Abstract
The relationship between endemic bacteriophages infecting E. coli O157:H7 (referred to as “phage”) and levels of shedding of E. coli O157:H7 by cattle was investigated in two commercial feedlots in southern Alberta, Canada. Between May and November 2007, 10 pens of cattle were monitored by collection of pooled fecal pats, water with sediment from troughs, manure slurry from the pen floor, and rectal fecal samples from individual animals (20 per pen) at two separate times. Bacteriophages infecting E. coli O157:H7 were detected more frequently (P < 0.001) after 18 to 20 h enrichment than by initial screening and were recovered in 239 of 855 samples (26.5% of 411 pooled fecal pats, 23.8% of 320 fecal grab samples, 21.8% of 87 water trough samples, and 94.6% of 37 pen floor slurry samples). Overall, prevalence of phage was highest (P < 0.001) in slurry. Recovery of phage from pooled fecal pats was highest (P < 0.05) in May. Overall recovery did not differ (P > 0.10) between fecal grab samples and pooled fecal pats. A higher prevalence of phage in fecal pats or water trough samples was associated (P < 0.01) with reduced prevalence of E. coli O157:H7 in rectal fecal samples. There was a weak but significant negative correlation between isolation of phage and E. coli O157:H7 in fecal grab samples (r = −0.11; P < 0.05). These data demonstrate that the prevalence of phage fluctuates in a manner similar to that described for E. coli O157:H7. Phage were more prevalent in manure slurry than other environmental sources. The likelihood of fecal shedding of E. coli O157:H7 was reduced if cattle in the pen harbored phage.
Bacteriophages are the most abundant biological entities on earth. An estimated 1030 marine bacteriophages are harbored in the ocean, and they significantly influence microbial communities and function (27). As resistance is an increasing challenge in antimicrobial therapy, the antimicrobial nature of bacteriophages is being more intensively studied (13, 15). Bacteriophages naturally inhabit the mammalian gastrointestinal tract (1, 8), and Escherichia coli-infecting bacteriophages are commonly isolated from sewage, hospital wastewater, and fecal samples from humans and animals (3). Ruminants have been shown to shed up to 107 bacteriophage per gram of feces (6), and in humans multiple types of bacteriophage exhibiting activity against E. coli have been isolated from a single fecal sample (7).
E. coli O157:H7 is an important zoonotic bacterium carried asymptomatically by cattle and readily isolated from manure, manure slurry, and drinking water in dairies and feedlots (11, 24, 30). Additionally, E. coli O157:H7 shedding by cattle has a seasonal pattern, peaking in the summer months (2, 25). Bacteriophage strains that infect E. coli O157:H7 have also been isolated from animal feces and have shown lytic activity against this bacterium in vivo and in vitro (5, 23, 28, 31). In recent studies, such phages were shown to be widely distributed in cattle and in feces on the pen floor within feedlots (4, 18). However, the relationships between the presence of E. coli O157:H7-infecting bacteriophage in cattle and their environment and the shedding of this bacterium by cattle are largely undefined. Consequently, the aims of the present study were (i) to determine the prevalence of endemic E. coli O157:H7-infecting bacteriophage (referred to as “phage”) in feedlots over a 7-month period and (ii) to compare the presence of phage to the occurrence of E. coli O157:H7 in cattle and their environment.
MATERIALS AND METHODS
Study design and feedlot description.
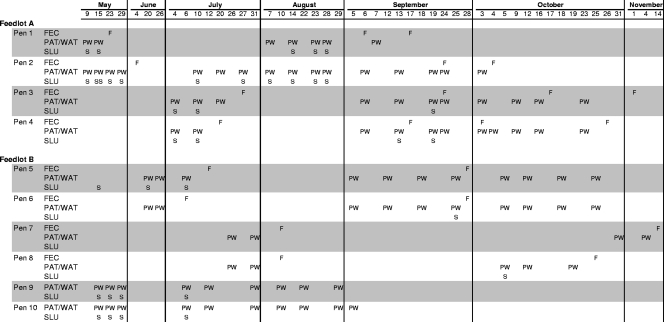
The study was conducted using 10 pens of cattle (18 to 24 months old; average initial weight, 524 kg) in two commercial feedlots (pens 1 to 4 in feedlot A and pens 5 to 10 in feedlot B) from May to November 2007. The average stocking density during this study was 174 head per pen. Pooled fecal pats were collected from 10 pens, as shown in Fig. 1. Samples were collected only when animals were present in the pens and at times when feedlot operators would grant access to the premises.
FIG. 1.
Schedule of sample collection in 10 pens in two southern Alberta feedlots. FEC and F, fecal material collected by rectal grab; PAT and P, pooled fecal pats from pen floor; WAT and W, water with sediment from drinking troughs; SLU and S: manure slurry from pen floors. On May 9 and 15, single fecal pats were collected from pens 1, 2, 9, and 10. Subsequently, five pats were collected per pen surveyed to produce the pooled fecal pats. On May 15, the presence of two separate slurry ponds in pen 2 enabled collection of two SLU samples (SS).
Sample collection.
To evaluate phage presence and E. coli O157:H7 shedding by individuals, rectal fecal samples were collected on two occasions from 20 cattle in each of pens 1 to 8. Approximately 10 g of feces per animal was collected by digital retrieval into a sterile Whirl-Pac bag (VWR International, Edmonton, Alberta, Canada). Samples were collected midway through the feeding period (S1) and just prior to shipment to market (S2) (Fig. 1). For S1 samples, each pen was sampled on a single visit, between May and August. Regarding the S2 samples, cattle in feedlot A were marketed in different groups, and thus, several sampling dates were required to collect individual fecal samples from all 20 animals within each pen, whereas at feedlot B, all cattle within a pen were marketed on the same date, and thus, samples from all 20 animals could be collected in a single visit to the feedlot.
Environmental samples from these eight pens were collected once weekly for at least two weeks before collection of fecal grab samples (Fig. 1). Environmental samples included fecal pats from the pen floor (pats), water with sediment from troughs (trough), and, when available, fecal slurry from the pen floor (slurry).
For pat samples, ∼100 g of feces was collected from five fresh, widely spaced fecal pats from each pen and pooled into a sterile Whirl-Pac bag and hand mixed to ensure homogeneity. Five composite pat samples were produced from 25 fecal pats in each pen sampled, except for sampling in pens 1 and 2 (May 9 and 15) and pens 9 and 10 (May 15), when a single composite pat was collected. For trough samples, trough water was thoroughly mixed by hand, and ∼120 ml was collected into a sterile specimen container (Fisher Scientific, Edmonton, Alberta, Canada). In addition, two sterile gauze sponges (5 cm by 5 cm; Johnson & Johnson Medical Ltd., Gargrave, United Kingdom) were used to swab a 100-cm2 area at the water-trough interface. One sponge was placed in the container with the water sample and the other was placed in 45 ml of modified EC broth containing 2% novobiocin (mECnov; EM Science, Gibbstown, NJ) for detection of E. coli O157:H7.
When present, slurry from the pen floor (up to 120 ml) was collected in a sterile specimen container. All samples were then transported to the laboratory, refrigerated at 4°C, and analyzed within 48 h.
Sample processing for recovery of phage.
Fecal subsamples (pats or rectal fecal material, 2 g) were mixed thoroughly with 8 ml of sterile lambda diluent (10 mM Tris Cl [pH 7.5], 8 mM MgSO4) and held at room temperature for at least 30 min. A subsample (3.6 ml) was withdrawn from the top of the diluted sample, centrifuged at 11,000 × g for 10 min, and filtered through a 0.22-μm syringe filter (Pall, Newquay, Cornwall, United Kingdom). The filtrate was then analyzed for phage. Trough subsamples (15 ml) were concentrated by ultrafiltration (Centriprep-30; Millipore, Bedford, MA) to a volume of 3 to 5 ml, with the concentrated fraction being used for phage detection. Slurry subsamples (10 ml) were suspended in 10 to 20 ml sterile lambda diluent for 15 min at room temperature. The suspension was centrifuged (5,250 × g; 25 min) and filtered through a sterile 50-ml disposable vacuum filtration unit (0.2 μm; Millipore, Billerica, MA), and the filtrate was analyzed for phage.
Detection of phage by initial phage screening.
E. coli O157:H7 R508, a bovine phage type 14 strain from the Laboratory for Foodborne Zoonoses, Public Health Agency of Canada, Guelph, Ontario, Canada, was used as the host for detection of phage. Aliquots (450 μl) of filtrate from pats and rectal fecal material, centrifuged slurry filtrate, or concentrated trough water were incubated for 1 h at 37°C with 50 μl E. coli O157:H7 R508 in early log phase (optical density at 600 nm ∼ 0.2 to 0.3). The filtrate-bacterium mixtures (20 μl) were then spotted in quadruplicate onto modified nutrient agar containing 8.5 g/liter NaCl, 1 mM CaCl2, 1 mM FeCl3, and 4 mM MgSO4. The presence or absence of plaques was recorded after 18 to 24 h incubation at 37°C.
Detection of phage by enrichment.
For enrichment, ∼1 ml of filtrate or trough concentrate was added to a 5-ml early-log-phase (optical density at 600 nm ∼ 0.2 to 0.3) culture of E. coli O157:H7 R508 in tryptic soy broth with 10 mM MgSO4 and further incubated at 37°C in a rotary shaker (150 rpm) for 18 to 20 h. A 1.8-ml subsample of the overnight culture was then centrifuged at 11,000 × g for 10 min and filtered through a 0.22-μm syringe filter. The filtrates were then tested by the soft-agar overlay plaque assay described by Sambrook and Russell (19), using E. coli O157:H7 R508 as the host.
Detection and enumeration of E. coli O157:H7.
All rectal fecal samples, all trough-water interface sponges, and one pooled fecal pat per pen were tested for the presence of E. coli O157 using enrichment and immunomagnetic separation (IMS). Fecal subsamples (∼1 g) were suspended in 9 ml of mECnov and incubated at 37°C for 6 h. Twenty microliters of anti-E. coli O157 magnetic beads (Dynabeads; Invitrogen, Burlington, Ontario, Canada) was incubated for 30 min with 1 ml of enrichment culture. The beads were washed three times in sterile phosphate buffer solution (PBS) containing 0.05% Tween 20 and resuspended in 100 μl of sterile PBS. A 50-μl aliquot of the resuspension was plated onto sorbitol MacConkey agar supplemented with (per liter) 0.05 mg cefixime and 2.5 mg tellurite (CT-SMAC; Dalynn Biologicals, Calgary, Alberta, Canada) and incubated for 18 to 24 h at 37°C. One to three non-sorbitol-fermenting colonies from each plate were tested for the presence of the O157 antigen by agglutination with an E. coli O157 latex kit (Oxoid, Nepean, Ontario, Canada). E. coli O157 was enumerated by direct plating of fecal pat or rectal fecal samples that were positive by IMS. Fecal material from the original rectal grab or pooled pat samples, which had been held at 4°C, was diluted serially in PBS, and duplicate 100-μl volumes were plated onto CT-SMAC. After incubation for 18 to 24 h at 37°C, plates displaying 30 to 300 colonies were used for determining numbers of E. coli O157. The presence of E. coli O157 at the water-trough interface was determined by incubation of the sponge sample in mECnov for 18 to 24 h at 37°C and testing by IMS as described above. Final confirmation of E. coli O157:H7 in all samples was based on the presence of genes encoding verotoxins (vt), intimin (eaeA), and H7 antigen (fliC), as determined by a multiplex PCR assay (10).
Statistical analysis.
A sample was considered positive for phage when initial screening and/or enrichment methods yielded at least one visible plaque. A sample was considered negative for phage only when both initial screening and enrichment showed no plaques. A sample was considered positive for E. coli O157:H7 when enrichment and IMS yielded one or more multiplex PCR-confirmed isolates. The Cohen kappa statistic (κ) was used to assess agreement between initial screening and enrichment methods. Correlations between isolation of E. coli O157:H7 and phage were assessed using the Pearson product-moment correlation coefficient. Odds ratios were generated for detection of phage using the GLIMMIX procedure. All analyses were conducted using the SAS system for Windows, version 9.1 (21).
RESULTS
Prevalence of phage.
Bacteriophages active against E. coli O157:H7 were found in all types of samples collected from all pens at feedlots A and B (Table 1). Overall, 239 (28.0%) of all 855 samples were positive for phage by initial screening and/or enrichment. The prevalence of phage in pats was higher (P < 0.05) in feedlot A (32.2%) than in feedlot B (20.3%), whereas no difference in rate of detection of phage in rectal fecal samples was observed between feedlots A (22.5%) and B (25.0%).
TABLE 1.
Likelihood of recovery of phage-positive samples among those collected from two feedlots between May and November 2007a
| Sample typeb | No. of positive samples/no. tested (%)c
|
Odds ratio | 95% confidence interval | P value | ||
|---|---|---|---|---|---|---|
| Feedlot A | Feedlot B | Total | ||||
| PAT | 69/214 (32.2) | 40/197 (20.3) | 109/411 (26.5) | 0.02 | 0.02-0.06 | <0.001 |
| FEC | 36/160 (22.5) | 40/160 (25.0) | 76/320 (23.8) | 0.02 | 0.01-0.05 | <0.001 |
| WAT | 8/46 (17.4) | 11/41 (26.8) | 19/87 (21.8) | 0.02 | 0.01-0.05 | <0.001 |
| SLU | 21/23 (91.3) | 14/14 (100) | 35/37 (94.6) | |||
The percentage of phage-positive samples in slurry samples was used as the referent.
PAT, pooled fecal pats; FEC, rectally collected fecal material; WAT, water with sediment from troughs; SLU, manure slurry.
The status of samples was determined by initial screening and/or enrichment followed by plaque assay.
Bacteriophages active against E. coli O157:H7 were isolated from 19 (21.8%) of 87 trough samples, with no difference between feedlots (P = 0.56). Eight of the 10 pens yielded phage-positive trough samples at least once. In general, phage were isolated more frequently from troughs in May than in other months (Table 2). The percentage of slurry samples positive for phage (94.6%) was greater than was observed for other sample types (P < 0.001), with no difference between feedlots (P = 1.0). All slurry samples from feedlot B were phage positive. Slurry samples were mainly available in May and sporadically in July after a period of wet weather (Table 2). Consequently, collection of slurry was possible only in 9 of 10 pens during the period from May to October. Odds ratios comparing the frequencies of detection of phage in fecal pats, rectal fecal material, or trough sample with frequency of detecting phage in slurry (Table 1) indicated that the former three sample types were 98% less likely (P < 0.001) than slurry to be phage positive.
TABLE 2.
Monthly prevalence of phage-positive environmental samples collected from feedlots between May and November 2007a
| Month | No. of positive samples/no. tested
|
||
|---|---|---|---|
| PAT | WAT | SLU | |
| May | 24/36 | 9/12 | 14/14 |
| June | 4/20 | 0/4 | 1/1 |
| July | 26/95 | 6/19 | 9/9 |
| August | 47/70 | 0/14 | 6/8 |
| September | 25/110 | 2/22 | 4/4 |
| October | 13/75 | 1/15 | 1/1 |
| November | 3/5 | 0/1 | NS |
Phage refers to naturally occuring bacteriophage capable of infecting, E. coli O157:H7. Sample status was determined by initial screening and/or by enrichment followed by plaque assay. PAT, pooled fecal pats; WAT, water with sediment from troughs; SLU, pen floor manure slurry; NS, no sample available.
Variation of phage prevalence in pooled fecal pats over time.
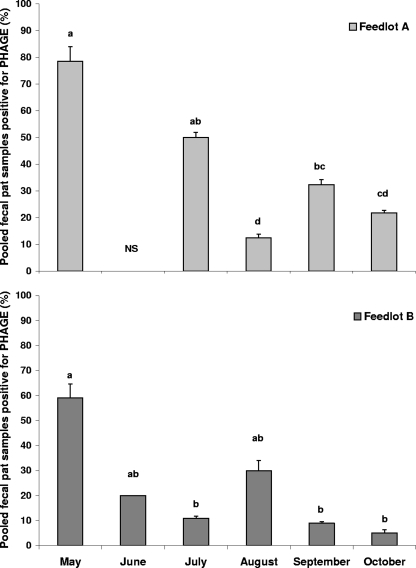
At both feedlots, the prevalence of phage in fecal pat samples was greatest (P < 0.05) in May (Fig. 2). At feedlot A, phage were more frequently detected in fecal pats in May (78.6%) than in August, September, or October (P < 0.01), and prevalence was higher (P < 0.05) in July (50.0%) and September (32.3%) than in August. At feedlot B, phage were more frequently (P < 0.05) detected in pats in May (59.1%) than in July (10.9%), September (8.9%), or October (5.0%).
FIG. 2.
Percentage, across pens, of pooled fecal pats testing positive for bacteriophage infecting E. coli O157:H7 between May and October in feedlots A and B, by the initial screening method and/or by enrichment followed by plaque assay. Error bars indicate standard errors. Within a feedlot, means with different letters (a to d) differ significantly (P < 0.05). No samples were collected in June at feedlot A (NS).
The prevalence of phage in fecal pats in May (67.7%) was higher (P < 0.001) than that in any other month. Prevalence declined to 24.4% in July and 26.0% in August. In September, phage were isolated from only 16.6% of fecal pats, but this value was not different (P = 0.59) from prevalences in July and August.
Prevalence of E. coli O157:H7 and phage.
Across samplings S1 and S2, the prevalence of phage in rectal fecal samples (20 per pen) ranged from 5.0% to 47.5% (Table 3). Interestingly, 19 of 20 animals from pen 2 (feedlot A) were positive for phage at S1 in June, but all 20 animals from this pen were negative for phage at S2 in September and October.
TABLE 3.
Prevalence of E. coli O157:H7 and of bacteriophage infecting E. coli O157:H7 (phage) in samplesa collected from two southern Alberta feedlots
| Organism type and sample origin | No. of animals per pen | Prevalence of organism (no. of samples positiveb/no. of samples tested)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1c
|
S2c
|
Total
|
||||||||
| PAT | WAT | FEC | PAT | WAT | FEC | PAT | WAT | FEC | ||
| E. coli O157:H7d | ||||||||||
| Feedlot A | ||||||||||
| Pen 1 | 152 | 0/2 | 0/2 | 9/20 | 0/2 | 0/3 | 12/20 | 0/4 | 0/5 | 21/40 |
| Pen 2 | 152 | 0/2 | 0/2 | 0/20 | 2/4 | 0/4 | 11/20 | 4/6 | 0/6 | 11/40 |
| Pen 3 | 253 | 0/2 | 2/2 | 12/20 | 1/6 | 0/6 | 14/20 | 1/8 | 2/8 | 26/40 |
| Pen 4 | 153 | 1/2 | 1/2 | 10/20 | 1/7 | 0/7 | 4/20 | 2/9 | 1/9 | 14/40 |
| Feedlot B | ||||||||||
| Pen 5 | 235 | 1/1 | 2/2 | 12/20 | 0/2 | 0/2 | 13/20 | 1/3 | 2/4 | 25/40 |
| Pen 6 | 151 | 0/2 | 2/2 | 12/20 | 2/2 | 0/2 | 12/20 | 2/4 | 2/4 | 24/40 |
| Pen 7 | 138 | 2/2 | 1/2 | 15/20 | 1/2 | 0/2 | 2/20 | 3/4 | 1/4 | 17/40 |
| Pen 8 | 149 | 0/2 | 1/2 | 15/20 | 0/2 | 0/1 | 4/20 | 0/4 | 1/3 | 19/40 |
| Phage | ||||||||||
| Feedlot A | ||||||||||
| Pen 1 | 152 | 1/2 | 1/2 | 7/20 | 5/15 | 0/3 | 0/20 | 6/17 | 1/5 | 7/40 |
| Pen 2 | 152 | 9/10 | 2/2 | 19/20 | 1/20 | 1/4 | 0/20 | 10/30 | 3/6 | 19/40 |
| Pen 3 | 253 | 2/10 | 0/2 | 3/20 | 12/30 | 0/6 | 5/20 | 14/40 | 0/8 | 8/40 |
| Pen 4 | 153 | 7/10 | 2/2 | 1/20 | 9/35 | 0/7 | 1/20 | 16/45 | 2/9 | 2/40 |
| Feedlot B | ||||||||||
| Pen 5 | 235 | 0/10 | 0/2 | 3/20 | 0/10 | 0/2 | 3/20 | 0/20 | 0/4 | 6/40 |
| Pen 6 | 151 | 4/10 | 0/2 | 6/20 | 0/10 | 1/2 | 13/20 | 4/20 | 1/4 | 19/40 |
| Pen 7 | 138 | 0/10 | 0/2 | 7/20 | 3/10 | 0/2 | 4/20 | 3/20 | 0/4 | 11/40 |
| Pen 8 | 149 | 2/10 | 2/2 | 1/20 | 1/10 | 0/2 | 3/20 | 3/20 | 3/4 | 4/40 |
PAT, pooled fecal pats; WAT, water with sediment from troughs; FEC, rectally collected fecal material. In each pen, the PAT and WAT samples were collected during the 16 days immediately preceding each of the two FEC samplings.
The presence of E. coli O157:H7 was determined by enrichment and IMS. The presence of phage was determined by initial screening and/or by enrichment followed by plaque assay.
Rectal fecal samples were collected on two occasions (in eight pens; 20 animals per pen) to evaluate E. coli O157:H7 and phage shedding by individuals. FEC samples were collected during cattle processing during implant of growth promotant (S1) and at final weigh-out prior to slaughter (S2), an interval of approximately 3 to 4 months.
Assessment for E. coli O157:H7 in PAT samples was conducted on one randomly selected PAT from the five prepared per pen.
Pooled fecal pats collected within 16 days of rectal fecal grab sampling were used to compare the frequencies of phage in pooled fecal pats and rectal fecal samples during S1 and S2 (Table 3). Overall, the prevalences of phage in these two sample types did not differ across sampling times, except for pen 4 in feedlot A, where the prevalence of phage was higher (P < 0.01) in pats from the pen floor than in rectal fecal material.
Pooled fecal pats and trough samples collected within 16 days of fecal grab sampling were used to determine the impact of phage on the prevalence of E. coli O157:H7 in cattle (rectal fecal samples at S1 and S2) (Fig. 1; Table 3). Across the two time periods and feedlots, the prevalence of E. coli O157:H7 in rectal fecal samples was 38.8% (124 of 320). Overall, phage were detected in 56 (26.4%) of 212 fecal pats and 10 (22.7%) of 44 trough samples collected just prior to S1 and S2. Among the eight pens monitored, higher prevalence of phage in pats or trough samples was associated (P < 0.01) with reduced prevalence of E. coli O157:H7 in fecal grab samples.
Analysis of the prevalence of E. coli O157:H7 and phage in rectal fecal samples revealed that 16.9% of 320 fecal samples were culture negative for E. coli O157:H7 but positive for phage, and only 6.9% were positive for both E. coli O157:H7 and phage. Isolation of phage was 43% less likely (95% confidence interval = 0.32 to 0.99; P < 0.05) from E. coli O157:H7 culture-positive fecal samples than from those testing negative. Bacteriophage were isolated more (P < 0.05) frequently from samples testing positive for E. coli O157:H7 via direct plating (12 of 42) than from those testing positive via IMS (10 of 82). Populations of E. coli O157:H7 in phage-positive rectal fecal samples averaged 4.6 log10 CFU/g by direct plating. In contrast to the results for rectal fecal samples, isolation of phage across sampling times and feedlots was unrelated (P > 0.1) to detection of E. coli O157:H7 in fecal pats or water trough samples.
Initial screening versus enrichment for phage detection.
Agreement between initial screening and enrichment for detecting phage was fair (κ = 0.29 to 0.39) within and across feedlots, irrespective of sampling type or time, and was slight to fair among different sample types (κ = 0.19 to 0.26) across feedlots. Overall, the proportions of samples testing phage positive without enrichment were 27 of 411 fecal pats (6.6%), 18 of 320 fecal grab samples (5.6%), 10 of 87 water trough samples (11.5%), and 28 of 37 slurries (75.7%). The percentage of slurry samples testing phage positive by initial screening was higher (P < 0.001) than that of other sample types, which did not differ from one another. Enrichment increased detection rates by 20.0%, 18.1%, 10.3%, and 18.9% for pats, rectal feces, trough samples, and slurry samples, respectively. In total, enrichment detected approximately twice as many phage-positive isolates as did initial screening (156 versus 83).
Odds ratios comparing detection of phage by initial screening and enrichment are presented in Table 4. Across sample types, overall isolation of phage was 69.0% less likely (P < 0.001) by initial screening than after enrichment. As well, across feedlots and sampling times, initial screening was 79.0 to 80.0% (P < 0.001) less likely than enrichment to recover phage either from pooled fecal pats or from rectal fecal samples.
TABLE 4.
Likelihood of detecting E. coli O157:H7-infecting bacteriophage from feedlot samples using initial screening compared to using enrichment
| Sample typea | No. of samples
|
Odds ratio | 95% confidence interval | P value | |||
|---|---|---|---|---|---|---|---|
| Tested | Positive
|
||||||
| Total | By initial screening | By enrichment | |||||
| PAT | 411 | 109 | 27 | 102 | 0.21 | 0.14-0.33 | <0.001 |
| FEC | 320 | 76 | 18 | 73 | 0.20 | 0.12-0.35 | <0.001 |
| WAT | 87 | 19 | 10 | 13 | 0.74 | 0.30-1.80 | 0.50 |
| SLU | 37 | 35 | 28 | 33 | 0.38 | 0.10-1.39 | 0.14 |
| Feedlot A | 443 | 134 | 39 | 127 | 0.24 | 0.16-0.35 | <0.001 |
| Feedlot B | 412 | 105 | 44 | 94 | 0.40 | 0.27-0.60 | <0.001 |
| Total (A+B) | 855 | 239 | 83 | 221 | 0.31 | 0.23-0.41 | <0.01 |
PAT, pooled fecal pats; FEC, rectal fecal samples; WAT, water with sediment from trough; SLU, manure slurry.
DISCUSSION
This study was the first to monitor over time the prevalence of phage in cattle and their environment. Bacteriophages targeting E. coli O157:H7 were detected in 26.5% of fecal pats and 23.8% of rectal fecal samples. These results confirm earlier findings (4, 18) that phage active against E. coli are likely ubiquitous within feedlots. Using the standard lab strain E. coli B as a host, Callaway et al. (4) isolated phage from 15% of rectal fecal samples and from 55% of feedlot pens. Oot et al. (18) reported that 65% of fecal pats in 10 pens were phage positive after enrichment using E. coli O157:H7 NCTC 12900 and E. coli B as hosts. In contrast to these two point-prevalence studies, we conducted a longitudinal investigation over months when shedding of E. coli O157:H7 was expected to be highest. This study is also the first to document isolation of phage from fecal slurry (94.6%) and water troughs (21.8%), demonstrating the wide distribution of phage in the feedlot environment.
In the present study, we selected E. coli O157:H7 R508 as the host strain for phage detection because it is a member of phage type 14. In Canada, phage types 14 and 14a are the predominant phenotypes of bovine and nonbovine E. coli O157:H7 isolates, and both have been associated with numerous disease outbreaks (36; R. Ahmed and W. Demczuk, personal communication). It was hypothesized that this approach would increase the likelihood of our isolating phage that exhibited activity against those strains of E. coli O157:H7 that are most likely to cause human clinical disease. However, this selective approach may also have restricted our ability to isolate active phage against other strains or phage types of E. coli O157:H7. We are in the process of screening the 255 phage isolates obtained in the present study against our collection of ruminant and human E. coli O157:H7 isolates. Phage isolates that exhibit a low multiplicity of infection in a wide range of E. coli O157:H7 hosts will be selected for further study.
The frequency of phage isolation was noticeably high among slurry samples, with this detection frequently occurring without enrichment. Slurry comprises a mixture of urine, feces, water, spilled feed, and bedding (37). Slurry is high in moisture and nutrient content (9, 22), conditions that could promote host growth and the proliferation and persistence of phage (34). Slurry could serve as a phage reservoir and may contribute to a reduction of the prevalence of E. coli O157:H7 within a pen, although the benefits of the presence of a phage-rich slurry would have to be balanced against the reduced growth of cattle housed in wet pens (26).
The present data indicate that the prevalence of phage in fecal pats was greatest in May. April and May were the two wettest months of 2007; June, July, and August were drier (http://www.climate.weatheroffice.ec.gc.ca/advanceSearch/searchHistoricData_e.html). Previous studies suggested that soilborne bacteriophages may persist longer in wetter environments (12, 33). Presumably, phage persistence in May could have been enhanced in the well-moistened fecal pats or the fecal slurry that was present in the feedlot pens. More frequent isolation of phage from water troughs in May also suggests a higher overall environmental prevalence of phage during this month. The prevalence of phage in feedlot A also increased from 12.5% in August to 32.3% in September, a period that coincided with an increase in rainfall (12.3 to 46.3 mm).
Interestingly, July at feedlot A showed the second highest prevalence of phage among the months sampled. July had the highest average ambient temperature (22.8°C), a factor that could also lead to increased growth of E. coli O157:H7 within the feedlot environment (2, 29) and, possibly, to the proliferation of phage. Higher isolation rates during warmer months have also been reported for bacteriophages against O149 enterotoxigenic E. coli (14). The monthly variation of phage in fecal pat samples was consistent with the predator-prey relationship that likely exists between phage and their host both in cattle and their environment. When E. coli O157:H7 was abundant, phage increased and E. coli O157:H7 populations subsequently declined. With low numbers of host, phage declined, leading to a recovery of the E. coli O157:H7 population. The role that these fluctuations in predator-prey relationships play in the seasonal prevalence of E. coli O157:H7 (2, 25) remains to be determined.
In contrast to feedlot A, phage prevalence in feedlot B did not demonstrate the same monthly fluctuation, possibly because of more frequent turnover of animals within pens. Moreover, the pens in feedlot B were apparently better drained, given their less frequent accumulation of slurry compared with feedlot A. These conditions may have resulted in a lower overall prevalence of phage in feedlot B than in feedlot A. In seven of the eight pens, the overall prevalence of phage was similar between fecal pats and rectal fecal samples across two sample times (S1 and S2), suggesting that the shedding of phage from individual animals contributed directly to the observed prevalence in pats on the pen floor. Thus, for the majority of pens, there was no difference in the prevalence of phage in fecal pats versus rectal fecal grab samples.
The association between phage prevalence in fecal pats or water trough samples and reduced E. coli O157:H7 prevalence in rectal fecal material indicates that phage in the environment may reduce E. coli O157:H7 populations. Williams Smith et al. (35) demonstrated that treatment of litter (102 organisms/12 m2 floor area) with phage active against enteropathogenic E. coli prevented diarrhea in calves. Sheng et al. (23) added phage to drinking water at 106 PFU/ml and suggested that this practice reduced the shedding of E. coli O157:H7 in cattle. Thus, administering phage on pen floors or in water troughs may be a convenient and effective means of controlling E. coli O157:H7 populations in cattle. The negative correlation between phage isolation and populations of its host E. coli O157:H7 in rectal fecal samples is in accord with the work of Oot et al. (18) and suggests that endemic phage attenuate populations of E. coli O157:H7. This study used a random subset of rectal fecal samples collected from a much larger investigation of E. coli O157:H7 in feedlots A and B (T. P. Stephens, unpublished data). In this study, the overall prevalence of E. coli O157:H7 in fecal grab samples (23.9%) was high but not unlike that reported previously (20). The high numbers of host persisting during the study period likely increased phage prevalence. Callaway et al. (5) demonstrated that naturally occurring phage isolated from cattle were capable of reducing E. coli O157:H7 populations in experimentally inoculated sheep. In the present study, phage isolation from rectal fecal material was more frequent in association with greater (4.6 log10 CFU/g) than with smaller (<1.0 log10 CFU/g) populations of E. coli O157:H7, which supports the theory of greater amplification of phage when more host is present. An in vitro study with bacteriophage T4 that exhibited activity against E. coli showed that amplification did not occur when numbers of the host dropped below 103 cells/ml (32). Consequently, E. coli O157:H7 may persist within cattle or the environment when phage proliferation is limited due to inadequate hosts, inappropriate physiological states for replication, or an infrequent contact with target (7). Failure of phage to eliminate E. coli O157:H7 in cattle (17, 23) may be a result of suboptimal host-to-phage ratios. Callaway et al. (5) found that an initial ratio (1:1) of phage to host was most effective for reducing numbers of E. coli O157:H7 in cecal and rectal contents from sheep, illustrating that phage efficacy depends on the density of host cells.
Dilution of E. coli O157:H7 and phage may reduce the effectiveness of this approach for controlling this pathogen in large volumes of water. Our laboratory showed previously that bacteriophage are not effective in controlling E. coli O157:H7 inoculated into water microcosms (16). Even though phage was not effective in controlling E. coli O157:H7 in water, Sheng et al. (23) found that phage administered through water decreased shedding of E. coli O157:H7 by cattle. The necessity of host-phage interaction may make phage therapy more effective at mitigating E. coli O157: H7 in the animal than in the environment.
Improved detection of phage after enrichment compared to initial screening confirmed the findings of Oot et al. (18) and also showed that low levels of phage may persist within the environment. Given its utility for detecting phage-positive samples, the enrichment technique is strongly recommended as a means of determining prevalence of endemic phage in cattle and their environment.
In conclusion, this study demonstrated monthly fluctuations in populations of E. coli O157:H7-infecting bacteriophage. Bacteriophage against E. coli O157:H7 were more commonly isolated from pen floor slurry than from other sources examined. Bacteriophages isolated from the feedlot environment may prove to be efficacious for controlling E. coli O157:H7 in feedlot cattle. Observations in this study suggest that environmental factors such as moisture levels and temperature likely influence the prevalence of E. coli O157:H7-infecting phage. The prevalence of these bacteriophages in fecal pats from the pen floor was similar to that in fecal samples collected from the rectum. Most importantly, endemic phage both in individual animals and in their environment were associated with a reduced prevalence of E. coli O157:H7 in cattle. Increased knowledge of the ecology of phage could aid in refining this therapy as a means of controlling E. coli O157:H7 in feedlot cattle. Further work is required to define the genetic diversity of E. coli O157:H7-infecting bacteriophage isolates within pens and over time, as well as the degree of infectivity of these phages for a variety of strains of the host E. coli O157:H7.
Acknowledgments
Financial support for this research project was provided by the Food Safety Initiative of Alberta Agriculture and Rural Development.
We gratefully acknowledge J. Graham, J. Hoffarth, and A. Stronks for sample collection and thank Public Health Agency of Canada, Guelph, Ontario, Canada, for the kind provision of the E. coli O157:H7 R508 host. We appreciate the excellent cooperation of the commercial feedlots that participated in this study.
Footnotes
Published ahead of print on 9 January 2009.
REFERENCES
- 1.Ackermann, H.-W., and H. M. Krisch. 1997. A catalogue of T4-type bacteriophages. Arch. Virol. 142:2329-2345. [DOI] [PubMed] [Google Scholar]
- 2.Bonardi, S., E. Maggi, A. Bottarelli, M. L. Pacciarini, A. Ansuini, G. Vellini, S. Morabito, and A. Caprioli. 1999. Isolation of verocytotoxin-producing Escherichia coli O157:H7 from cattle at slaughter in Italy. Vet. Microbiol. 67:203-211. [DOI] [PubMed] [Google Scholar]
- 3.Brüssow, H. 2005. Bacteriophage therapy: the Escherichia coli experience. Microbiology 151:2133-2140. [DOI] [PubMed] [Google Scholar]
- 4.Callaway, T. R., T. S. Edrington, A. D. Brabban, J. E. Keen, R. C. Anderson, M. L. Rossman, M. J. Engler, K. J. Genovese, B. L. Gwartney, J. O. Reagan, T. L. Poole, R. B. Harvey, E. M. Kutter, and D. J. Nisbet. 2006. Fecal prevalence of Escherichia coli O157, Salmonella, Listeria, and bacteriophage infecting E. coli O157:H7 in feedlot cattle in the southern plains region of the United States. Foodborne Pathog. Dis. 3:234-244. [DOI] [PubMed] [Google Scholar]
- 5.Callaway, T. R., T. S. Edrington, A. D. Brabban, R. C. Anderson, M. L. Rossman, M. J. Engler, M. A. Carr, K. J. Genovese, J. E. Keen, M. L. Looper, E. M. Kutter, and D. J. Nisbet. 2008. Bacteriophage isolated from feedlot cattle can reduce Escherichia coli O157:H7 populations in ruminant gastrointestinal tracts. Foodborne Pathog. Dis. 5:183-191. [DOI] [PubMed] [Google Scholar]
- 6.Chibani-Chennoufi, S., A. Bruttin, M.-L. Dillmann, and H. Brüssow. 2004. Phage-host interaction: an ecological perspective. J. Bacteriol. 186:3677-3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chibani-Chennoufi, S., J. Sidoti, A. Bruttin, M.-L. Dillmann, E. Kutter, F. Qadri, S. A. Sarker, and H. Brüssow. 2004. Isolation of Escherichia coli bacteriophages from the stool of pediatric diarrhea patients in Bangladesh. J. Bacteriol. 186:8287-8294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dabrowska, K., K. Switala-Jelen, A. Opolski, B. Weber-Dabrowska, and A. Gorski. 2005. Bacteriophage penetration in vertebrates. J. Appl. Microbiol. 98:7-13. [DOI] [PubMed] [Google Scholar]
- 9.Eghball, B., J. Wienhold, B. L. Woodbury, and R. A. Eigenberg. 2005. Phosphorus management—plant availability of phosphorus in swine slurry and cattle feedlot manure. Agron. J. 97:542-548. [Google Scholar]
- 10.Gannon, V. P., S. D'Souza, T. Graham, and R. K. King. 1997. Specific identification of Escherichia coli O157:H7 using multiplex PCR assay. Adv. Exp. Med. Biol. 412:81-82. [DOI] [PubMed] [Google Scholar]
- 11.Hancock, D. D., T. E. Besser, D. H. Rice, E. D. Ebel, D. E. Herriott, and L. V. Carpenter. 1998. Multiple sources of Escherichia coli O157 in feedlots and dairy farms in the northwestern USA. Prev. Vet. Med. 35:11-19. [DOI] [PubMed] [Google Scholar]
- 12.Hurst, C. J., C. P. Gerba, and I. Cech. 1980. Effects of environmental variables and soil characteristics on virus survival in soil. Appl. Environ. Microbiol. 40:1067-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inal, J. M. 2003. Phage therapy: a reappraisal of bacteriophages as antibiotics. Arch. Immunol. Ther. Exp. (Warsaw) 51:237-244. [PubMed] [Google Scholar]
- 14.Jamalludeen, N., R. P. Johnson, R. Friendship, A. M. Kropinski, E. J. Lingohr, and C. L. Gyles. 2007. Isolation and characterization of nine bacteriophages that lyse O149 enterotoxigenic Escherichia coli. Vet. Microbiol. 124:47-57. [DOI] [PubMed] [Google Scholar]
- 15.Matsuzaki, S., M. Rashel, J. Uchiyama, S. Sakurai, T. Ujihara, M. Kuroda, M. Ikeuchi, T. Tani, M. Fujieda, H. Wakiguchi, and S. Imai. 2005. Bacteriophage therapy: a revitalized therapy against bacterial infectious diseases. J. Infect. Chemother. 11:211-219. [DOI] [PubMed] [Google Scholar]
- 16.McAllister, T. A., S. J. Bach, and R. P. Johnson. 2005. Use of bacteriophage to control Escherichia coli O157:H7 in beef cattle and the environment. Final report, project 1.31. Beef Cattle Research Council, Calgary, Alberta, Canada.
- 17.Niu, Y. D., Y. Xu, T. A. McAllister, E. Rozema, T. P. Stephens, S. J. Bach, R. P. Johnson, and K. Stanford. 2008. Comparison of fecal versus rectoanal mucosal swab sampling for detecting Escherichia coli O157:H7 in experimentally inoculated cattle used in assessing bacteriophage as a mitigation strategy. J. Food Prot. 71:691-698. [DOI] [PubMed] [Google Scholar]
- 18.Oot, R. A., R. R. Raya, T. R. Callaway, T. S. Edrington, E. M. Kutter, and A. D. Brabban. 2007. Prevalence of Escherichia coli O157 and O157:H7-infecting bacteriophages in feedlot cattle feces. Lett. Appl. Microbiol. 45:445-453. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 20.Sanderson, M. W., J. M. Sargeant, X. Shi, T. G. Nagaraja, L. Zurek, and M. J. Alam. 2006. Longitudinal emergence and distribution of Escherichia coli O157 genotypes in a beef feedlot. Appl. Environ. Microbiol. 72:7614-7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SAS Institute. 1999. SAS users' guide: statistics, version 9.1. SAS Institute, Inc., Cary, NC.
- 22.Schröder, J. J., A. G. Jansen, and G. J. Hilhorst. 2005. Long-term nitrogen supply from cattle slurry. Soil Use Manage. 21:196-204. [Google Scholar]
- 23.Sheng, H., H. J. Knecht, I. T. Kudva, and C. J. Hovde. 2006. Application of bacteriophages to control intestinal Escherichia coli O157:H7 levels in ruminants. Appl. Environ. Microbiol. 72:5359-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shere, J. A., K. J. Bartlett, and C. W. Kaspar. 1998. Longitudinal study of Escherichia coli O157:H7 dissemination on four dairy farms in Wisconsin. Appl. Environ. Microbiol. 64:1390-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanford, K., D. Croy, S. J. Bach, G. L. Wallins, H. Zahiroddini, and T. A. McAllister. 2005. Ecology of Escherichia coli O157:H7 in commercial dairies in southern Alberta. J. Dairy Sci. 88:4441-4451. [DOI] [PubMed] [Google Scholar]
- 26.Step, L. D., and R. A. Smith. 2006. Nonrespiratory diseases of stocker cattle. Vet. Clin. N. Am. Food Anim. Pract. 22:413-434. [DOI] [PubMed] [Google Scholar]
- 27.Suttle, C. A. 2005. Viruses in the sea. Nature 437:356-361. [DOI] [PubMed] [Google Scholar]
- 28.Tanji, Y., T. Shimada, M. Yoichi, K. Miyanaga, K. Hori, and H. Unno. 2004. Toward rational control of Escherichia coli O157:H7 by a phage cocktail. Appl. Microbiol. Biotechnol. 64:270-274. [DOI] [PubMed] [Google Scholar]
- 29.Van Donkersgoed, J., J. Berg, A. Potter, D. Hancock, T. Besser, D. Rice, J. LeJeune, and S. Klashinsky. 2001. Environmental sources and transmission of Escherichia coli O157 in feedlot cattle. Can. Vet. J. 42:714-720. [PMC free article] [PubMed] [Google Scholar]
- 30.Vernozy-Rozand, C., M. P. Montet, F. Lequerrec, E. Serillon, B. Tilly, C. Bavai, S. Ray-Gueniot, J. Bouvet, C. Mazuy-Cruchaudet, and Y. Richard. 2002. Prevalence of verotoxin-producing Escherichia coli (VTEC) in slurry, farmyard manure and sewage sludge in France. J. Appl. Microbiol. 93:473-478. [DOI] [PubMed] [Google Scholar]
- 31.Viscardi, M., G. A. Perugini, C. Auriemma, F. Capuano, S. Morabito, K. P. Kim, J. M. Loessner, and G. Iovane. 2008. Isolation and characterization of two novel coliphages with high potential to control antibiotic-resistant pathogenic Escherichia coli (EHEC and EPEC). Int. J. Antimicrob. Agents 31:152-157. [DOI] [PubMed] [Google Scholar]
- 32.Wiggins, B. A., and M. Alexander. 1985. Minimum bacterial density for bacteriophage replication: implications for significance of bacteriophages in natural ecosystems. Appl. Environ. Microbiol. 49:19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williamson, K. E., M. Radosevich, and K. E. Wommack. 2005. Abundance and diversity of viruses in six Delaware soils. Appl. Environ. Microbiol. 71:3119-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williamson, S. J., and J. H. Paul. 2004. Nutrient stimulation of lytic phage production in bacterial populations of the Gulf of Mexico. Aquat. Microb. Ecol. 36:9-17. [Google Scholar]
- 35.Williams Smith, H., H. B. Huggins, and K. M. Shaw. 1987. The control of experimental Escherichia coli diarrhoea in calves by means of bacteriophages. J. Gen. Microbiol. 133:1111-1126. [DOI] [PubMed] [Google Scholar]
- 36.Woodward, D. L., C. G. Clark, R. A. Caldeira, R. Ahmed, and F. G. Rodgers. 2002. Verotoxigenic Escherichia coli (VTEC): a major public health threat in Canada. Can. J. Infect. Dis. 13:321-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woolcock, J. B. 1991. Microbiology of animals and animal products. Elsevier Science Publishing Company, Inc., New York, NY.