Abstract
We have previously shown that the HSV-2 anti-apoptotic protein ICP10PK is delivered by the replication incompetent virus mutant ΔRR and prevents kainic acid (KA)-induced epileptiform seizures and neuronal cell loss in the mouse and rat models of temporal lobe epilepsy. The present studies used ΔRR and the ICP10PK deleted virus mutant ΔPK, to examine the mechanism of neuroprotection. ΔRR-infected neuronal cells expressed a chimeric protein in which ICP10PK was fused in frame to LacZ (p175) while retaining ICP10PK kinase activity. ΔPK-infected neuronal cells expressed a mutant ICP10 protein that is deleted in PK domain and is kinase negative (p95). p175 and p95 were expressed in CA3 (86±3%) and CA1 (69±7%) cells from ΔRR or ΔPK-infected organotypic hippocampal cultures (OHC) and 80–85% of the ICP10 positive cells co-stained with antibody to βIII Tubulin (neuronal marker). ΔRR, but not ΔPK, inhibited KA-induced cell death and caspase-3 activation in CA3 neurons and inhibition was seen whether ΔRR was delivered 2 days before, or 2 days after KA administration (95 % neuroprotection). Neuroprotection was associated with ERK and Akt activation and was abrogated by simultaneous treatment with the MEK (U0126) and PI3-K (LY294002) inhibitors. Increased expression of the anti-apoptotic protein Bag-1 and the transcription factor CREB, and decreased expression of the pro-apoptotic protein Bad were associated with ΔRR-mediated neuroprotection and the surviving neurons retained normal synaptic function. The data indicate that ΔRR is a promising platform for neuroprotection from excitotoxic injury.
Keywords: ICP10PK, kainic acid, organotypic hippocampal cultures, apoptosis
1. INTRODUCTION
Neuronal apoptosis is a component of both acute and chronic neurodegenerative diseases. It is a tightly regulated, energy dependent, irreversible process that is mediated by cysteine proteases (caspases) which are activated by the sequential cleavage of inactive zymogens (Thornberry and Lazebnik, 1998). In the central nervous system (CNS), apoptosis is triggered by a variety of stress conditions including virus infection, ischemia, withdrawal of neurotrophic factors, and excitotoxin release. Cascades of neuronal death emerge gradually, providing a period available for therapeutic intervention. Several strategies were proposed to interrupt the apoptotic cascade, including gene therapy with growth factors or anti-apoptotic proteins (Natsume et al., 2001; Spencer et al., 2001; Harvey et al., 2003). However, these genes had relatively narrow neuroprotective profiles, neuronal survival was often limited and did not correlate with retention of functional integrity, and some strategies were associated with detrimental effects (Wiessner et al., 1999; Dumas et al., 2000; Spencer et al., 2001; Manoonkitiwongsa et al, 2004). The identification of genes with therapeutically effective neuroprotective profiles and the generation of optimal delivery vectors present considerable clinical challenges that have limited the success of this therapeutic approach.
Survival pathways play pivotal roles in apoptosis inhibition. One major pathway initiates with activation of the membrane bound small GTPase Ras, which, in turn, activates the downstream kinases mitogen activated protein kinase kinase (MEK) and extracellular signal regulated kinase (ERK). In neurons, the activated MEK/ERK pathway can override pro-apoptotic signals (Anderson et al, 1999; Perkins et al., 2003), and ERK activation in the hippocampus is associated with long term potentiation (LTP), an important component of learning and memory (Sweatt, 2001). Another Ras initiated pathway results from binding of Ras-GTP to the catalytic subunit of phosphatidyl-inositol-3-kinase (PI3-K) leading to Akt activation (Hemmings, 1997; Rubio et al., 1997). Differential activation of these two pathways is cell type and stimulus specific (Colucci-D’Amato et al, 2003; Rössler et al, 2004).
The large subunit of HSV-2 ribonucleotide reductase (R1, also known as ICP10) is a chimera that contains a serine-threonine protein kinase function, which is located within its amino-terminal domain (known as ICP10PK) (Smith et al., 1998). ICP10PK has anti-apoptotic activity in virus infected neuronal cells, which seems to be required for virus latency reactivation (Aurelian and Smith, 2000; Gober et al., 2005). In primary hippocampal cultures, ICP10PK activates the Ras-dependent Raf-1/MEK/ERK pathway, thereby overriding apoptosis triggered by infection with HSV-1 (Perkins et al., 2003). Recent studies indicate that a replication compromised HSV-2 vector, which retains ICP10PK (known as ΔRR), prevents kainic acid (KA) induced epileptiform seizures and neuronal loss, in both the rat and mouse models (Laing et al., 2006). The growth compromised HSV-2 vector ΔPK, which is deleted in ICP10PK does not inhibit KA-induced disease/apoptosis, indicating that neuroprotection is due to ICP10PK. However, the mechanism of neuroprotection in this paradigm of neuronal cell death, is still unknown. The studies described in this report were designed to address this question.
2. RESULTS
2.1 The ICP10 mutated proteins p175 and p95 are expressed in neuronal cells, but only p175 has kinase activity
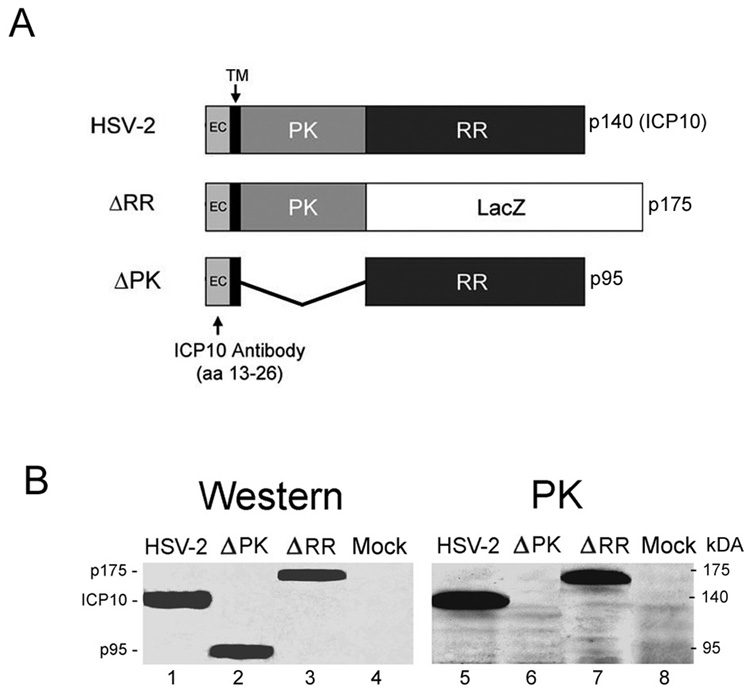
The large subunit of the HSV-2 ribonucleotide reductase (RR) (also known as R1 or ICP10) is a 140kDa protein that consists of an amino-terminal domain which has protein kinase (PK) activity and a carboxy-terminal domain, which has RR activity. The PK domain is preceded by a transmembrane (TM) domain and a short extracellular (EC) domain that retains amino acids 13–26, which are recognized by the ICP10 antibody (Luo et al., 1991; Smith et al., 1994; Smith et al., 1998). In ΔRR, the RR domain of ICP10 was replaced with LacZ, which was fused in frame with ICP10PK, giving rise to a 175kDa protein (p175). P175 retains the TM and EC domains of the wild type ICP10 protein and it is under the direction of the authentic ICP10 promoter. In ΔPK, the PK domain of ICP10 was deleted, giving rise to a 95kDa protein (p95), which also retains the authentic EC and TM domains and is driven by the same wild type ICP10 promoter (Smith et al., 1998). This is schematically represented in Fig. 1A.
Fig. 1. Expression and kinase activated of mutant ICP10 proteins in neuronal cells.
A. Schematic representation of the ICP10 and mutant proteins. The wild type ICP10 protein expressed by HSV-2 is a 140kDa chimera that contains an amino-terminal PK domain and a carboxy-terminal RR domain. In ΔRR, the RR domain was replaced with the β–galactosidase gene (LacZ) which was fused in frame to the ICP10PK domain, giving rise to a 175kDa protein (p175). In ΔPK, the PK domain of ICP10 was deleted giving rise to a 95kDa protein (p95). All three proteins (ICP10, p175 and p95) retain the transmembrane (TM) and extracellular (EC) domains and amino acids 13–26, which are recognized by the ICP10 antibody. B. SK-NSH cells infected with HSV-2, ΔRR, ΔPK or PBS (mock-infected) were collected at 18 hrs after infection and cell extracts were assayed for protein expression (western) and ICP10 kinase activity (PK) using immunoblotting and immunocomplex kinase assays with ICP10 antibody, respectively.
To confirm that p175 and p95 are expressed in neuronal cells, SK-NSH cultures were given ΔRR or ΔPK (10 PFU/cell) and cell extracts obtained at 18 hrs post infection were immunoblotted with the ICP10 antibody. Cells similarly infected with HSV-2 or mock-infected with PBS served as controls. A 140-kDa protein, consistent with the wild type ICP10 protein (Smith et al., 1998) was recognized by the ICP10 antibody in HSV-2 infected cells (Fig. 1B, lane 1). In cells infected with ΔPK, the antibody recognized a 95-kDa protein (p95) (Fig. 1B, lane 2) and in cells infected with ΔRR, it recognized a 175-kDa protein (p175) (Fig. 1A, lane 3). Mock-infected cells were negative (Fig. 1B, lane 4), as was preimmune IgG (data not shown). Immunocomplex PK assays with ICP10 antibody identified a 140-kDa phosphorylated protein consistent with the autophosphorylated ICP10 in HSV-2 infected cells (Fig. 1B, lane 5). Kinase activity was retained by p175, which was also autophosphorylated (Fig. 1B, lane 7), but not by p95, as evidenced by the absence of phosphorylated proteins in the ΔPK-infected SK-NSH cells (Fig. 1B, lane 6). Phosphorylated proteins were not seen in the immunocomplex PK assays using extracts from mock- infected cells (Fig. 1, lane 8). Consistent with previous reports for ΔRR or ΔPK infected Vero cells (Smith et al., 1998), p95 retained ribonucleotide reductase (RR) activity, also in SK-NSH cells, but p175 was RR negative (data not shown). The data support previous conclusions that the PK and RR domains of ICP10 function independently of each other (Smith et al., 1998), and indicate that the p175 protein expressed by ΔRR retains the ICP10 kinase activity, also in neuronal cells.
2.2 The ICP10 mutant protein p175 and p95 are expressed in the absence of virus replication
We have previously shown that the RR and PK domains of ICP10 are independently required for virus growth in non-replicating cells, including neurons, and the ΔRR and ΔPK viruses are replication incompetent in OHC (Smith et al., 1998; Perkins et al., 2002; Laing et al., 2006). To examine whether p175 and p95 (repectively expressed by ΔRR and ΔPK) are expressed in OHC in the absence of virus replication, OHC were infected with 105 pfu of ΔRR or ΔPK, or mock infected with PBS, and stained with the fluorescent LacZ substrate C12FDG (live cells) or ICP10 antibody (fixed cells) at 1, 2 and 7 days after infection. The % staining cells was calculated relative to DAPI, as described in materials and methods. Because LacZ was fused in frame with ICP10PK to generate p175, C12FDG staining reflects expression of ICP10PK. The ΔRR- infected OHC stained with C12FDG (Fig. 2A, panel 1), but C12FDG staining was not seen in mock-infected (Fig. 2A, panels 3,4) or ΔPK-infected OHC. Staining was localized primarily in the CA3 and CA1 fields (86 ± 3% and 68 ± 7% staining cells, respectively) (Fig. 2A, panel 4). Both ΔRR and ΔPK infected OHC stained with ICP10 antibody, and staining was again primarily localized in the CA3 and CA1 fields, as shown for the ΔPK infected cultures in Fig 2A, Panel 2.
Fig. 2. ΔRR and ΔPK-infected OHC express mutated ICP10 proteins in the absence of virus replication.
A, OHC were infected with 105 pfu of ΔRR (panel 1) or ΔPK (panel 2) or mock infected with PBS (panel 3, 4) and the ΔRR (panel 1) and mock (panel 3)-infected OHC were stained with the β-galactosidase substrate, C12-fluorescein di-β-D-galactopyranoside (C12FDG), which identifies the ICP10PK-LacZ fusion protein p175, on day 2 post infection (p.i). C12FDG staining was followed by treatment with 5% triton X-100 and staining with DAPI (panel 4). Similar staining patterns were obtained for FITC-labeled ICP10 antibody. ΔPK-infected slices were examined for p95 expression by staining with FITC-labeled ICP10 antibody (panel 3). The mean % p175+ cells ± SEM in the ΔRR-infected OHC was calculated for the dentate gyrus (DG), CA3 and CA1 fields (panel 5) relative to DAPI staining cells. B, Duplicate of ΔRR and ΔPK-infected OHC from A were stained with ethidium homodimer (measures cell death) on day 4 p.i. Staining was followed by treatment with 5% triton X-100 and staining with DAPI. C, Extracts of OHC mock infected or infected with ΔRR (105 pfu) for 7 days were immunoblotted with ICP10 antibody. The blots were stripped and sequentially re-probed with antibodies to VP5 (viral capsid protein) and actin. Extracts of 24hrs HSV-2 infected Vero cells (moi = 2) served as control. D, OHC infected with ΔRR as in A, were stained with Alexa-fluor labeled ICP10 antibody or preimmune IgG and FITC-labeled antibodies to β-tubulin III (Neurons), CD11b (Microglia), or GFAP (Astrocytes) at 48hrs p.i. Blue stain is DAPI.
To confirm that staining reflects expression of p175 and document that it is independent of virus replication, extracts of OHC collected at 2 and 7 days were immunoblotted with antibodies to ICP10 or the major capsid protein VP5, the expression of which depends on virus DNA replication (Roizman and Sears, 1996). p175 was robustly expressed in the ΔRR-infected OHC as late as 7 days p.i, but the cultures were negative for VP5 (Fig. 2B). The failure to detect VP5 is not an artifact due to re-probing of stripped gels, as evidenced by additional stripping and re-probing with actin-specific antibody. It is also not due to problems with the VP5 antibody, because both VP5 and ICP10 were expressed in extracts from cultures infected with HSV-2 (Fig 2B). Collectively, the data confirm previous reports that the mutated ICP10 proteins expressed by the ΔRR and ΔPK viruses are expressed both in OHC and in vivo, in the absence of virus replication (Laing et al., 2006). This presumably reflects the use of the authentic ICP10 promoter to drive these proteins, because it is regulated by AP-1 and is independent of other viral genes (Wymer et al., 1989; Laing et al., 2006),).
2.3 p175 and p95 are expressed in CA3 and CA1 neurons
Having seen that the mutated ICP10 protein p175 (ΔRR) and p95 (ΔPK) are expressed in the CA3 and CA1 fields, we wanted to know whether expression is restricted to neurons. OHC were infected with ΔRR or ΔPK and stained by double immunofluorescence with FITC-labeled ICP10 antibody and Alexa-Fluor labeled antibodies to βIII Tubulin (neurons), CD11b (microglia) or GFAP (astrocytes). As summarized in Fig. 2C for ΔRR infected OHC, the majority of the cells staining with ICP10 antibody (80–85%) also stained with antibody to βIII Tubulin. By contrast, ICP10 staining was only seen in 12–15% of the CD11b+ cells and virtually all the GFAP+ cells (98–99%) were ICP10 negative. Preimmune serum was negative (Fig. 2C). The data indicate that p175 and p95 are expressed primarily in neurons.
2.4 ΔRR delivered ICP10PK protects from KA-induced excitotoxicity
Having previously seen that ΔRR, but not ΔPK, has neuroprotective activity in animal models of KA-induced temporal lobe epilepsy (Laing et al., 2006), we wanted to confirm that protection is mediated by ICP10PK, which is retained by the fusion protein p175 (Fig. 1A,B). OHC were treated with KA (3 µM) in the presence or absence of ΔRR (or ΔPK control) and examined for neuronal cell death by staining with ethidium homodimer. Compared to untreated OHC, KA caused a time-dependent increase in the % CA3 dead cells (4 ± 1% and 75 ± 8 % cells, respectively on day 3 post treatment) (Fig 3A). ΔRR infection (105 pfu) 2 days before KA, caused a significant reduction in the % dead cells (3 ± 1%; p < 0.001, by ANOVA) (Fig. 3B,D). Protection was due to ICP10PK because: (i) 81 ± 7 % of the CA3 cells in the same cultures stained with C12FDG and there was no co-localization between p175 expression and cell death (Fig. 3B), and (ii) protection was not seen in OHC infected with ΔPK (70 ± 8% cell death), although staining with ICP10 antibody indicated that p95 was expressed as well as p175 (Fig. 3C,D).
Fig. 3. ΔRR protects OHC from KA-induced cell death.
A, OHC untreated (no treat) or treated with KA (3 µM) for 24 hrs, were washed, re-fed with fresh growth medium, and stained with ethidium homodimer immediately thereafter (day 1) and at daily intervals thereafter (days 2, 3). Cell death was quantified for the CA3 field as described in materials and methods and the results are expressed as % dead cells ± SEM. B, OHC infected with ΔRR (105 pfu) or mock-infected with PBS were exposed to KA (3µM; 24hrs) and examined for cell death on day 3 post KA as in A (day 5 after ΔRR infection). Sections were stained with C12FDG (p175) and eithidium homodimer (cell death) and the images were digitally merged. DAPI was used as control. C, Duplicate cultures infected with ΔPK (105 pfu) and treated with KA were stained with ethidium homodimer, and DAPI on day 3 post KA. D, The levels of cell death in OHC from B and C were quantified for the CA3 field and the results are expressed as % dead cells ± SEM.
2.5 ΔRR inhibits KA-induced caspase-3 activation
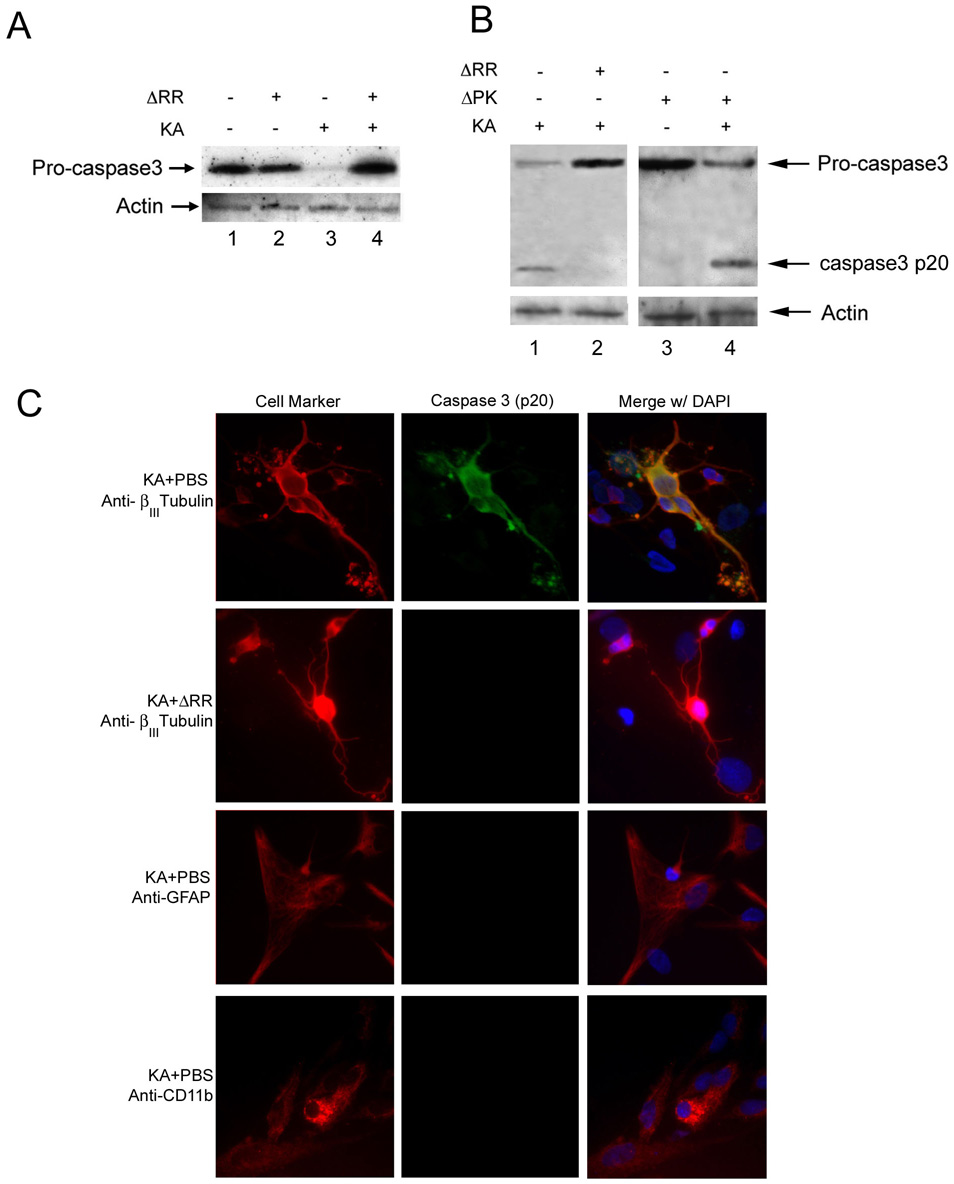
Previous studies had shown that KA causes caspase 3–dependent neuronal cell apoptosis (Faherty et al., 1999; Niquet and Wasterlain, 2004). Two series of experiments were done in order to examine whether the neuroprotective activity of ICP10PK is through apoptosis inhibition. In the first series of experiments, extracts from OHC treated or not with KA in the presence or absence of ΔRR or ΔPK were examined for caspase activation by immunoblotting with an antibody that recognizes both the inactive zymogen (procaspase-3) and its cleaved p20 product. Caspase-3 activation was seen in OHC treated with KA, as evidenced both by the loss of procaspase-3 (Figs. 4A, lane 3; 4B, lane 1), which is indicative of cleavage/activation (Schroeter et al., 2001), and the appearance of the caspase-3p20 cleavage product (Fig. 4B, lane 1). Caspase-3 was also activated in OHC treated with KA in the presence of ΔPK (Fig. 4B, lane 4), but not in OHC given KA in the presence of ΔRR (Fig. 4A, lane 4; 4B, lane 2). Caspase-3 was not activated in OHC given ΔRR or ΔPK alone (Fig. 4A, lane 2; 4B, lane 3). In a second series of experiments, duplicate OHC cultures were stained by double immunofluorescence with Alexa–Fluor labeled antibodies to βIII Tubulin, CD11b or GFAP and FITC-labeled antibody to activated caspase-3 (caspase-3p20). Consistent with previous reports that KA causes caspase-dependent apoptosis in neuronal cells (Faherty et al., 1999; Niquet and Wasterlain, 2004), all the βIII Tubulin+ cells (neurons) in the KA treated OHC, also stained with caspase-3p20 antibody (Fig. 4C). This percentage was significantly decreased in OHC given KA with ΔRR (4± 1%) (Fig. 4C), but not ΔPK (96 ± 3%). Collectively, the data indicate that ICP10PK inhibits KA-induced neuronal cell apoptosis.
Fig. 4. ΔRR, but not ΔPK inhibits KA-induced caspase-3 activation.
A. OHC were infected with ΔRR or ΔPK (105 pfu) or mock-infected with PBS with or without KA (3 µM; 24 hr) and cell extracts obtained 48hrs after initiation of KA treatment were separated by SDS-PAGE and transferred to nitrocellulose membranes and immunoblotted with caspase-3 antibody. Blots were stripped and re-probed with actin antibody. B. Duplicates of the assays in A were transferred to PVDF membranes and immunoblotted with caspase-3 antibody. C. OHC mock-infected with PBS or ΔRR (105 pfu) together with KA were stained in double immunofluorescence with FITC-labeled antibody to activated caspase-3 (caspase-3p20) and Alexa-Fluor labeled antibodies to βIII Tubulin (neurons), CD11b (microglia) or GFAP (astrocytes). Blue stain is DAPI.
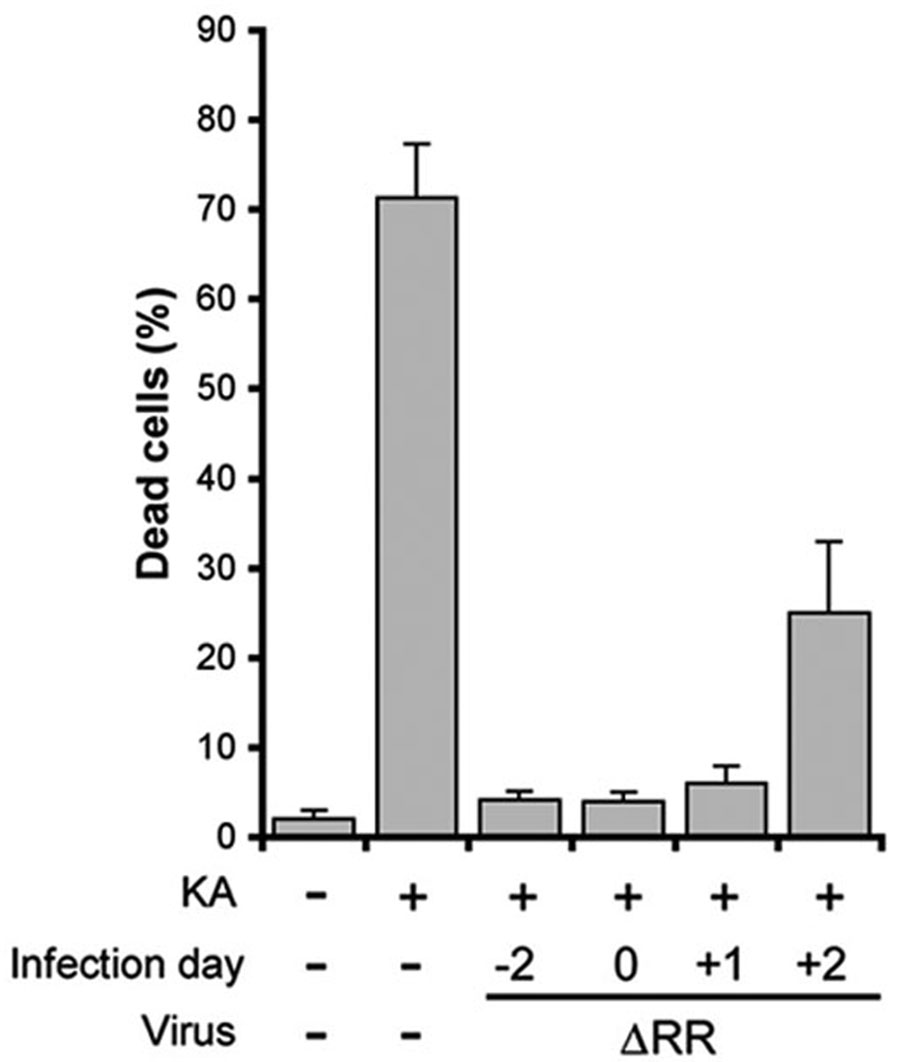
2.6 ΔRR has a relatively wide therapeutic window
To determine the therapeutic window of ΔRR, OHC were infected with ΔRR on day −2, 0, +1, or +2 relative to the initiation of KA treatment (day 0) and examined for cell death 3 days later by staining with ethidium homodimer. ΔRR infection on day −2 indicates that virus was added 2 days prior to the addition of KA, while infection on day −2 signifies virus was added 2 days after addition of KA. In all cultures, KA was given at 3 µM for 24 hrs. In mock infected cultures, 71 ± 5% of the cells in the CA3 field died in response to KA while only background levels of cell death (5 ±1 %) were seen in OHC infected with ΔRR on day −2. ΔRR infection on day 0 (together with KA) or on day +1 also resulted in nearly complete protection from KA-induced cell death (4 ± 1 % and 6 ± 2% dead cells, respectively; ~96% neuroprotection determined as described in materials and methods). Significant levels of neuroprotection (~94 %) were also seen in OHC infected with ΔRR on day +2 (26±9% and 28±3% cell death for KA+ΔRR and background, respectively) (Fig. 5). The data indicate that ΔRR has a relatively wide therapeutic window, with significant levels of neuroprotection still seen as late as 2 days after KA administration. Because neurotoxic stimuli (including KA), induce AP-1 transcription factors (Pennypacker et al., 1995; Gober et al., 2005), the rapid response of the ΔRR–delivered ICP10PK is likely due to the AP-1 responsive ICP10 promoter (Wymer et al., 1992; Zhu and Aurelian 1997).
Fig. 5. ΔRR has a wide therapeutic window.
OHC were infected with ΔRR (105 pfu) on day −2, 0, +1, or +2 relative to initiation of KA treatment. Infection on day −2 indicates virus was added 2 days prior to KA treatment, while infection on day +2 signifies virus was added 2 days after KA treatment. Staining with ethidium homodimer was done on day +3 for all cultures and the data are expressed as % dead cells ± SEM. Neuroprotection was calculated as described in materials and methods relative to background cell death at the time of ΔRR infection and shown in Fig. 3A.
2.7 ΔRR neuroprotection is MEK/ERK and PI3-K/Akt dependent
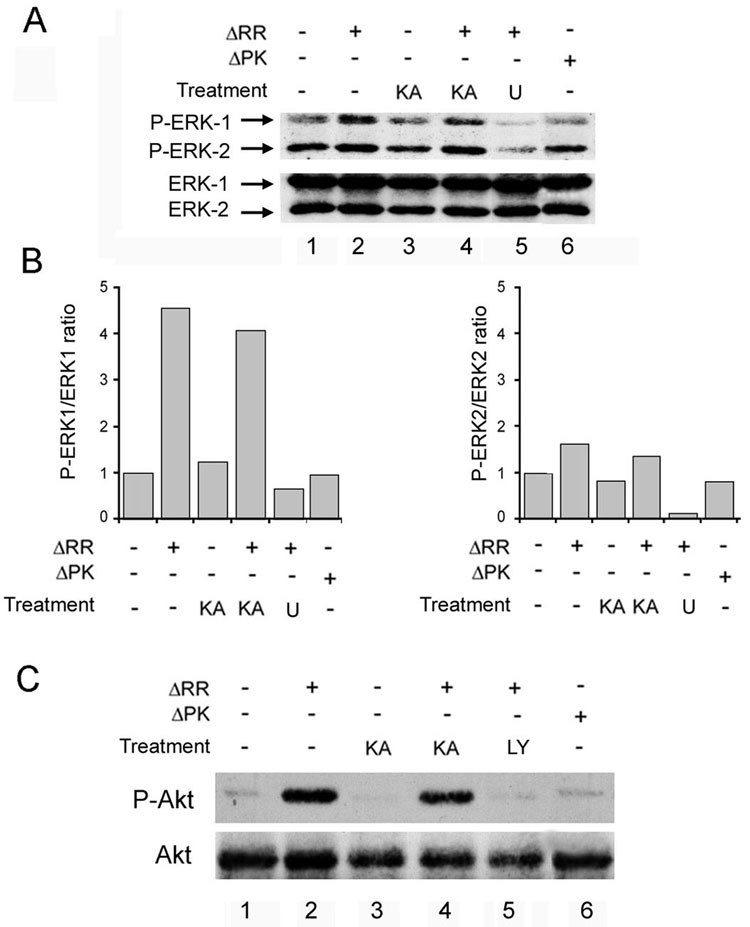
We have previously shown that ICP10PK has anti-apoptotic activity in virus infected hippocampal cultures, though MEK/ERK activation (Perkins et al., 2002; Perkins et al., 2003). However, the regulation of signaling and apoptotic pathways is cell type and stimulus specific (Colucci-D’Amato et al., 2003; Rössler et al., 2003), and recent studies have shown that ERK is also activated by the PI3–K/Akt pathway (Zhuang, 2004). To examine the mechanism of ICP10PK-mediated anti-apoptotic activity in KA-treated OHC, the cultures were infected with ΔRR or ΔPK (105 pfu) together with KA (3 µM) and cultured (24 hrs) in the presence or absence of the MEK inhibitor U0126 (20 µM) or the PI3-K inhibitor LY294002 (100 µM). Cell extracts obtained at this time were immunoblotted with antibody to phosphorylated (activated) ERK 1/2 (P-ERK 1/2) using antibody to total ERK1/2 as control. The results are expressed as P-ERK/ERK ratios determined by densitometric scanning and normalized to the ratio in mock-infected OHC. The levels of P-ERK1 (p44) were significantly higher (4.5-fold) in ΔRR–infected than uninfected cells, both in the presence and absence of KA (Fig 6A, lanes 2,4). Activation was completely inhibited by U0126 (Fig. 6A, lane 5), indicating that it is MEK-dependent. The levels of P-ERK2 (p42) were only minimally increased in ΔRR as compared to mock-infected OHC (less than 2-fold), also subject to U0126 inhibition. Activation was mediated by ICP10PK, because it was not seen in OHC infected with ΔPK (Fig. 6A, lane 6).
Fig. 6. ICP10PK activates the MEK/ERK and PI3K/Akt survival pathways.
A, OHC were infected with ΔRR or ΔPK (105 pfu) (or mock infected) and simultaneously exposed to KA (3µM; 24 hrs). U0126 (U; 20µM) was added at the time of infection and remained in the culture for the entire experiment. Extracts were harvested at 24 hrs p.i. and immunoblotted with antibody to phosphorylated ERK1/2 (P-ERK1/2). The blots were stripped and reprobed with antibody to total ERK1/2. B, Data from A expressed as fold-increase in P-ERK/ERK ratio relative to mock infected OHC for ERK1 and ERK2, respectively. C, ΔRR or mock-infected OHC treated with KA as in A were incubated with LY294002 (LY; 100 µM) at the time of infection and extracts obtained at 24 hrs p.i. were immunoblotted with antibody to phosphorylated Akt (P-Akt). Blots were stripped and re-probed with Akt antibody.
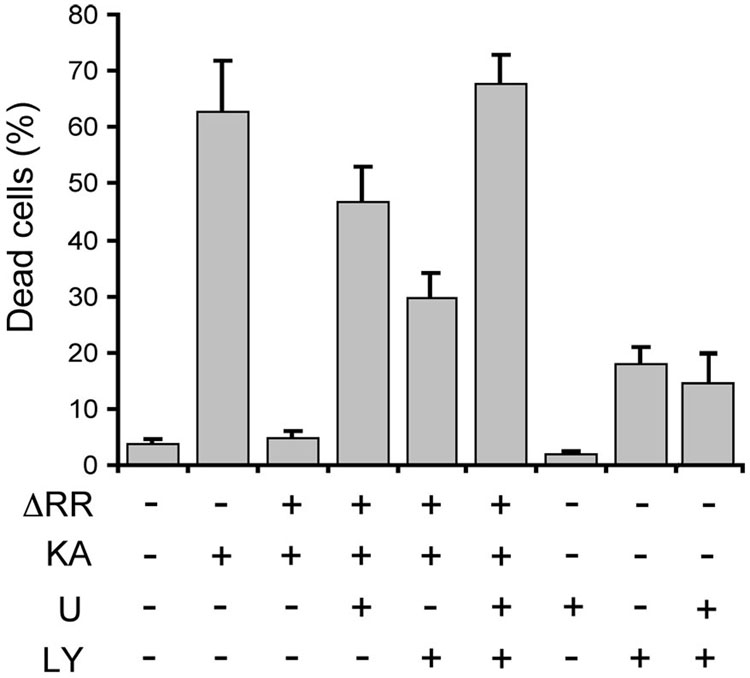
Significantly, when the cell extracts were immunoblotted with antibody to activated (phosphorylated) Akt (using antibody to total Akt as control), we found that the levels of P-Akt were also higher in ΔRR- than mock- (Fig. 6C lanes 2, 4) or ΔPK- (Fig. 6C, lane 6) infected OHC. However, Akt activation was blocked with LY294002 (Fig. 6C, lane 5), indicating that it was PI3-K-dependent. The data indicate that ICP10PK activates both the MEK/ERK and PI3-K/Akt pathways, also in the presence of KA. We conclude that both pathways are involved in ΔRR-mediated neuroprotection, because: (i) cell death was increased by culture (3 days) with U0126 (20 µM) or LY294002 (100µM) (47 ± 5% and 30 ± 4 %, respectively, as compared to 5 ± 1 % in the absence of the inhibitors), and (ii) protection was abrogated only by treatment with both inhibitors (70 ± 8 % and 71 ± 5% cell death for ΔRR + inhibitors and KA alone, respectively). Inhibitor toxicity was not responsible for the loss of neuroprotection, as cell death was not seen in cultures treated with the inhibitors alone or in combination, but in the absence of KA (2±1 %, 13± 5 % and 11±7 % for U0126, LY294002, and U0126+LY294002, respectively) (Fig. 7).
Fig. 7. Activation of the MEK and PI3-K pathways is required for ΔRR neuroprotection.
OHC were infected with ΔRR (105 pfu) or mock infected with PBS and exposed to KA (3µM; 24 hrs). They were washed, re-fed fresh growth medium, and incubated (36 °C) for an additional 48 hrs. U0126 (U; 20µM), LY294002 (LY; 100µM) or both were added at the time of infection and were replenished every 24 hr to ensure adequate inhibition. OHC were stained with ethidium homodimer on day 3 p.i. Cell death was quantified as described in materials and methods and the results are expressed as % dead cells ± SEM.
2.8 Activated MEK and PI3-K pathways upregulate Bag-1 and downregulate Bad expression
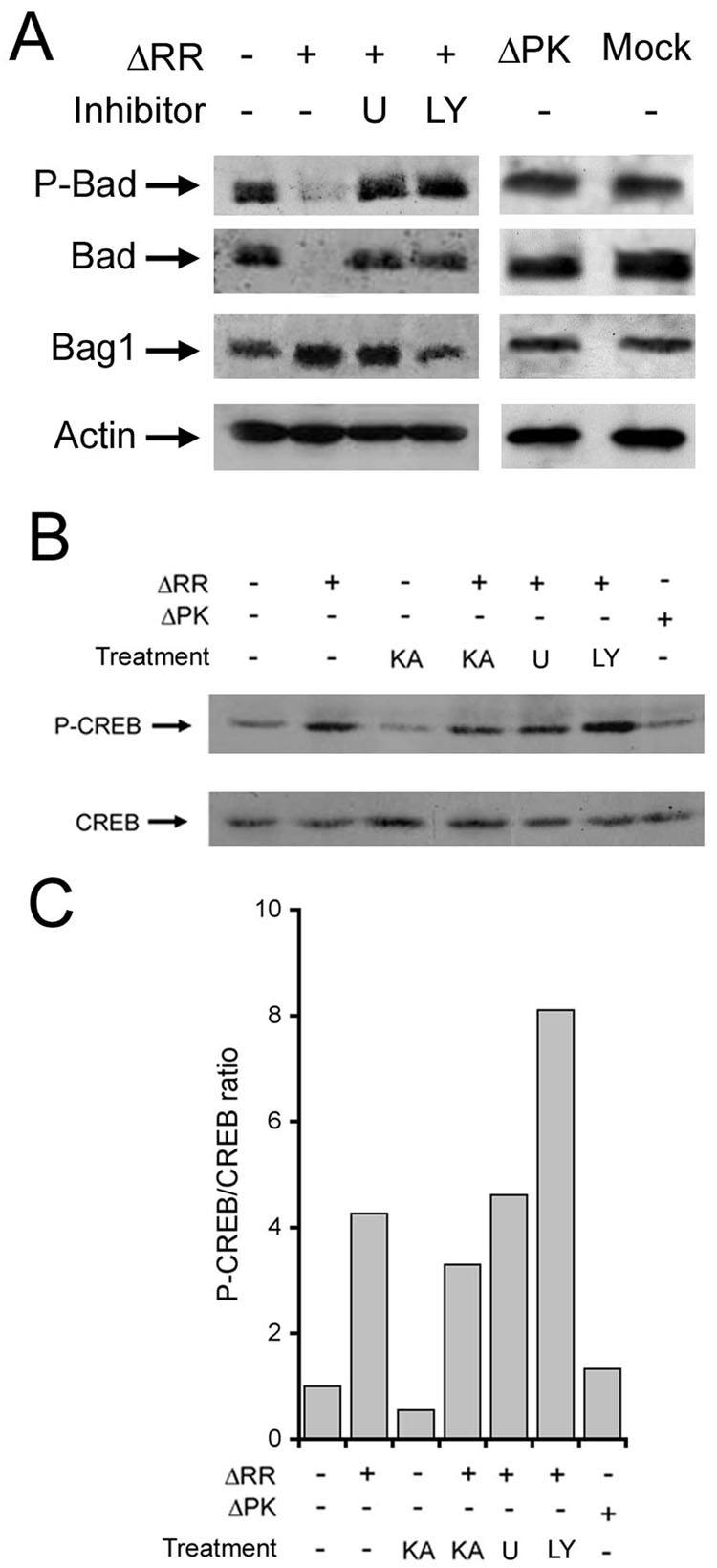
Signaling pathways impart survival by transcription-dependent and/or independent mechanisms (Zha et al., 1996; Bossy-Wetzel et al., 1997). To further elucidate the mechanism of ICP10PK anti-apoptotic activity, OHC were infected with ΔRR (105 pfu) or mock-infected with PBS (24 hrs; 36 °C) in the presence or absence of U0126 (20 µM) or LY294002 (100 µM) and cell extracts were immunoblotted with antibodies to Bag-1, Bad or phosphorylated Bad (P-Bad). These molecules were targeted because: (i) Bag-1 is a Bcl-2 and c-Raf-1 regulatory protein (Wang et al., 1996; Tankayama et al., 1995) that promotes survival in virus infected primary hippocampal cultures (Perkins et al., 2003), (ii) Bad is a pro-apoptotic Bcl-2 family member that is involved in KA-induced caspase-3 activation and apoptosis (Niquet and Wasterlain 2004), and (iii) Bad inhibition (through phosphorylation on Ser112) was associated with neuroprotection (Bergmann, 2002). OHC infected with ΔPK were studied in parallel and served as control. As summarized in Fig. 8A, Bad and P-Bad were seen in mock-infected OHC, but they were virtually absent from OHC infected with ΔRR. Bad expression was restored by treatment with U0126 or LY294002, indicating that its inhibition is MEK and PI3-K-dependent. By contrast to Bad, the levels of Bag-1 were increased by ΔRR and the increase was not seen in cultures treated with LY294002, indicating that it is PI3-K-dependent. Bad and Bag-1 expression were not altered by ΔPK (Fig. 8A). Bad phosphorylation was not affected, independent of its expression. Collectively, the data indicate that ICP10PK downregulates Bad and upregulates Bag-1 expression through activation of the MEK and/or PI3-K pathways.
Fig. 8. ΔRR modulates Bad and Bag-1 expression through activation of the MEK and PI3-K pathways and activates CREB independent of these pathways.
A, OHC were infected with ΔRR or ΔPK (105 pfu) or mock infected with PBS and cultured with U0126 (U; 20µM) or LY294002 (LY; 100 µM). Extracts obtained at 24 hrs p.i. were immunoblotted with antibody to phosphorylated Bad (P-Bad). The blots were stripped and sequentially re-probed with antibodies to Bad, Bag-1 and actin. B, Duplicates of the extracts in Fig 6 were immunobloted with antibody to phosphorylated CREB (P-CREB). Blots were stripped and re-probed with CREB antibody. C, Data from B expressed as fold increase in P-CREB/CREB ratios relative to mock-infected OHC.
2.9 ICP10PK activates CREB independent of the MEK and PI3-K pathways
Because CREB activation had been previously shown to be involved in neuronal function (Davis et al., 1996), we wanted to know whether it is activated in ΔRR–infected OHC. OHC were exposed to ΔRR or ΔPK (105 pfu) at the time of KA treatment (3 µM), cultured (24 hrs) in the presence or absence of U0126 (20 µM) or LY294002 (100 µM) and the cell extracts were immunoblotted with antibody specific for phosphorylated (activated) CREB (P-CREB). Antibody to total CREB was used as control and the results are expressed as P-CREB/CREB ratios determined by densitometric scanning and normalized to the ratio in mock–infected OHC. KA caused a significant reduction in the levels of P-CREB in mock-treated OHC. By contrast, the P-CREB/CREB ratios were increased 4.3-fold in ΔRR infected cultures, even in the presence of KA. They were not altered by U0126 and were somewhat increased by LY294002 (Fig. 8B,C), suggesting that CREB activation is independent of MEK and PI3-K. However, activation was mediated by ICP10PK, because P-CREB levels were not increased in OHC infected with ΔPK.
2.10 ΔRR protected neurons retain functional integrity
A major limitation of various neuroprotective strategies is that prevention of neuronal cell death does not correlate with retention of normal synaptic physiology (Dumas et al., 2000; Spencer et al., 2001). To determine whether neurons that survive KA-induced cell death due to ΔRR infection retain functional integrity, the amplitude of field excitatory postsynaptic potentials (fEPSP) was recorded in stratum radiatum of area CA3 in response to dentate gyrus stimulation. OHC were exposed to ΔRR on the same day as KA (3 µM; 24 hrs) and fEPSP recordings were taken 3 days later. We also measured paired pulse ratios, using paired stimuli delivered with an interstimulus interval of 65ms, in order to assay neurotransmitter release probability. As shown in Fig 9, both the amplitude of the responses and their paired-pulse ratios, in the ΔRR infected cultures were indistinguishable from uninfected cultures, whereas it was impossible to elicit any detectable fEPSPs in OHC treated with KA alone. The data indicate that neurons surviving KA-induced cell death through ΔRR infection retain synaptic function.
Fig. 9. ΔRR protected neurons exhibit normal field EPSPs and paired pulse ratios.
OHC were infected with ΔRR and/or treated with KA as in Fig. 3B. At 72 hrs after initiation of KA treatment field excitatory postsynaptic potentials (fEPSP) were recorded in the stratum radiatum in response to granule cell stimulation as described in materials and methods. The fEPSP amplitude and paired pulse ratios were expressed as the mean ± SEM. Paired pulse ratios for cultures given KA alone could not be calculated due to the absence of fEPSP amplitudes.
3. DISCUSSION
We have previously shown that the HSV-2 protein ICP10PK has anti-apoptotic activity in virus infected hippocampal cultures (Perkins et al., 2003) and prevents neuronal cell death and KA-induced epileptiform seizures in amimal models through infection with a growth compromised HSV-2 mutant known as ΔRR (Laing et al., 2006). The studies described in this report were designed to examine the mechanism of neuroprotection from KA-induced cell death. The salient features of the data are the observations that neuronal survival is through activation of the MEK/ERK and PI3-K/Akt pathways and the surviving neurons retain synaptic function. The following comments seem pertinent with respect to these findings.
The construction of the growth compromised vector ΔRR and its ability to deliver ICP10PK to the hippocampus and prevent KA-induced epileptiform seizures and neuronal loss in animal models, were previously described (Laing et al., 2006). ΔPK, used as control for ΔRR, was also previously described and shown to lack neuroprotective activity (Smith et al., 1998; Perkins et al., 2002; Laing et al, 2006). ΔPK is a particularly stringent control, because: (i) it was established from the same HSV-2 strain as ΔRR, (ii) it is deleted in ICP10PK but retains all other viral proteins, (iii) expression of the mutant ICP10 proteins p175 and p95 is under the control of the same authentic ICP10 promoter and both mutants retain the authentic EC and TM domains of ICP10, and (iv) ΔRR and ΔPK are equally growth compromised in neuronal cells (Smith et al., 1998). We showed that the p175 and p95 proteins are expressed equally well in OHC, respectively infected with ΔRR or ΔPK but only p175 retains the ICP10 kinase activity.
Protein expression was determined by staining with ICP10 antibody and, in the case of p175, also by staining with the LacZ substrate C12FDG which is fused in frame with ICP10PK and is therefore controlled by the ICP10 promoter and reflects ICP10PK expression. LacZ is well known to lack neuroprotective activity (Natsume et al., 2001; Harvey et al. 2003) and was used as a marker to trace ICP10PK expression in live cells. The RR activity retained by ΔPK (Smith et al., 1998) is irrelevant from the standpoint of neuroprotection since ΔPK was unable to protect primary hippocampal cultures from virus induced apoptosis (Perkins et al., 2003) and did not prevent KA induced apoptosis in animal models (Laing et al., 2006), or OHC, as shown in our studies. p175 and p95 were expressed for at least 7 days and independent of virus growth, as evidenced by the failure to detect VP5, the expression of which requires viral DNA replication (Roizman and Sears, 1996). This is likely due to the use of the ICP10 promoter, which is regulated with IE kinetics and is independent of other viral proteins, while responding to AP-1 (Wymer et a., 1992; Zhu and Aurelian, 1997; Smith et al., 2000; Gober et al., 2005; Laing et al., 2006). Expression was primarily localized in CA3 and CA1 neurons, as determined by the finding that 80–85% of the βIII Tubulin+ cells stained with antibody to ICP10.
Consistent with previous reports (Faherty et al., 1999; Niquet and Wasterlain, 2004), KA caused a time-dependent increase in the % dead cells in the CA3 field, with maximal levels (72±7%) seen on day 3 after exposure. KA induced cell death was associated with caspase-3 activation (apoptosis), as determined both by immunoblotting with caspase-3 antibody and by double immunofluorescent staining with antibodies to activated caspase-3 and the βIII Tubulin, which indicates that virtually all the cells positive for activated caspase-3 were neurons. ΔRR caused a significant decrease (p<0.001) in the % dead CA3 cells and blocked KA-induced activation of caspase-3 in neurons. Protection was due to ICP10PK, because: (i) cell death and p175 expression were mutually exclusive, and (ii) OHC infected with ΔPK and studied in parallel were not protected. We assume that ICP10PK-mediated neuroprotection is due to its direct effect on neuronal survival, because caspase-3 activation was inhibited in ΔRR-infected neurons, which express the kinase positive p175 protein. However, double immunofluorescent staining using antibodies to ICP10 and glial cell markers indicated that p175 was also expressed in 12–15% microglia and 1–2% astrocytes. While glial cell replication was blocked with mitotic inhibitors, we cannot exclude the possibility that these cells contributed to neuroprotection, for example through the production of cytokines/chemokines (Lokensgard et al., 2001) that play pivotal roles in neuronal fate determination (Streit, 2005). Ongoing studies are designed to examine the role of ΔRR-induced glial cell factors in neuroprotection, if any.
Significantly, very high levels of neuroprotection were still seen when ΔRR was given 2 days after KA treatment. We suggest that rapid neuroprotection (day 1 after ΔRR infection) is related to the generation of an AP-1 feedback amplification loop induced by neurotoxic stimuli (Pennypacker et al., 1994; Smith et al., 2000; Gober et al., 2005) Interestingly, however, caspase-3 activation occurred on day 2 after initiation of KA treatment, suggesting that ICP10PK can also block apoptosis downstream of caspase activation, likely through upregulation of a member of the inhibitor of apoptosis protein (IAP) family. Studies are ongoing to examine whether LacZ replacement with a cellular anti-apoptotic gene that functions at a different location along the apoptotic cascade than ICP10PK may achieve synergistic anti-apoptotic effects (Eberhardt et al., 2000), for example by reducing the vector dose required for protection.
How does ICP10PK protect from KA-induced apoptosis at the level of the ΔRR infected neurons? We have previously shown that ICP10PK activates Ras (Nelson et al., 1996; Smith et al.,2000; Smith, 2005), and it, in turn, activates the c-Raf/MEK/ERK pathway, also in primary hippocampal cultures (Smith et al., 2000; Perkins et al., 2002; Perkins et al., 2003) Consistent with these earlier findings, our data indicate that ΔRR, but not ΔPK, had ICP10 kinase activity, which activated the MEK/ERK pathway in OHC, and approximately 70% of the ΔRR neuroprotective activity was blocked by the MEK inhibitor, U0126. We conclude that ERK1 played a pivotal role in neuroprotection, because it was not activated by ΔPK, which lacks neuroprotective activity, and ERK2, which was previously implicated in KA excitotoxicity (Goodenough et al., 2004), was minimally activated by ΔRR. The differential activation of the two ERK isotypes may reflect the contribution of scaffolding proteins, which facilitate the association of different members of the MEK/ERK pathway (Morrison et al., 2003). However, its relevance to neuroprotection is still unclear. Interestingly, we found that Akt was also activated in ΔRR-, but not ΔPK-infected OHC, and PI3-K/Akt provided the remaining 30% of the ΔRR neuroprotective activity that was not covered by MEK/ERK, as determined by its inhibition with the PI3-K inhibitor LY294002. The PI3-K/Akt pathway is also activated by Ras (Rubio et al., 1997), providing a likely interpretation for its activation by ICP10PK (Fig. 10). However, our observation that PI3-K/Akt is involved in ΔRR-mediated neuroprotection from KA-induced apoptosis is in contrast to previous reports for virus induced apoptosis in primary hippocampal cultures (Perkins et al., 2002), in which protection was mediated solely by the activated Raf-1/MEK/ERK pathway (Perkins et al., 2003). This difference presumably reflects the developemental age of the OHC (6-day old pups, and 14 days of in vitro growth) as compared to the primary hippocampal cultures (16–19 day-old embryos, and 6 days of in vitro growth) (Vogt Weisenhorn et al., 2001) and is consistent with the well known stimulus and cell type specificity of apoptotic and signaling responses (Colucci-D’Amato et al., 2003; Rössler et al., 2004). Notwithstanding, the finding that ICP10PK activates both the MEK/ERK and PI3-K/Akt survival pathways underscores its versatility and its ability to adapt to distinct conditions in order to prevent neuronal cell death.
Fig. 10. Survival pathways activated by ICP10PK in ΔRR infected OHC.
Schematic representation based on previous (Smith et al., 1994; Nelson et al., 1996; Smith et al., 2000; Perkins et al., 2003; Smith et al., 2005) and present findings. Arrows represent activation while reversed “T” symbols represent inhibition. The arrow with the asterisk is data from Wales and Aurelian, in preparation. Dotted line is a postulated pathway that is still under investigation in our laboratory.
Signaling pathways impart survival by transcription-dependent and independent mechanisms (Bazan et al., 2005). We reason that ICP10PK functions primarily through a transcription-dependent mechanism, because it upregulated Bag-1 expression through PI3-K/Akt activation presumably involving the ability of the Bag-1 promoter (which contains AP-1 binding motifs) to respond to AP-1 factors upregulated/activated by the PI3-K/Akt pathway (Yang et al., 1999; Duan et al., 2002; Yamada et al., 2005). ICP10PK also decreased Bad expression, but not its phosphorylation, through activation of the MEK/ERK and PI3-K/Akt pathways. This could be due to pathway induced upregulation of inhibitory transcription factors, or proteasomal degradation, as suggested for other pro-apoptotic Bcl-2 family members the expression of which is downregulated by MEK/ERK and PI3-K/Akt activation (Shinijyo et al., 2001; Ley et al., 2003). The exact contribution of these respective proteins to ICP10PK mediated neuroprotection is still unclear. Bag-1 has neuroprotective activity (Liman et al., 2005). It inhibits apoptosis by itself or by collaborating with the anti-apoptotic protein Bcl-2 (Takayama et al., 1995), which is also associated with neuroprotection (Schulz et al., 1997), is stabilized by Bag-1 and is proteolytically inactivated by other apoptosis-inducing viruses (Peng et al., 2001). Bag-1 also binds and stimulates the c-Raf-1 kinase activity (Wang et al., 1996), and the cytoprotective heat shock protein Hsp70 (Vogt Weisenhorn et al., 2001). Bad is a pro-apoptotic Bcl-2 family member the increased expression of which has been shown to kill cells (Bergmann, 2002). KA induces its disassociation from the 14-3-3 chaperone, thereby freeing it to activate the intrinsic apoptotic cascade (Niquet and Wasterlain, 2004). Classically, Bad is inhibited by phosphorylation (Wang et al., 1996), but its downregulation has also been reported (Munger and Roizman, 2001; Bazan et al., 2005). However, the ability of ICP10PK to alter the balance of these apoptosis regulatory proteins through transcription-dependent mechanisms and in favor of apoptosis inhibition is consistent with previous reports for virus-inefected hippocampal cultures (Perkins et al., 2003).
Cascades of neuronal cell death that accompany neurodegenerative diseases emerge gradually, and are, therefore, amenable to therapeutic intervention. Several gene therapy strategies were proposed to interrupt the apoptotic cascade (Natsume et al., 2001; Harvey et al., 2003). However, the genes had relatively narrow and limited (25–40%) neuroprotective profiles, and some strategies were detrimental (Natsume et al., 2001; Spencer et al., 2001; Wiessner et al., 1999; Manoonkitiwongsa et al., 2004). Most importantly, neuronal survival did not correlate with retention of functional integrity thereby limiting the clinical relevance of the therapeutic approach (Dumas et al., 2000). Our studies have addressed this issue using OHC, a widely used in vitro culture system that retains much of the in vivo hippocampal anatomy and synaptic physiologic connections (Gähwiler et al., 1997; Debanne et al., 1998) thereby allowing for examination of not only the mechanism of neuroprotection, but more importantly, whether normal synaptic function is preserved. Our data indicate that ICP10PK protected neurons from KA-induced cell death and that the protected neurons retained their normal synaptic functions. Indeed, the paired pulse ratios, a measure of presynaptic Ca2+ entry and neurotransmitter release probability, were normal in KA-treated cultures protected by ΔRR. Previous studies have shown that activated (phosphorylated) CREB was associated with increased synaptic strength via upregulation of presynaptic neurotransmitter release (Bossy-Wetzel et al., 1997), and thus may be required for the retention of neuronal function. Interestingly, although CREB was activated by ICP10PK, it was through a pathway other than MEK/ERK or PI3-K/Akt. We propose that PKA is involved in CREB activation (Fig 10), because: (i) P-CREB levels were slightly increased by LY294002 and PI3-K inhibits PKA (Fujino et al., 2005), and (ii) ICP10PK activates PKA in PC12 cells deprived of trophic growth support (unpublished observation). P-CREB is not involved in neuronal survival, because the latter is fully dependent on the MEK/ERK and PI3-K/Akt pathways. Ongoing studies are designed to verify the role of CREB in ΔRR mediated retention of neuronal function and to examine the contribution of the glial cells to either neuroprotection or retention of function.
4. MATERIALS AND METHODS
4.1 Viruses
HSV-1 (strain F), HSV-2 (strain G) and the HSV-2 mutants ΔPK and ΔRR were previously described (Smith et al., 1998; Perkins et al., 2003; Laing et al., 2006). The HSV-2 protein ICP10, which is encoded by the viral gene UL39, has PK and RR activities, which function independently of each other (Smith et al., 1998). The PK activity is located within sequences encoded by the 5’-end of UL39, while the R1 activity is located within sequences encoded by the 3’-end of UL39 (Chung et al., 1989). Both the PK and R1 functions are required for virus growth in non-replicating cells, including neurons (Goldstein and Weller, 1988; Smith et al., 1998). To generate the ΔRR vector, the 3’-end R1-encoding sequences of UL39 were deleted. LacZ was fused in frame with ICP10PK in order to facilitate ΔRR tracing in live cells, giving rise to a 175kDa (p175) mutant protein (Smith et al., 1998; Laing et al, 2006). ΔPK, was generated from ΔRR by deletion of the UL39 5’-end sequences that encode ICP10PK and are poorly conserved in HSV-1 (Nikas et al., 1986), giving rise to a 95kDa protein (p95) (Smith et al., 1998). The p175 and p95 proteins that are repectively encoded by the mutant UL39 gene in ΔRR and ΔPK (Smith et al., 1998) are driven by the authentic ICP10 promoter, which is regulated with IE kinetics (independent of virus replication) (Wymer et al., 1989) and responds to AP-1 transcription factors (Wymer et al., 1992; Zhu and Aurelian, 1997; Gober et al., 2005) upregulated/activated by neurotoxic stress stimuli (viz. KA) (Pennypacker et al., 1994; Gober et al., 2005; Laing et al., 2006).
4.2 Cell culture
Vero (African green monkey kidney) and SK-NSH (human neuroblastoma) cells were grown in minimal essential medium (MEM), supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 100 µM non-essential amino acids and 10% fetal bovine serum (FBS). OHC were prepared and maintained for 2 weeks in roller tubes, as described previously (Gähwiler et al., 1998). In brief, hippocampi were dissected from 5- to 7-day-old Sprague Dawley rat pups, sliced with a tissue chopper at 400µm thickness, and attached to glass coverslips in a chicken plasma clot. The coverslips and slices were placed in individual sealed test tubes and cultured on a roller-drum in an incubator at 36°C in medium consisting of 50% Basal Eagle Medium (Invitrogen, Carlsbad, CA), 25% Hank’s balanced salt solution, and 25% heat inactivated horse serum, and supplemented with 55.6 mM glucose and 1mM L-glutamine. After 5 days in culture, the mitotic inhibitors cytosine-β-D-arabino-furanoside (Sigma), uridine (Sigma), and 5-fluoro-2’-deoxyuridine (Sigma) were added at final concentrations of 0.8 µg/ml to inhibit glial cell growth.
4.3 Antibodies, pharmacologic inhibitors and reagents
The generation and specificity of the rabbit ICP10 antibody were previously described (Smith et al., 1998; Smith et al., 2000; Perkins et al., 2003; Gober et al., 2005). It recognizes amino acids 13 to 26, which are retained by both p175 and p95 (respectively expressed by ΔRR and ΔPK) (Smith et al., 1998; Perkins et al., 2003; Laing et al., 2006). The following antibodies were purchased and used according to the manufacturer’s instructions. Polyclonal antibodies to caspase-3 (recognizes procaspase-3 and the p20 cleavage product), ERK, Bad and Bag-1 (Santa Cruz Biotechnology, Santa Cruz, CA)], P-ERK1/2 (phosphorylated ERK 1/2 on Tyr183) and β Tubulin (III) (Promega, Madison, WI), P-Akt (phosphorylated Akt on Ser473), Akt, CREB and P-CREB (phosphorylated CREB on Ser133), P–Bad (phosphorylated Bad on Ser112) (Cell Signaling Technology, Beverly, MA), HSV VP5 (Virusys Corporation, Sykesville, MD), GFAP (Sigma) and CD11b (Mac-1αm chain-biotin conjugated; Leinco, St. Louis, MO). Secondary antibodies used in immunofluorescent staining were FITC-conjugated goat anti rabbit IgG (BD Biosciences, San Jose, CA); FITC-conjugated goat anti mouse IgG (Jackson Labs; West Grove, PA) Alexa-Fluor-labeled goat anti-rabbit IgG (Molecular Probes, Eugene OR) and FITC-conjugated Streptavidin (Vector, Burlinghame, CA). Preimmune rabbit or mouse IgG was routinely used as negative control (Perkins et al., 2003a; Smith et al., 1998; Gober et al., 2005; Smith et al., 2000). The MEK specific inhibitor U0126 (Promega) and the PI3-K specific inhibitor LY294002 (EMD Biosciences, San Diego, CA) were used according to manufacturer’s instructions. The glutamate analogue KA was obtained from A.G. Scientific, San Diego, CA.
4.5 Immunofluorescence and Immunohistochemistry
OHC were fixed (4 °C; 18 hrs) in 4% paraformaldehyde in PBS (w/v) followed by permeabilization (4 °C; 24 hrs) in PBS + 0.4% triton X-100. Cultures were blocked in 10% horse serum and exposed overnight (4°C) to the primary antibody. For immunofluorescent staining, the immunolabeled cells were subsequently detected by incubation (25 °C; 1 hr) with FITC or Alexa-Fluor conjugated secondary antibody or FITC-conjugated streptavidin (biotinconjugated CD11b) and the slides were mounted in VectaShield with DAPI (Vector). For immunohistochemistry, the secondary antibody was biotinylated and the cultures were subsequently exposed to avidin conjugated alkaline phosphatase and alkaline phosphatase substrate [Dako LSAB-2 AP kit (Dako Corp, Carpenteria, CA)] according to manufacturer’s instructions.
4.6 LacZ expression and cell death
Live cells that express p175 were identified by staining with the green fluorescent β-galactosidase substrate, C12-luorescein di-β-D-galactopyranoside (C12FDG; Molecular Probes, Eugene, OR) according to the manufacturer’s instructions. Dead cells were identified by staining with ethidium homodimer (Molecular Probes), a red fluorescent nuclear stain that penetrates dead cells and increases intensity after binding to DNA. Staining was done according to the manufacturer’s instructions. C12FDG and ethidium stained cells were counted in 5 randomly selected microscopic fields (at least 250 cells) and the percentage of positive cells was calculated relative to the total number of cells visualized by permeabilizing the culture with 5 % triton X-100 for 30 seconds followed by incubation with the fluorescent nuclear stain, DAPI (Sigma). Data are expressed as the mean % positive cells ± SEM. Neuroprotection is calculated according to the formula: % protection =[(KA−(RR−B))/KA] × 100; where KA is % dead cells in OHC given KA alone, RR is % dead cells in ΔRR infected OHC, and B is the % dead cells at the time of virus infection (background).
4.7 Immunoblotting
OHC were briefly frozen on dry ice to facilitate their separation from the surrounding chicken plasma clot. Cultures were lysed using Laemmli buffer (Sigma) supplemented with protease and phosphatase inhibitor cocktails (Sigma) and extracts from 4 individual cultures were pooled to minimize variability. They were incubated at 95 °C for 5 min for protein denaturation and proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose or 0.2µm pore PVDF membranes (used to capture the caspase-3 p20 fragment). For immunoblotting with antibodies to P-ERK 1/2 and P-Akt, blots were blocked (25°C; 1 hr) with blocking buffer [TN-T buffer (0.01M Tris-HCl pH 7.4, 0.15M NaCl, 0.05% Tween-20) containing 1% bovine serum albumin (Sigma)] and incubated with antibody for 12 hrs at 4 °C. For all other antibodies the blots were incubated 1 hr at room temperature in blocking buffer containing 5% nonfat milk (w/v). After 3 washes in TN-T buffer, blots were incubated (25 °C; 1 hr) with HRP-conjugated secondary antibody diluted in blocking buffer followed by 4 additional washes. Detection was with ECL reagents (Amersham Life Science, Arlington Heights, IL) followed by exposure to high performance chemiluminescence film (Hyperfilm ECL, Amersham). Quantitation was by densitometry using the Bio Rad GS-700 Imaging Densitometer.
4.8 Immunocomplex kinase assay
ICP10 kinase assays were as previously described (Luo and Aurelian 1992; Smith et al. 1996). Briefly, cell extracts in lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1% NP-40 and protease and phosphatase inhibitor cocktails) were standardized for protein concentration and incubated with 10 µl of ICP10 antibody (1 hr, 4°C) and 100 µl of protein A-sepharose CL4B beads (50% v/v) (30 min, 4°C). The beads were washed (3×x) with RIPA buffer followed by TS buffer [20mM Tris-Hcl (pH 7.4), 0.15 M NaCl], resuspended in 50 µl kinase reaction buffer consisting of 10 µCi [32P]-ATP (0.1 µM, 3000 Ci/mmol, NEN), 5 mM MgCl2, 2 mM MnCl2, 20 mM Tris-HCl (pH 7.4), and incubated at 30°C for 30 min. Samples were washed in 20 mM Tris-HCl (pH 7.4) with 0.15 M NaCl and boiled for 5 min. after addition of 100 µl denaturing solution and proteins were resolved by SDS-PAGE.
4.9 Electrophysiology
OHC were placed in a recording chamber and perfused with extracellular saline containing (in mM) 137 NaCl, 2.8 KCl, 2 CaCl2, 1 MgCl2, 11.6 NaHCO3, 0.4 NaH2PO4, and 5.6 glucose at approximately 1 ml/min. Extracellular stimuli (−2 to −90 V for 100 µs) were delivered in the dentate gyrus using a 2 MΩ patch pipette filled with extracellular saline. Field EPSPs were recorded in stratum radiatum of area CA3 with an Axopatch 200B amplifier and Clampex 7 software (Axon Instruments). Evoked field potentials were elicited with pairs of stimuli at an interval of 65ms, repeated every 6s. Fifteen maximal amplitude responses were averaged in each culture.
ACKNOWLEDGEMENT
These studies were supported by NINDS, National Institutes of Health (NIH) public health service grant NS45169 to LA and NS40338 to SMT. MDG and JML were supported by grant ES07263 from NIEHS, NIH.
REFERENCES
- Anderson CN, Tolkovsky AM. A role for MAPK/ERK in sympathetic neuron survival: protection against a p53-dependent, JNK-independent induction of apoptosis by cytosine arabinoside. J Neurosci. 1999;19:664–673. doi: 10.1523/JNEUROSCI.19-02-00664.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aureliana L, Smith CC. Herpes simplex type 2 growth and latency reactivation by cocultivation are inhibited with antisense oligonucleotides complementary to the translation initiation site of the large subunit of ribonucleotide reductase (RR1) Antisense Nucleic Acid Drug Dev. 2000;10:77–85. doi: 10.1089/oli.1.2000.10.77. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Marcheselli VL, Cole-Edwards K. Brain response to injury and neurodegeneration: endogenous neuroprotective signaling. Ann N Y Acad Sci. 2005;1053:137–147. doi: 10.1196/annals.1344.011. [DOI] [PubMed] [Google Scholar]
- Bergmann A. Survival signaling goes BAD. Dev Cell. 2002;3:607–608. doi: 10.1016/s1534-5807(02)00328-3. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Bakiri L, Yaniv M. Cell cycle-dependent variations in c-Jun and JunB phosphorylation: a role in the control of cyclin D1 expression. EMBO J. 1997;16:1695–1709. doi: 10.1093/emboj/19.9.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung TD, Wymer JP, Smith CC, Kulka M, Aurelian L. Protein kinase activity associated with the large subunit of the herpes simplex virus type 2 ribonucleotide reductase (ICP10) J Virol. 1989;63:3389–3398. doi: 10.1128/jvi.63.8.3389-3398.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci-D'Amato L, Perrone-Capano C, di Porzio U. Chronic activation of ERK and neurodegenerative diseases. Bioessays. 2003;25:1085–1095. doi: 10.1002/bies.10355. [DOI] [PubMed] [Google Scholar]
- Davis GW, Schuster CM, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. III. CREB is necessary for presynaptic functional plasticity. Neuron. 1996;17:669–679. doi: 10.1016/s0896-6273(00)80199-3. [DOI] [PubMed] [Google Scholar]
- Debanne D, Gahwiler BH, Thompson SM. Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J Physiol. 1998;507:237–247. doi: 10.1111/j.1469-7793.1998.237bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan R, Xie W, Li X, McDougal A, Safe S. Estrogen regulation of c-fos gene expression through phosphatidylinositol-3-kinase-dependent activation of serum response factor in MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2002;294:384–394. doi: 10.1016/S0006-291X(02)00499-0. [DOI] [PubMed] [Google Scholar]
- Dumas TC, McLaughlin JR, Ho DY, Lawrence MS, Sapolsky RM. Gene therapies that enhance hippocampal neuron survival after an excitotoxic insult are not equivalent in their ability to maintain synaptic transmission. Exp Neurol. 2000;166:180–189. doi: 10.1006/exnr.2000.7500. [DOI] [PubMed] [Google Scholar]
- Eberhardt O, Coelln RV, Kugler S, Lindenau J, Rathke-Hartlieb S, Gerhardt E, Haid S, Isenmann S, Gravel C, Srinivasan A, Bahr M, Weller M, Dichgans J, Schulz JB. Protection by synergistic effects of adenovirus-mediated X-chromosome-linked inhibitor of apoptosis and glial cell line-derived neurotrophic factor gene transfer in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. J. Neurosci. 2000;20:9126–9134. doi: 10.1523/JNEUROSCI.20-24-09126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faherty CJ, Xanthoudakis S, Smeyne RJ. Caspase-3-dependent neuronal death in the hippocampus following kainic acid treatment. Brain Res Mol Brain Res. 1999;70:159–163. doi: 10.1016/s0169-328x(99)00143-6. [DOI] [PubMed] [Google Scholar]
- Fujino H, Salvi S, Regan JW. Differential regulation of phosphorylation of the cAMP response element-binding protein after activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. Mol Pharmacol. 2005;68:251–259. doi: 10.1124/mol.105.011833. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Thompson SM, McKinney RA, Debanne D, Robertson RT. Organotypic slice cultures of neural tissue. In: Banker G, Goslin K, editors. Culturing Nerve Cells. 2nd Ed. Cambridge, MA: MIT Press; 1998. pp. 461–498. [Google Scholar]
- Gober MD, Wales SQ, Hunter JC, Sharma BK, Aurelian L. Stress upregulates neuronal expression of the HSV-2 large subunit of ribonucleotide reductase (R1; ICP10) by activating AP-1. J Neurovirol. 2005;11:329–336. doi: 10.1080/13550280591002423. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Weller SK. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology. 1988;166:41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Goodenough S, Conrad S, Skutella T, Behl C. Inactivation of glycogen synthase kinase-3beta protects against kainic acid-induced neurotoxicity in vivo. Brain Res. 2004;1026:116–125. doi: 10.1016/j.brainres.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Harvey BK, Chang CF, Chiang YH, Bowers WJ, Morales M, Hoffer BJ, Wang Y, Federoff HJ. HSV amplicon delivery of glial cell line-derived neurotrophic factor is neuroprotective against ischemic injury. Exp. Neurol. 2003;183:47–55. doi: 10.1016/s0014-4886(03)00080-3. [DOI] [PubMed] [Google Scholar]
- Hemmings BA. Akt signaling: linking membrane events to life and death decisions. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- Laing JL, Gober MD, Golembewski EK, Yarowsky PJ, Thompson SM, Gyure KA, Aurelian LA. Intranasal Administration of the Growth-Compromised HSV-2 Vector DeltaRR Prevents Kainate-Induced Seizures and Neuronal Loss in Rats and Mice. Mol Ther. 2005 Feb 22; doi: 10.1016/j.ymthe.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- Liman J, Ganesan S, Dohm CP, Krajewski S, Reed JC, Bahr M, Wouters FS, Kermer P. Interaction of BAG1 and Hsp70 mediates neuroprotectivity and increases chaperone activity. Mol Cell Biol. 2005;25:3715–3725. doi: 10.1128/MCB.25.9.3715-3725.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokensgard JR, Hu S, Sheng W, van Oijen M, Cox D, Cheeran MC, Peterson PK. Robust expression of TNF-alpha, IL-1beta, RANTES, and IP-10 by human microglial cells during nonproductive infection with herpes simplex virus. J Neurovirol. 2001;7:208–219. doi: 10.1080/13550280152403254. [DOI] [PubMed] [Google Scholar]
- Luo JH, Smith CC, Kulka M, Aurelian L. A truncated protein kinase domain of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) expressed in Escherichia coli. J Biol Chem. 1991;266:20976–20983. [PubMed] [Google Scholar]
- Manoonkitiwongsa PS, Schultz RL, McCreery DB, Whitter EF, Lyden PD. Neuroprotection of ischemic brain by vascular endothelial growth factor is critically dependent on proper dosage and may be compromised by angiogenesis. J Cereb Blood Flow Metab. 2004;24:693–702. doi: 10.1097/01.WCB.0000126236.54306.21. [DOI] [PubMed] [Google Scholar]
- Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- Munger J, Roizman B. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc. Natl. Acad. Sci. U S A. 2001;98:10410–10415. doi: 10.1073/pnas.181344498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsume A, Mata M, Goss J, Huang S, Wolfe D, Oligino T, Glorioso J, Fink DJ. Bcl-2 and GDNF delivered by HSV-mediated gene transfer act additively to protect dopaminergic neurons from 6-OHDA-induced degeneration. Exp Neurol. 2001;169:231–238. doi: 10.1006/exnr.2001.7671. [DOI] [PubMed] [Google Scholar]
- Nelson J, Zhu J, Smith CC, Kulka M, Aurelian L. ATP and SH3 binding sites in the protein kinase of the large subunit of herpes simplex virus type 2 of ribonucleotide reductase (ICP10) J Biol Chem. 1996;271:17021–17027. doi: 10.1074/jbc.271.29.17021. [DOI] [PubMed] [Google Scholar]
- Nikas I, McLauchlan J, Davison AJ, Taylor WR, Clements JB. Structural features of ribonucleotide reductase. Proteins. 1986;1:376–384. doi: 10.1002/prot.340010411. [DOI] [PubMed] [Google Scholar]
- Niquet J, Wasterlain CG. Bim, Bad, and Bax: a deadly combination in epileptic seizures. J. Clin Invest. 2004;113:960–962. doi: 10.1172/JCI21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Sadusky T, Li Y, Coulton GR, Zhang H, Archard LC. Altered expression of Bag-1 in Coxsackievirus B3 infected mouse heart. Cardiovasc Res. 2001;50:46–55. doi: 10.1016/s0008-6363(00)00323-0. [DOI] [PubMed] [Google Scholar]
- Pennypacker KR, Thai L, Hong JS, McMillian MK. Prolonged expression of AP-1 transcription factors in the rat hippocampus after systemic kainate treatment. J Neurosci. 1994;14:3998–4006. doi: 10.1523/JNEUROSCI.14-07-03998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D, Pereira EF, Gober M, Yarowsky PJ, Aurelian L. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J Virol. 2002;76:1435–1449. doi: 10.1128/JVI.76.3.1435-1449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D, Pereira EF, Aurelian L. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) functions as a dominant regulator of apoptosis in hippocampal neurons involving activation of the ERK survival pathway and upregulation of the antiapoptotic protein Bag-1. J Virol. 2003;77:1292–1305. doi: 10.1128/JVI.77.2.1292-1305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B, Sears AE. Herpes simplex viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Virology. 3rd Ed. Philadelphia, PA: Lippincott-Raven Publishers; 1996. pp. 2231–2295. [Google Scholar]
- Rössler OG, Giehl KM, Thiel G. Neuroprotection of immortalized hippocampal neurones by brain-derived neurotrophic factor and Raf-1 protein kinase: role of extracellular signal-regulated protein kinase and phosphatidylinositol 3-kinase. J Neurochem. 2004;88:1240–1252. doi: 10.1046/j.1471-4159.2003.02255.x. [DOI] [PubMed] [Google Scholar]
- Rubio I, Rodriguez-Viciana P, Downward J, Wetzker R. Interaction of Ras with phosphoinositide 3-kinase gamma. Biochem J. 1997;326:891–895. doi: 10.1042/bj3260891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter H, Spencer JP, Rice-Evans C, Williams RJ. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3c. Biochem J. 2001;358:547–557. doi: 10.1042/0264-6021:3580547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JB, Bremen D, Reed JC, Lommatzsch J, Takayama S, Wullner U, Loschmann PA, Klockgether T, Weller M. Cooperative interception of neuronal apoptosis by BCL-2 and BAG-1 expression: prevention of caspase activation and reduced production of reactive oxygen species. J Neurochem. 1997;69:2075–2086. doi: 10.1046/j.1471-4159.1997.69052075.x. [DOI] [PubMed] [Google Scholar]
- Shinjyo T, Kuribara R, Inukai T, Hosoi H, Kinoshita T, Miyajima A, Houghton PJ, Look AT, Ozawa K, Inaba T. Downregulation of Bim, a proapoptotic relative of Bcl-2, is a pivotal step in cytokine-initiated survival signaling in murine hematopoietic progenitors. Mol Cell Biol. 2001;21:854–864. doi: 10.1128/MCB.21.3.854-864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Luo JH, Hunter JC, Ordonez JV, Aurelian L. The transmembrane domain of the large subunit of HSV-2 ribonucleotide reductase (ICP10) is required for protein kinase activity and transformation-related signaling pathways that result in ras activation. Virology. 1994;200:598–612. doi: 10.1006/viro.1994.1223. [DOI] [PubMed] [Google Scholar]
- Smith CC, Peng T, Kulka M, Aurelian L. The PK domain of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) is required for immediate-early gene expression and virus growth. J Virol. 1998;72:9131–9141. doi: 10.1128/jvi.72.11.9131-9141.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Nelson J, Aurelian L, Gober M, Goswami BB. Ras-GAP binding and phosphorylation by herpes simplex virus type 2 RR1 PK (ICP10) and activation of the Ras/MEK/MAPK mitogenic pathway are required for timely onset of virus growth. J Virol. 2000;74:10417–10429. doi: 10.1128/jvi.74.22.10417-10429.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC. The herpes simplex virus type 2 protein ICP10PK: a master of versatility. Front Biosci. 2005;10:2820–2831. doi: 10.2741/1738. [DOI] [PubMed] [Google Scholar]
- Spencer B, Agarwala S, Gentry L, Brandt CR. HSV-1 vector-delivered FGF2 to the retina is neuroprotective but does not preserve functional responses. Mol Ther. 2001;3:746–756. doi: 10.1006/mthe.2001.0307. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia and neuroprotection: implications for Alzheimer's disease. Brain Res Brain Res Rev. 2005;48:234–239. doi: 10.1016/j.brainresrev.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Takayama S, Sato T, Krajewski S, Kochel K, Irie S, Millan JA, Reed JC. Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell. 1995;80:279–284. doi: 10.1016/0092-8674(95)90410-7. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Vogt Weisenhorn DM, Roback LJ, Kwon JH, Wainer BH. Coupling of cAMP/PKA and MAPK signaling in neuronal cells is dependent on developmental stage. Exp Neurol. 2001;169:44–55. doi: 10.1006/exnr.2001.7651. [DOI] [PubMed] [Google Scholar]
- Wang HG, Takayama S, Rapp UR, Reed JC. Bcl-2 interacting protein, BAG-1, binds to and activates the kinase Raf-1. Proc Natl Acad Sci U S A. 1996;93:7063–7068. doi: 10.1073/pnas.93.14.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiessner C, Allegrini PR, Rupalla K, Sauer D, Oltersdorf T, McGregor AL, Bischoff S, Bottiger BW, van-der-Putten H. Neuron-specific transgene expression of Bcl-XL but not Bcl-2 genes reduced lesion size after permanent middle cerebral artery occlusion in mice. Neuroscience Letters. 1999;268:119–122. doi: 10.1016/s0304-3940(99)00392-4. [DOI] [PubMed] [Google Scholar]
- Wymer JP, Chung TD, Chang Y-N, Hayward GS, Aurelian L. Identification of immediate-early-type cis-response elements in the promoter for the ribonucleotide reductase large subunit from herpes simplex virus type 2. J Virol. 1989;63:2773–2784. doi: 10.1128/jvi.63.6.2773-2784.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymer JP, Aprhys CM, Chung TD, Feng CP, Kulka M, Aurelian L. Immediate early and functional AP-1 cis-response elements are involved in the transcriptional regulation of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) Virus Res. 1992;23:253–270. doi: 10.1016/0168-1702(92)90112-m. [DOI] [PubMed] [Google Scholar]
- Yamada K, Ishiguro H, Ichino N, Nishii K, Sawada H, Hida T, Nagatsu T. Expression levels of Rab2, a G protein, and Bag-1, a Bcl-2 binding protein are controlled by withdrawal of nicotine from cultured pheochromocytoma PC12 cells. J Neural Transm. 2005;112:633–639. doi: 10.1007/s00702-005-0294-4. [DOI] [PubMed] [Google Scholar]
- Yang X, Pater A, Tang SC. Cloning and characterization of the human BAG-1 gene promoter: upregulation by tumor-derived p53 mutants. Oncogene. 1999;18:4546–4553. doi: 10.1038/sj.onc.1202843. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- Zhu J, Aurelian L. AP-1 cis-response elements are involved in basal expression and Vmw110 transactivation of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) Virology. 1997;231:301–312. doi: 10.1006/viro.1997.8522. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]