well as the relatively high number of hypoxia-attenuated Tn mutants identified in this model compared to the mouse aerosol model, we next tested the activity of metronidazole against M. tuberculosis in the mouse hollow fiber model. Beginning 14 days after fiber implantation, mice received daily (5 days per week) therapy with isoniazid (25 mg/kg), rifampin (10 mg/kg), pyrazinamide (150 mg/kg), or metronidazole (100 mg/kg), and control mice received vehicle by gavage. As demonstrated in Figure 5, metronidazole had no significant activity against bacilli during the first 56 days after infection. Isoniazid had the greatest bactericidal activity during the first 28 days after fiber implantation (p<0.01), with no activity thereafter (p=0.47). Conversely, rifampin showed minimal activity during the first 28 days (p=0.7), but reduced intra-fiber CFU counts by 2 log10 by Day 56 after implantation (p<0.05). Consistent with data from the standard mouse model [36], pyrazinamide alone had no bactericidal activity against hollow fiber-encapsulated M. tuberculosis.
Figure 5.
Activity of metronidazole against bacilli in the mouse implantable hollow fiber model. Mice were treated with metronidazole 100 mg/kg/day (Met), isoniazid 25 mg/kg/day (INH), rifampin 10 mg/kg/day (RIF), and pyrazinamide 150 mg/kg/day (PZA) by esophageal cannulation beginning 14 days after hollow fiber implantation. CFU counts were log10-converted and error bars represent standard deviations.
DISCUSSION
Exposure of M. tuberculosis to progressive hypoxia induces a non-replicating persistent state characterized by reduced susceptibility to isoniazid and rifampin, which is analogous to human LTBI. However, this in vitro model has been criticized because the organisms become susceptible to metronidazole, yet this antibiotic lacks tuberculocidal activity in the murine model [12, 13]. Data are lacking to support its activity against human LTBI. In addition, in vitro models cannot accurately simulate the complex host-pathogen interactions involved in bacillary containment during human LTBI. We have developed a novel in vivo granuloma model of LTBI (mouse hollow fiber model), in which bacillary growth containment is partially immune-mediated, and the organisms exhibit a dormancy phenotype characteristic of human LTBI [16]. Consistent with the presence of microaerophilic conditions in this model, the organisms show significant upregulation of the DosR regulon. We used the hypoxia-specific marker pimonidazole to directly demonstrate reduced tissue oxygen content in this in vivo model. The findings of M. tuberculosis dose-dependent pimonidazole staining, as well as the absence of cellular inflammation and pimonidazole staining surrounding fibers containing heat-killed bacilli, are consistent with active secretion and extra-fiber diffusion of M. tuberculosis soluble factors leading to host inflammatory cell recruitment, peri-fiber granuloma formation, and ensuing tissue hypoxia. Consistent with previous data [37], our data suggest a role for hypoxia in promoting M. tuberculosis dormancy beyond Day 28 in this model, during which the organisms become phenotypically resistant to isoniazid, but susceptible to rifampin.
To our knowledge, this is the first study in which the accuracy of stress-induced M. tuberculosis gene expression in predicting gene function was tested systematically on a large scale. We investigated the role of various hypoxia-induced genes in mycobacterial survival under microaerophilic conditions thought to be present in human caseous TB lesions. Identification of M. tuberculosis genes essential for hypoxic survival potentially provides a basis for rational drug design targeting dormant organisms in human LTBI. Approximately 69% (74/107) of all hypoxia-induced genes were found to be essential for survival during progressive hypoxia. This percentage is significantly higher than that observed using random Tn mutant pools under various stress conditions (25–30%, unpublished data), consistent with our original hypothesis that mycobacterial gene induction predicts gene essentiality under the same stress conditions.
Among the M. tuberculosis genes identified as essential for bacillary survival under hypoxia were Rv3134c, Rv0823c, Rv1129, Rv1894c, and Rv0020c. Rv3134c is directly upstream of both genes of the two-component response regulator, dosR/dosS, raising the possibility that its disruption might have polar effects on downstream genes of this operon, which is required for M. bovis survival under hypoxic conditions [38]. The genes Rv0823c and Rv1129c encode putative transcriptional regulators, which may be involved in DosR-independent hypoxic gene regulation. The Rv0020c-encoded protein is predicted to have a forkhead-associated domain, which is a phosphopeptide binding motif and putative nuclear signaling domain present in many regulatory proteins. The conserved hypothetical protein Rv1894c has weak similarity to some oxidoreductases. Additionally, each Tn mutant tested containing an interruption of a member of the DosR regulon (Rv0079, Rv0081, Rv0569, Rv1736c, Rv2029c, Rv2031c, Rv2626c, and Rv3134c) was attenuated under hypoxia, further corroborating the importance of the DosR regulon in M. tuberculosis adaptation to hypoxia.
Consistent with greater hypoxia in the mouse hollow fiber model, more hypoxia-attenuated mutants were detected in this model relative to the mouse and guinea pig aerosol models. Surprisingly, the guinea pig model was not superior to the standard mouse model in detecting such mutants. One explanation is potential variability in pathology and tissue microenvironments throughout guinea pig lungs, such that distinct bacillary populations experience different oxygen tensions. Another possibility is that granuloma caseation was not complete at the output sample time points selected, and that more hypoxia-attenuated mutants would be detected at later time points.
The findings of metronidazole inactivity in the murine model of TB [12, 13] have been criticized by proponents of the antituberculous activity of the drug, since mouse TB lesions do not achieve the degree of hypoxia necessary for drug activation [14, 15]. In this study, we report no antituberculous activity of metronidazole in a novel in vivo granuloma model of M. tuberculosis dormancy, despite immunohistochemical and mutant survival evidence of tissue hypoxia. There are several potential explanations for our results. Since intra-fiber metronidazole concentrations were not directly measured, it is possible that intra-fiber bacilli received suboptimal drug exposures. In this study, isoniazid and rifampin showed activity against intra-fiber bacilli, suggesting bioavailability of these drugs following gavage. The oral metronidazole dose we used (100 mg/kg) equals or exceeds that used in previous murine TB studies [12, 13], and is greater than that shown to have activity against Bacteroides fragilis and Neisseria gonorrhoeae [39, 40] subcutaneous abscesses in mice, suggesting bioavailability of the drug in the subcutaneous space following oral administration. Alternatively, metronidazole may have poor penetration into granulomatous lesions. In that case, however, the drug would be expected to have minimal activity against persistent bacilli in human TB infection, since these organisms are postulated to inhabit the caseous and necrotic cores of granulomas. Finally, tissue oxygen levels in the mouse hollow fiber model may be microaerophilic, but not sufficiently hypoxic to permit reductive activation of metronidazole.
It remains to be conclusively demonstrated if persistent bacilli in human TB infection dwell in caseous and necrotic lesions, and whether these lesions are sufficiently hypoxic to permit metronidazole activation. Ultimately, the utility of metronidazole as an antituberculous drug can only be addressed directly in a prospective, randomized clinical trial.
Supplementary Material
Acknowledgments
This work was supported by NIH grants: AI043846, AI37856, AI36973, AI007608, and contract AI30036 to William R. Bishai.
NIH grant: AI064229 to Petros C. Karakousis
Potts Memorial Foundation: Fellowship to Lee G. Klinkenberg.
Footnotes
The authors of this manuscript have no conflicts of interest to report.
Portions of this work were presented at: 46th annual meeting of ICAAC poster number B-1321
2007 Keystone Symposia Tuberculosis poster numbers 260 and 267.
45th annual meeting of IDSA poster number 343.
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Lillebaek T, Dirksen A, Baess I, Strunge B, Thomsen VO, Andersen AB. Molecular evidence of endogenous reactivation of Mycobacterium tuberculosis after 33 years of latent infection. J Infect Dis. 2002;185:401–404. doi: 10.1086/338342. [DOI] [PubMed] [Google Scholar]
- 3.Tufariello JM, Chan J, Flynn JL. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect Dis. 2003;3:578–590. doi: 10.1016/s1473-3099(03)00741-2. [DOI] [PubMed] [Google Scholar]
- 4.Nuermberger E, Bishai WR, Grosset JH. Latent tuberculosis infection. Semin Respir Crit Care Med. 2004;25:317–336. doi: 10.1055/s-2004-829504. [DOI] [PubMed] [Google Scholar]
- 5.Opie EL, Aronson JD. Tubercle bacilli in latent tuberculous lesions and in lung tissue without tuberculous lesions. Arch Pathol Lab Med. 1927:1–21. [Google Scholar]
- 6.Adler JJ, Rose DN. Transmission and Pathogenesis of Tuberculosis. In: Rom WNGS, editor. Tuberculosis. 1. Boston: Little, Brown and Company; 1996. p. 1002. [Google Scholar]
- 7.Canetti G. The tubercle bacillus in the pulmonary lesion of man; histobacteriology and its bearing on the therapy of pulmonary tuberculosis. American rev. New York: Springer Pub. Co.; 1955. [Google Scholar]
- 8.Wayne LG, Hayes LG. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun. 1996;64:2062–2069. doi: 10.1128/iai.64.6.2062-2069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wayne LG. Synchronized replication of Mycobacterium tuberculosis. Infect Immun. 1977;17:528–530. doi: 10.1128/iai.17.3.528-530.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wayne LG, Sramek HA. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1994;38:2054–2058. doi: 10.1128/aac.38.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards DI. Nitroimidazole drugs--action and resistance mechanisms. I. Mechanisms of action. J Antimicrob Chemother. 1993;31:9–20. doi: 10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- 12.Brooks JV, Furney SK, Orme IM. Metronidazole therapy in mice infected with tuberculosis. Antimicrob Agents Chemother. 1999;43:1285–1288. doi: 10.1128/aac.43.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhillon J, Allen BW, Hu YM, Coates AR, Mitchison DA. Metronidazole has no antibacterial effect in Cornell model murine tuberculosis. Int J Tuberc Lung Dis. 1998;2:736–742. [PubMed] [Google Scholar]
- 14.Aly S, Wagner K, Keller C, et al. Oxygen status of lung granulomas in Mycobacterium tuberculosis-infected mice. J Pathol. 2006 doi: 10.1002/path.2055. [DOI] [PubMed] [Google Scholar]
- 15.Tsai MC, Chakravarty S, Zhu G, et al. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8:218–232. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 16.Karakousis PC, Yoshimatsu T, Lamichhane G, et al. Dormancy phenotype displayed by extracellular Mycobacterium tuberculosis within artificial granulomas in mice. J Exp Med. 2004;200:647–657. doi: 10.1084/jem.20040646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voskuil MI, Schnappinger D, Visconti KC, et al. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med. 2003;198:705–713. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamichhane G, Zignol M, Blades NJ, et al. A postgenomic method for predicting essential genes at subsaturation levels of mutagenesis: application to Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2003;100:7213–8. doi: 10.1073/pnas.1231432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin EJ, Akerley BJ, Novik VN, Lampe DJ, Husson RN, Mekalanos JJ. In vivo transposition of mariner-based elements in enteric bacteria and mycobacteria. Proc Natl Acad Sci USA. 1999;96:1645–1650. doi: 10.1073/pnas.96.4.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muttucumaru DG, Roberts G, Hinds J, Stabler RA, Parish T. Gene expression profile of Mycobacterium tuberculosis in a non-replicating state. Tuberculosis (Edinb) 2004;84:239–46. doi: 10.1016/j.tube.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Park HD, Guinn KM, Harrell MI, et al. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol. 2003;48:833–843. doi: 10.1046/j.1365-2958.2003.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherman DR, Voskuil M, Schnappinger D, Liao R, Harrell MI, Schoolnik GK. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha -crystallin. Proc Natl Acad Sci USA. 2001;98:7534–7539. doi: 10.1073/pnas.121172498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voskuil MI, Visconti KC, Schoolnik GK. Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis (Edinb) 2004;84:218–27. doi: 10.1016/j.tube.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Rosenkrands I, Slayden RA, Crawford J, Aagaard C, Barry CE, III, Andersen P. Hypoxic response of Mycobacterium tuberculosis studied by metabolic labeling and proteome analysis of cellular and extracellular proteins. J Bacteriol. 2002;184:3485–3491. doi: 10.1128/JB.184.13.3485-3491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollingshead MG, Alley MC, Camalier RF, et al. In vivo cultivation of tumor cells in hollow fibers. Life Sci. 1995;57:131–41. doi: 10.1016/0024-3205(95)00254-4. [DOI] [PubMed] [Google Scholar]
- 26.Xu ZQ, Hollingshead MG, Borgel S, Elder C, Khilevich A, Flavin MT. In vivo anti-HIV activity of (+)-calanolide A in the hollow fiber mouse model. Bioorg Med Chem Lett. 1999;9:133–8. doi: 10.1016/s0960-894x(98)00713-6. [DOI] [PubMed] [Google Scholar]
- 27.Ausubel FM. Current protocols in molecular biology. New York: Greene Pub. Associates and Wiley-Interscience: J. Wiley; 1991. [Google Scholar]
- 28.Lamichhane G, Tyagi S, Bishai WR. Designer arrays for defined mutant analysis to detect genes essential for survival of Mycobacterium tuberculosis in mouse lungs. Infect Immun. 2005;73:2533–2540. doi: 10.1128/IAI.73.4.2533-2540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arteel GE, Thurman RG, Yates JM, Raleigh JA. Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br J Cancer. 1995;72:889–95. doi: 10.1038/bjc.1995.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacManus MP, Maxwell AP, Abram WP, Bridges JM. The effect of hypobaric hypoxia on misonidazole binding in normal and tumour-bearing mice. Br J Cancer. 1989;59:349–52. doi: 10.1038/bjc.1989.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenner RG, Young RA. Insights into host responses against pathogens from transcriptional profiling. Nat Rev Microbiol. 2005;3:281–94. doi: 10.1038/nrmicro1126. [DOI] [PubMed] [Google Scholar]
- 33.Lenaerts AJ, Hoff D, Aly S, et al. Location of persisting mycobacteria in a Guinea pig model of tuberculosis revealed by r207910. Antimicrob Agents Chemother. 2007;51:3338–45. doi: 10.1128/AAC.00276-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–80. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hewinson RG, Michell SL, Russell WP, McAdam RA, Jacobs WR., Jr Molecular characterization of MPT83: a seroreactive antigen of Mycobacterium tuberculosis with homology to MPT70. Scand J Immunol. 1996;43:490–9. doi: 10.1046/j.1365-3083.1996.d01-78.x. [DOI] [PubMed] [Google Scholar]
- 36.Lalande V, Truffot-Pernot C, Paccaly-Moulin A, Grosset J, Ji B. Powerful bactericidal activity of sparfloxacin (AT-4140) against Mycobacterium tuberculosis in mice. Antimicrob Agents Chemother. 1993;37:407–13. doi: 10.1128/aac.37.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karakousis PC, Williams EP, Bishai WR. Altered expression of isoniazid-regulated genes in drug-treated dormant Mycobacterium tuberculosis. J Antimicrob Chemother. 2008;61:323–31. doi: 10.1093/jac/dkm485. [DOI] [PubMed] [Google Scholar]
- 38.Boon C, Dick T. Mycobacterium bovis BCG response regulator essential for hypoxic dormancy. J Bacteriol. 2002;184:6760–7. doi: 10.1128/JB.184.24.6760-6767.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brook I. Metronidazole and spiramycin therapy of mixed Bacteroides spp. and Neisseria gonorrhoeae infection in mice. Chemotherapy. 1989;35:105–12. doi: 10.1159/000238655. [DOI] [PubMed] [Google Scholar]
- 40.Joiner KA, Lowe BR, Dzink JL, Bartlett JG. Antibiotic levels in infected and sterile subcutaneous abscesses in mice. J Infect Dis. 1981;143:487–94. doi: 10.1093/infdis/143.3.487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.