Abstract
DNA barcoding is a diagnostic technique for species identification using a short, standardized DNA. An effective DNA barcoding marker would be very helpful for unraveling the poorly understood species diversity of dinoflagellates in the natural environment. In this study, the potential utility for DNA barcoding of mitochondrial cytochrome c oxidase 1 (cox1) and cytochrome b (cob) was assessed. Among several primer sets examined, the one amplifying a 385-bp cob fragment was most effective for dinoflagellates. This short cob fragment is easy to sequence and yet possess reasonable taxon resolution. While the lack of a uniform gap between interspecific and intraspecific distances poses difficulties in establishing a phylum-wide species-discriminating distance threshold, the variability of cob allows recognition of species within particular lineages. The potential of this cob fragment as a dinoflagellate species marker was further tested by applying it to an analysis of the dinoflagellate assemblages in Long Island Sound (LIS) and Mirror Lake in Connecticut. In LIS, a highly diverse assemblage of dinoflagellates was detected. Some taxa can be identified to the species and some to the genus level, including a taxon distinctly related to the bipolar species Polarella glacialis, and the large number of others cannot be clearly identified, due to the inadequate database. In Mirror Lake, a Ceratium species and an unresolved taxon were detected, exhibiting a temporal transition from one to the other. We demonstrate that this 385-bp cob fragment is promising for lineage-wise dinoflagellate species identification, given an adequate database.
DNA barcoding is a diagnostic technique for species identification using a short, standardized DNA (i.e., DNA barcode) (15). For microbial organisms, this PCR-based technique is useful not only for identifying cultured species but also for rapid retrieval and species identification for uncultured taxa from natural environments. A good DNA barcoding marker should be simple (easy to PCR amplify and sequence) and universal (effective for a wide range of lineages), with a high resolving power (high interspecific and low intraspecific variations). Therefore, an ideal DNA barcoding marker is a relatively short and reasonably variable gene fragment (for species discrimination) flanked by highly conserved sequences (for primer design). The pioneering DNA barcoding work used mitochondrial cytochrome c oxidase 1 (cox1) to identify animal species (9, 10). Mitochondrial genes are a good barcode choice for animals, because they are markedly more variable than nuclear genes (3, 32) and contain conserved regions for primer design. Among other organisms, cox1 has also been shown to be useful for barcoding other organisms, such as fungi (35). Initial attempts at cox1 barcoding for macroalgae (rhodophyte and phaeophyte) also showed good potential (21, 29, 34). In land plants, the mitochondrial genome evolves substantially more slowly than the nuclear genome (26, 27), rendering its genes less useful than genes from chloroplast (14, 15). The utility of cox1 or other mitochondrial genes for DNA barcoding is less clear for unicellular organisms, with few documented attempts (e.g., reference 7) for those living in the marine ecosystem.
Dinoflagellates are important unicellular organisms in the marine ecosystem because of their significant contribution to marine primary production, support of coral reef growth through symbiotic associations (31), micrograzing (25), and formation of harmful and often toxic algal blooms (1). Dinoflagellates are genetically diverse, with at least 2,000 documented extant species and 2,000 fossil species. Continual discovery of new species in the ocean (e.g., references 6, 12, 13, 17, 20, 22, 24, and 38) suggests that there are likely many more dinoflagellate lineages to be recognized. Identification of dinoflagellate species and discovery of species diversity by use of traditional morphological analysis is often hampered by high degrees of morphological similarity and a lack of unique characters between different species. A systematic survey of dinoflagellate diversity using a diagnostic molecular marker is highly desirable. To unravel species diversity and new taxa in natural environments, a DNA barcode would need to be specific for dinoflagellates in addition to the above-mentioned requirements.
In this study, the potential utilities of mitochondrial genes as DNA barcoding markers were assessed. Mitochondrial cox1 and cob (the gene coding for cytochrome b) from dinoflagellates were compared for PCR efficiency and resolving power. We demonstrated that while neither of the mitochondrial genes seems to be a good phylum-wide DNA barcoding marker, a cob primer set can be used to determine the species diversity of dinoflagellate flora in a lineage-by-lineage manner.
MATERIALS AND METHODS
Barcode primer development.
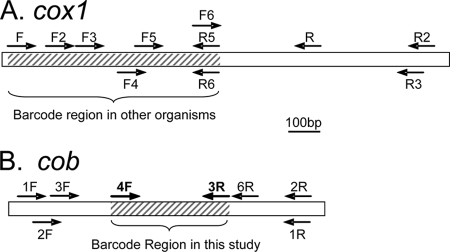
Available dinoflagellate mitochondrial cox1 and cob sequences were aligned using CLUSTAL W (version 1.8) (39). Primers were designed for conserved regions by using Beacon Designer 3.0 (Premier Biosoft International). Various sets of the primers for both cox1 and cob had been published elsewhere (44, 45), but two reverse primers for cox1 and one for cob were new, and all possible primer combinations with predicted amplicons of >300 bp were tested for both cox1 and cob in this study (Table 1 and Fig. 1). Among the cox1 primers, the Dinocox1F-Dinocox1R5 and Dinocox1F-Dinocox1R6 combinations embraced the gene fragment that had been commonly used for DNA barcoding in animals.
TABLE 1.
Primers used in the present study
| Primer name | Sequence (5′-3′) | Dinoflagellate application |
|---|---|---|
| Dinocox1Fa | AAAAATTGTAATCATAAACGCTTAGG | cox1 forward |
| Dinocox1F2b | GAATTATATCTCCTGAAAACCAGAACTTC | cox1 forward |
| Dinocox1F3b | TTATGATCTTCTTYTTWRTWATGCC | cox1 forward |
| Dinocox1F4b | TTTGGAGGTGGWWCWGGNTGGAC | cox1 forward |
| Dinocox1F5b | TGGTTGGACATTATATCCTCCATTATC | cox1 forward |
| Dinocox1F6b | GGTTCTTTGGACATCCTGAAGTTTA | cox1 forward |
| Dinocox1Rb | TGTTGAGCCACCTATAGTAAACATTA | cox1 reverse |
| Dinocox1R2b | AGTTATTCCTGATCCAATAGATGACAG | cox1 reverse |
| Dinocox1R3b | CTGATCCAATAGATGACAGAAAATTCC | cox1 reverse |
| Dinocox1R4b | CATGAYACWTATTATRTWRTWGCWCATTTCCA | cox1 reverse |
| Dinocox1R5c | TAAACTTCAGGRTGDCCRAARAACCA | cox1 reverse |
| Dinocox1R6c | GGAAKWATTARGATRTAAACTTCWGGATG | cox1 reverse |
| Dinocob1Fa | ATGAAATCTCATTTACAWWCATATCCTTGTCC | cob forward |
| Dinocob2Fb | ATWAATTYTTTYTGGAATHTBGGTTT | cob forward |
| Dinocob3Fb | GAATTACTATTRTTATHCARATHNTWACWGG | cob forward |
| Dinocob4Fb | AGCATTTATGGGTTATGTNTTACCTTT | cob forward |
| Dinocob1Ra | TCTCTTGAGGKAATTGWKMACCTATCCA | cob reverse |
| Dinocob2Rb | CGAGCATAAGATAKAAACWTCTCTTGAGG | cob reverse |
| Dinocob3Rb | AGCTTCTANDGMATTATCTGGATG | cob reverse |
| Dinocob6Rc | ATTGGCATAGGAAATACCATTCAGG | cob reverse |
FIG. 1.
Schematic diagram showing locations of primers designed for cox1 (A) and cob (B) barcoding markers. Lengths of genes are indicated by the scale bar in the middle. (A) The hatched area indicates a conventional DNA barcode region (embraced by primer set FR5 or FR6). The expected lengths of the PCR products for all primer sets are as follows: for FR2 and FR3, 1.4 kb; for F2R2 and F2R3, 1.2 kb; for F3R2 and F3R3, 1 kb; for FR5 and FR6, 0.7 kb; and for F2R5 and F2R6, 0.6 kb. (B) The hatched area indicates the cob region that is identified as a potential barcode region in this study. The expected lengths of the PCR products for all primer sets are as follows: for 1F1R and 1F2R, 1 kb; for 1F3R, 0.73 kb; for 1FR6, 0.79 kb; for 2F1R and 2F2R, 0.96 kb; for 2F3R, 0.69 kb; for 2FR6, 0.75 kb; for 3F1R and 3F2R, 0.92 kb; for 3F3R, 0.65 kb; for 3FR6, 0.71 kb; for 4F1R and 4F2R: 0.66 kb; for 4F3R, 0.38 kb; and for 4F6R, 0.44 kb.
Cultures.
Dinoflagellate species representing six major orders (Table 2) were used in this study as a database through which unknown samples could be compared. To maximize geographic coverage of dinoflagellate habitats, two polar species, Heterocapsa arctica (strain CCMP445) and Polarella glacialis (strain CCMP1383 from the Antarctic Ocean and CCMP2088 from the Arctic Ocean), were grown at 4°C with a light intensity of 30 microeinsteins m−2 s−1 and included in this study. Growth was monitored by removing a 1-ml sample every other day and examining cell concentration under a microscope with a Sedgwick-Rafter counting chamber. Once the cultures entered the exponential growth phase, about 1 million cells were harvested by centrifugation (3,000 × g), and cell pellets were kept at −80°C until DNA extraction.
TABLE 2.
Dinoflagellate taxa used in the distance matrix and phylogenetic analysis in this study
| Species | Strain or source | GenBank accession no.
|
|
|---|---|---|---|
| cox1 | cob | ||
| Adenoides eludens | EF036541 | ||
| Akashiwo sanguinea | LIS1 | EU126138 | AY456105 |
| Alexandrium affine | CCMP112 | EF377324 | |
| Alexandrium catenella | AB290124 | ||
| Alexandrium pseudogonyaulax | AB290130 | ||
| Alexandrium tamarense | CB307; D. M. Anderson | EU126139 | AY456116 |
| Alexandrium tamiyavanichi | AB290122 | ||
| Amphidinium carterae | CCMP1314 | EU126140 | EU126130 |
| Amphidinium operculatum | CCMP123 | EU126141 | EU126131 |
| Ceratium sp. | Mirror Lake | AY460573 | |
| Ceratium longipes | CCMP1770 | EU840164a | |
| Ceratocorys horrida | CCMP157 | EU840165a | |
| Crypthecodinium cohnii | WHd; M. Gray | AF186994 | AF403220 |
| Dinophysis acuminata | Narragansett Bay sample | EU130566 | EU130568 |
| Gambierdiscus toxicus | CCMP401 | EU840166a | |
| Gymnodinium catenatum | CCMP1937 | EU840167a | |
| Gyrodinium instriatum | JM010818-1D5 | AY786473 | |
| Heterocapsa arctica | CCMP445 | EU840168a | |
| Heterocapsa rotundata | CCMP1542 | EU126143 | EU126133 |
| Heterocapsa triquetra | CCMP449 | EU126142 | EU126132 |
| Karenia brevis | CCMP2229 | EU126144 | AY456104 |
| Karenia mikimotoi | CCMP429 | EU840169a | |
| Karlodinium veneficum | CCMP1975 | AF463416 | AY345908 |
| Lingulodinium polyedrum | CCMP405 | AY456109 | |
| Oxyrrhis marina | CCMP1795 | EU126146 | EU126134 |
| Peridinium aciculiferum | DQ094829 | ||
| DQ094825 | |||
| DQ094826 | |||
| DQ094827 | |||
| DQ094828 | |||
| Pfiesteria piscicida | CCMP1831 | AF463412 | AF357519 |
| Pfiesteria-like | CCMP1828 | EU126147 | AY456119 |
| Pfiesteria-like | CCMP1835 | AY456120 | |
| Pfiesteria-like | CCMP1827 | AY456117 | |
| Polarella glacialis | CCMP1383, CCMP2088 | EU840170a | |
| EU840171a | |||
| Prorocentrum cassubicum | LB1596 | EU126135 | |
| Prorocentrum dentatum | CCMP1517 | DQ336058 | |
| Prorocentrum donghaiense | S. Lü | DQ336055 | |
| Prorocentrum lima | CCMP1966 | EU840172a | |
| Prorocentrum micans | CCMP1589 | EU126148 | AY585525 |
| Prorocentrum minimum | PTPM | DQ336070 | |
| JA01 | DQ336064 | ||
| EXUV | DQ336061 | ||
| CCMP696 | AF463414 | AY030286 | |
| Prorocentrum nanum | LB1008 | EU126136 | |
| Protoceratium reticulatum | CCMP1721 | EU126137 | |
| Pseudopfiesteria shumwayae | T4; P. Tester | EU130570 | AF502593 |
| Pyrocystis lunula | J. W. Hastings | EU840173a | |
| Pyrocystis noctiluca | CCMP732 | EU840174a | |
| Pyrodinium bahamense | AY456114 | ||
| Scrippsiella hangoei | EF205036 | ||
| DQ094821 | |||
| DQ094822 | |||
| DQ094823 | |||
| DQ094824 | |||
| Scrippsiella sp. | LIS | EU130571 | AY743961 |
| Scrippsiella sweeneyae | CCMP280 | EU840175a | |
| Symbiodinium goreaui | CCMP2466 | EU130574 | |
| Symbiodinium microadriaticum | CCMP830 | EU130573 | AY456110 |
| Symbiodinium sp. | CCMP832 | EU130572 | AY456112 |
Sequence obtained in this study.
Environmental samples from LIS.
Water samples were collected from three stations (Table 3). The first two stations, A4 (40°52′21″N, 73°44′03″W) and K2 (41°14′04″N, 72°15′57″W), were located in the western and eastern sections of Long Island Sound (LIS), respectively, and were two of the stations regularly sampled in the Water Quality Monitoring Program by the Connecticut Department of Environment Protection. Samples were collected in February, April, July, and October 2002 from a depth of 0.5 m by using Niskin bottles mounted on a conductivity-temperature-depth rosette. The third station (AP; 41°18′55″N, 72°38′11″W) was located at the Avery Point campus of the University of Connecticut, off the east end of LIS (near K2), where surface samples were collected using a clean plastic bucket on 28 March, 23 May, 29 August, 26 September, and 21 November 2003 and 23 January 2004 (Table 3). From each station, subsamples of 250 ml were taken and fixed on site with Utermöhl's solution (40) at a final concentration of 2%. Upon arrival at our laboratory, the Utermöhl-preserved samples were either processed immediately (Avery Point samples) or stored at 4°C for a short period of time before DNA extraction (within 1 month).
TABLE 3.
Dinoflagellate taxa and genotypes of dinoflagellates from the three stations in LIS, based on cob barcode sequencea
| Station, sampling time, and taxon | No. of genotypes (total no. of clones obtained) |
|---|---|
| A4 | |
| February 2002 | |
| Karlodinium-like | 1 (1) |
| Gymnodinium-like | 4 (8) |
| S. sweeneyae | 1 (3) |
| Unknown9 | 2 (8) |
| Unknown10 | 1 (1) |
| April 2002 | |
| Suessiale-like | 1 (3) |
| Polarella-like | 1 (7) |
| Gymnodinium-like | 3 (4) |
| Unknown3 | 1 (1) |
| Unknown9 | 2 (5) |
| Unknown10 | 2 (2) |
| July 2002 | |
| Karlodinium-like | 1 (1) |
| Gymnodinium-like | 1 (1) |
| P. nanum-like | 1 (1) |
| P. micans-like | 1 (1) |
| Scrippsiella sp. | 1 (1) |
| D. acuminata-like | 1 (3) |
| Pyrocystis-like | 2 (6) |
| Unknown2 | 1 (4) |
| Unknown4 | 1 (1) |
| Unknown9 | 2 (7) |
| Unknown10 | 1 (1) |
| October 2002 | |
| Gymnodinium-like | 8 (3) |
| Gymnodiniales2 | 1 (1) |
| Pyrocystis-like | 1 (1) |
| Unknown9 | 12 (3) |
| K2 | |
| February 2002 | |
| Gymnodinium-like | 5 (10) |
| Gymnodiniales2 | 2 (2) |
| Karenia-like | 1 (2) |
| Unknown3 | 2 (3) |
| Unknown8 | 1 (2) |
| Unknown9 | 2 (4) |
| Unknown10 | 1 (2) |
| April 2002 | |
| Durinskia-like | 1 (3) |
| Karlodinium-like | 1 (2) |
| Gymnodinium-like | 3 (3) |
| Gymnodiniales1 | 1 (1) |
| S. sweeneyae | 1 (5) |
| Pyrocystis-like | 1 (2) |
| Unknown5 | 4 (3) |
| Unknown8 | 2 (2) |
| Unknown10 | 2 (1) |
| July 2002 | |
| Polarella-like | 1 (3) |
| Gymnodinium-like | 1 (1) |
| P. nanum-like | 1 (1) |
| Scrippsiella-like | 1 (1) |
| S. sweeneyae | 2 (4) |
| D. acuminata-like | 1 (1) |
| Ceratocorys-like | 2 (3) |
| Peridinium-like | 1 (1) |
| Unknown5 | 1 (1) |
| Unknown9 | 2 (3) |
| Unknown10 | 2 (3) |
| October 2002 | |
| Pyrocystis-like | 1 (2) |
| Unknown9 | 5 (15) |
| Unknown10 | 1 (1) |
| AP | |
| 28 March 2003 | |
| Karlodinium-like | 1 (2) |
| Karenia-like | 2 (2) |
| Gymnodinium-like | 4 (19) |
| Gymnodiniales1 | 2 (6) |
| Gymnodiniales2 | 1 (1) |
| D. acuminata | 1 (1) |
| Unknown2 | 2 (5) |
| Unknown5 | 1 (5) |
| Unknown9 | 1 (3) |
| Unknown10 | 1 (4) |
| 23 May 2003 | |
| Suessiale-like | 1 (1) |
| Polarella-like | 1 (4) |
| Karlodinium-like | 2 (6) |
| Karenia-like | 1 (1) |
| Gymnodinium-like | 3 (3) |
| Gymnodiniales1 | 1 (1) |
| D. acuminata-like | 2 (5) |
| Pyrocystis-like | 1 (1) |
| Ceratium-like | 1 (1) |
| Unknown2 | 1 (1) |
| Unknown3 | 13 (2) |
| Unknown7 | 1 (1) |
| Unknown8 | 1 (8) |
| Unknown10 | 1 (1) |
| 29 August 2003 | |
| Polarella glacialis | 1 (1) |
| Polarella-like | 1 (1) |
| Akashiwo-like | 2 (3) |
| A. sanguinea | 1 (9) |
| Karlodinium-like | 3 (5) |
| Gymnodinium-like | 5 (6) |
| Gymnodiniales2 | 4 (5) |
| Scrippsiella-like | 1 (1) |
| Scrippsiella sp. | 2 (2) |
| Ceratocorys-like | 1 (1) |
| Peridinium-like | 6 (10) |
| Unknown10 | 2 (2) |
| 26 September 2003 | |
| Akashiwo-like | 1 (1) |
| A. sanguinea | 1 (14) |
| Karlodinium-like | 1 (1) |
| Gymnodinium-like | 1 (1) |
| Gymnodiniales2 | 1 (1) |
| D. acuminata-like | 2 (2) |
| Ceratium-like | 1 (1) |
| Peridinium-like | 9 (16) |
| Unknown2 | 1 (1) |
| Unknown9 | 1 (1) |
| Unknown10 | 2 (4) |
| 21 November 2003 | |
| Suessiale-like | 1 (1) |
| Karenia-like | 1 (3) |
| Karlodinium-like | 1 (1) |
| Gymnodinium-like | 7 (8) |
| Gymnodiniales1 | 3 (3) |
| Gymnodiniales2 | 1 (4) |
| P. micans | 1 (1) |
| Lingulodinium-like | 1 (1) |
| Pfiesteria-like | 1 (1) |
| Unknown1 | 2 (6) |
| Unknown2 | 1 (1) |
| Unknown3 | 1 (4) |
| Unknown5 | 1 (1) |
| Unknown6 | 4 (6) |
| Unknown9 | 1 (1) |
| Unknown10 | 5 (1) |
| 23 January 2004 | |
| Gymnodinium-like | 4 (4) |
| Gymnodiniales1 | 1 (1) |
| Gymnodiniales2 | |
| Unknown7 | 1 (1) |
| Unknown9 | 2 (6) |
| Unknown10 | 6 (29) |
A4(40°52′21″N, 73°44′03″W) and K2 (41°14′04″N, 72°15′57″W) were two representative stations located in the western and eastern sections of the Sound, respectively. AP (41°18′55″N, 72°38′11″W) was located at the Avery Point campus of the University of Connecticut, off the east end of Long Island Sound, near K2. One taxon may contain multiple genotypes.
Samples from Mirror Lake.
To examine if the potential DNA barcoding marker would be useful for freshwater dinoflagellates, we sampled three times (1 and 28 June and 25 August 2008) from Mirror Lake, a retention pond constructed to receive overflow rainwater on the Storrs campus of the University of Connecticut (41°48′36″N, 72°15′36″W). At each sampling event, 5 liters of water was collected from the surface of the lake by using a clean bucket. Immediately, 500 ml was filtered onto a 5-μm Nuclepore filter membrane by using a handheld vacuum pump. Filtration was completed within 3 min. The filter retaining the plankton cells was immersed in 0.5 ml DNA buffer (0.1 M EDTA, 1% sodium dodecyl sulfate with 100 μg ml−1 proteinase K) in a microcentrifuge tube. Upon arrival in our laboratory (within 2 h), more proteinase K was added to reach a final concentration 200 μg ml−1 and the sample was processed for DNA extraction.
Microscopic analysis.
During each sampling, a subsample of 250 ml was taken and fixed on site with Utermöhl's solution for subsequent microscopic analysis of dinoflagellate identities and cell concentrations. In the laboratory, 100 ml of the Utermöhl-preserved samples was transferred to two 50-ml cone-bottom centrifuge tubes and allowed to settle for 3 days in the dark. The supernatant was aspirated down to 1 ml in each tube and combined together. One milliliter of the concentrated samples was placed in a Sedgwick-Rafter counting chamber for microscopic identification of phytoplankton species, following the method of Steidinger and Tengen (36). For the Avery Point station, water samples were observed for live dinoflagellates under the microscope and single cells were isolated into cultures.
DNA extraction.
Field-collected, Utermöhl-preserved samples from LIS were centrifuged (3,000 × g, 20 min), and the pellets were resuspended in 0.5 ml DNA extraction buffer containing 50 μg proteinase K. Similarly, cell pellets from cultures were resuspended in DNA extraction buffer. These samples, along with the Mirror Lake samples already in DNA buffer, were incubated overnight at 55°C. DNA was extracted using a cetyl trimethyl ammonium bromide protocol (43). To eliminate PCR-inhibitory compounds, each resultant DNA solution was further purified using a Zymo DNA cleanup and concentration column (Zymo Research, Orange, CA), eluted in 50 μl of 10 mM Tris-HCl buffer (pH 8.0), checked for DNA quality by universal 18S rRNA gene primers (43), and stored at −20°C.
PCR-based gene cloning and sequencing.
PCR for both cox1 and cob was performed with 25-μl reaction mixtures, using the following program: 1 min at 95°C, followed by 35 cycles of 20 s at 94°C, 30 s at 56°C, and 30 s at 72°C, and finally an elongation step of 7 min at 72°C. For PCRs that did not yield results, DNA quality was checked using universal 18S rRNA gene primers (43). A negative result was taken as indication of poor DNA quality, and DNA was repurified and PCR rerun. For DNA samples from monospecific cultures, PCR products were directly sequenced from both directions (in most of the cases) or cloned into a T vector before being sequenced (43). For field samples, PCR products were purified using a Zymo DNA cleanup and concentration column and cloned into a T vector (43). Colonies carrying a target gene fragment were randomly selected, and 20 to 50 clones were sequenced for each water sample by using a BigDye Terminator cycle sequencing kit (Applied Biosystems). A total of 450 clones were sequenced.
Sequence alignment, distance matrix, and phylogenetic analysis.
Sequences for each gene were aligned using CLUSTAL W (version 1.8) (39), inspected manually, and corrected when necessary for codon integrity. The data sets were run through ModelTest version 3.7 (28) to identify the best-fit nucleotide substitution model. The best-fit model (transversion model with gamma distributed rate variation among sites [TVM+G]) was then used to generate a distance matrix. For comparison, the default model in the Phylip DNADist program (F4) was also used to generate the distance matrix. On the basis of the best-fit distance matrix, a phylogenetic analysis was conducted using the unweighted-pair group method with arithmetic means (UPGMA) as previously reported for barcoding (e.g., reference 29), with a bootstrap value of 500 (cob) or 1,000 (cox1) replications. To verify the reliability of the tree topology, maximum-likelihood (ML) analysis was performed using PhyML (8), with a bootstrap value of 100 resamplings for the cultured species. To identify the dinoflagellate species in the field samples, the cob marker sequences from these field samples, along with those from cultured dinoflagellate species, were subjected to UPGMA analysis. An ML tree was not run, because the large data set made the analysis excessively slow.
Nucleotide sequence accession numbers.
The cob sequences obtained in this study were deposited in GenBank under accession numbers EU839995 to EU840054 (LIS stations A4 and K2), EU840055 to EU840151 (Avery Point), EU840152 to EU840163 (Mirror Lake), and EU840164 to EU840175 (laboratory cultures listed in Table 1).
RESULTS
Resolving power in phylogenetic analysis and PCR efficiency of the cox1 barcode region.
The alignment of sequences was fully colinear except for a small deletion in five basal species (see Fig. S1 in the supplemental material). The resolving power of cox1 was examined by inferring a distance-based phylogenetic tree, using recently reported sequences. The resulting UPGMA and ML trees (see Fig. S2A and S2B in the supplemental material) were congruent with trees reconstructed from a three-gene data set (45). In these trees, different species and genera in major orders of dinoflagellates were well separated whereas intraspecific strains exhibited low genetic divergence, indicating a potential for cox1 as a barcoding marker.
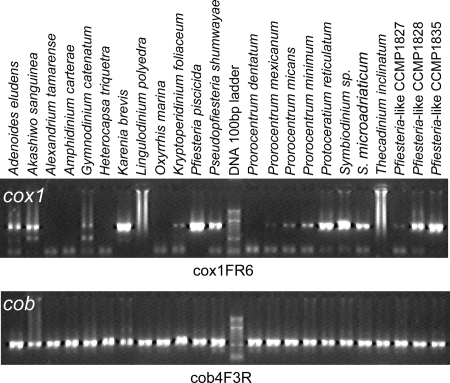
We tried to establish an effective dinoflagellate universal barcode primer set based on cox1. However, all the combinations of the primers tested (Table 1 and Fig. 1) gave similar results in PCR in that some taxa could not be amplified efficiently. As shown in Fig. 2 (top panel), when the Dinocox1F-Dinocox1R5 primer set, which flanked the conventional barcode region, was used, PCR either never or inconsistently amplified taxa such as Alexandrium tamarense, some strains of Amphidinium carterae, Heterocapsa triquetra, Lingulodinium polyedrum, Prorocentrum dentatum, and Thecadinium yashimaense, and only a faint PCR band was obtained for some others (e.g., Kryptoperidinium foliaceum strain CCMP1326, Prorocentrum mexicanum strain CCMP687, and the Pfiesteria-like strain CCMP1827).
FIG. 2.
Agarose gel analysis of cox1 and cob PCR of some dinoflagellates. The gel image shown for cox1 is the result of primer set FR6. Similar results were obtained for primer sets FR5, F2R5, and F2R6. The gel shown for cob was the result of primer set cob4F-3R.
PCR efficiency of the potential cob barcode region.
Four forward and four reverse primers were tested for cob (Table 1 and Fig. 1). Different combinations of these primers exhibited different efficiencies in PCR, and most of the primer sets failed to amplify some species. However, Dinocob4F-Dinocob3R consistently amplified a product of the expected size (385 bp, except for Oxyrrhis marina [388 bp] and the Pfiesteria-like strain CCMP1828 [370 bp]) from each of the dinoflagellate DNA samples tested (Fig. 2). Sequencing results verified that these products were genuine cob fragments, except for Gyrodinium uncatenatum CCMP1310, Thecadinium yashimaense CCMP1890, and Noctiluca scintillans NS3, in which the sequence was either a fusion of partial fragments of cob and mitochondrial cytochrome c oxidase 3, apparently a pseudogene, or a cob gene fragment from another species, likely a prey dinoflagellate that it had ingested (45). These sequences were excluded from further analyses. In all cases, no amplification was observed for nondinoflagellate DNA samples, indicating the specificity of the primers for dinoflagellates.
Resolving power of the potential cob barcode region.
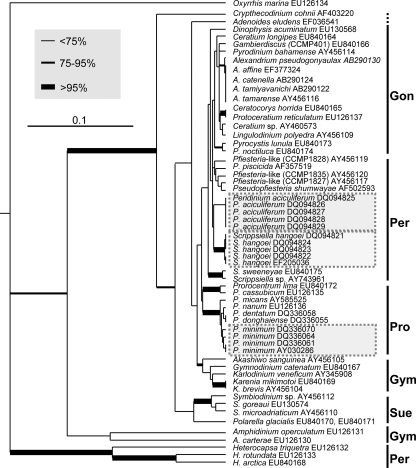
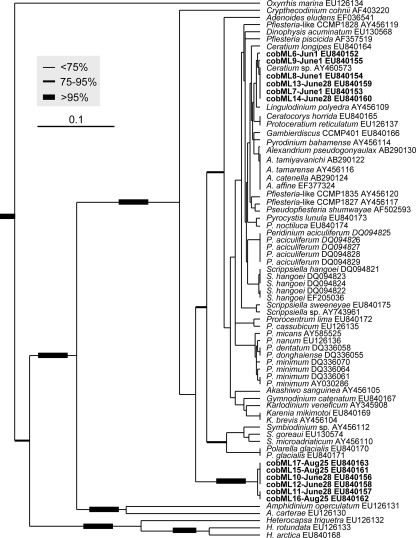
The alignment of cob sequences was fully colinear except for the small deletions in O. marina and the Pfiesteria-like strain mentioned above (see Fig. S3 in the supplemental material). Both the UPGMA and the ML phylogenetic analyses based on the cob fragment (334 bp after the primer regions were excluded from the original 385-bp fragment) produced trees (Fig. 3; also see Fig. S4 in the supplemental material) generally similar to trees based on cox1 (see Fig. S2A and S2B in the supplemental material) and congruent with trees using the long fragments (>0.9 kb) of cob (45); taxa from each of the major orders of dinoflagellates formed a monophyletic cluster (e.g., Gonyaulacales, Peridiniales, Prorocentrales, Gymnodiniales, and Suessiales). The only exception was Ceratium, in which the marine species Ceratium longipes was separated from the Mirror Lake Ceratium sp. isolate, suggesting genetic differentiation between marine and freshwater species of Ceratium. In this phylogenetic tree, two strains of P. glacialis, the bipolar dinoflagellate, were placed within the order Suessiales. In cases where multiple intraspecific strains were available for analysis, sequences from those strains were either identical (Peridinium aculiferum) or very similar (Prorocentrum minimum and Scrippsiella hangoei), indicative of low intraspecific divergence. In contrast, interspecific divergence was markedly higher (e.g., Prorocentrum spp., Scrippsiella spp., and Symbiodinium spp.), with exceptions for Alexandrium and Karenia species (Fig. 3).
FIG. 3.
UPGMA phylogram based on cob barcode region for cultured dinoflagellate species. Bootstrap values were based on 500 replications; the thickest branches denote bootstrap values of >95%, medium-thick branches values of 75 to 95%, and thin branches values of <75%. Major groups of dinoflagellates were identified to the right: Gon, Gonyaulacales; Per, Peridiniales; Pro, Prorocentrales; Gym, Gymnodiniales; Sue, Suessiales. Highlighted in boxes are intraspecific strains that were highly identical (>99.5%). The scale bar indicates the substitution rate per nucleotide (0.1).
The interspecific and intraspecific divergences were further analyzed using the cob distance matrix derived from the TVM+G model (Table 4). Similar to what was observed on the phylogenetic tree, a greater distance was consistently observed between species than between intraspecific strains within each lineage examined (Table 4). With all dinoflagellate lineages considered together, intraspecific variation ranged from 0 to 0.008982 and interspecific variation was >0.008982. However, some exceptions to this pattern were found, e.g., in the genera Alexandrium, Karenia, and Pyrocystis (Table 4).
TABLE 4.
Distances between species in the same genus and between strains within species derived from cob, based on the TVM+G model
| Group | Divergence
|
No. of sequences | No. of taxa | ||
|---|---|---|---|---|---|
| Range | Mean | SD | |||
| Congeneric, interspecific | |||||
| Alexandrium | 0-0.00299 | 0.0009 | 0.0014 | 5 | 21 |
| Amphidinium | 0.23652 | 0.2365 | 0.0000 | 2 | 1 |
| Ceratium | 0.02096 | 0.0210 | 0.0000 | 2 | 1 |
| Heterocapsa | 0.11976-0.25150 | 0.3962 | 0.0240 | 3 | 3 |
| Karenia | 0 | 0.0000 | 0.0000 | 2 | 1 |
| Prorocentrum (P. lima and P. micans vs others) | 0.00898-0.06587 | 0.0309 | 0.0242 | 7 | 35 |
| Pyrocystis | 0.00898 | 0.0090 | 0.0000 | 2 | 1 |
| Scrippsiella | 0.00898-0.04790 | 0.0057 | 0.0131 | 3 | 11 |
| Symbiodinium | 0.02695-0.04491 | 0.0389 | 0.0363 | 3 | 3 |
| Intraspecific | |||||
| Peridinium aciculiferum | 0 | 0.0000 | 0.0000 | 5 | 10 |
| Polarella glacialis | 0 | 0 | 0 | 2 | 1 |
| Prorocentrum dentatum/P. donghaiense | 0 | 0 | 0 | 2 | 1 |
| Prorocentrum minimum | 0.00599-0.00898 | 0.006487 | 0.0035 | 4 | 6 |
| Scrippsiella hangoei | 0-0.00299 | 0.0099 | 0.0139 | 5 | 10 |
Dinoflagellate taxa and species diversity in LIS as revealed by microscopic analysis.
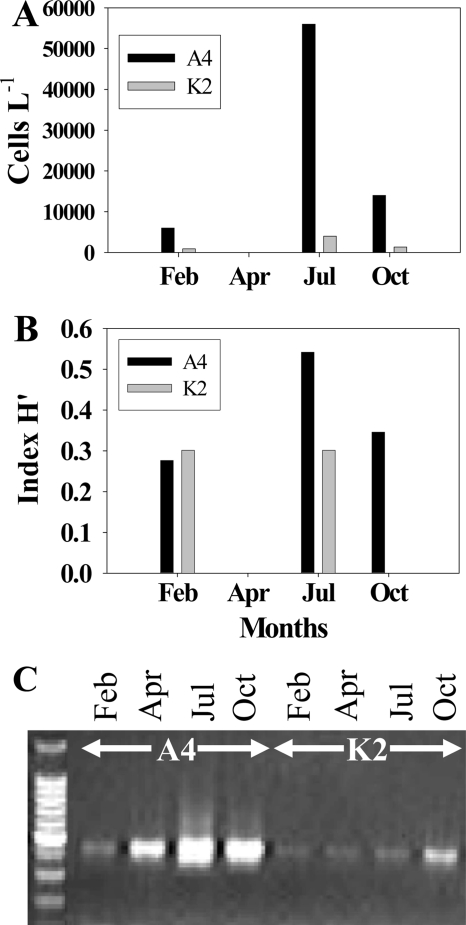
A diversity of dinoflagellates was observed microscopically. At Avery Point station, this resulted in successful isolation of Akashiwo sanguinea and a Scrippsiella sp. into cultures (44, 45). For stations A4 and K2, spatial variations were noted for abundance and genetic diversity of dinoflagellates. Generally, dinoflagellates were more abundant at the A4 station than at the K2 station throughout the whole sampling period by microscopic cell counts (Fig. 4A). Similarly, species diversity was higher in A4 than in K2. Throughout the study period, seven dinoflagellate lineages (Akashiwo, Scrippsiella, Gymnodinium, Dinophysis, Heterocapsa, Prorocentrum, and Protoperidinium) were identified at the A4 station and only three lineages (Gyrodinium, Prorocentrum, and Scrippsiella) were detected at the K2 station. The Shannon-Weaver diversity index (H′) derived from the microscopic counts showed the same spatial pattern (Fig. 4B). At station A4, dinoflagellate species diversity and total abundance were higher in July than at other sampling times.
FIG. 4.
Relative abundance and diversity levels of dinoflagellates in LIS for seasonal samples from the A4 and K2 stations. Overall abundance was higher at the A4 station than at the K2 station and higher in July and October than in February and April. (A) Abundance of dinoflagellates, based on microscopic cell count. (B) H′ values calculated from microscopic analysis results. (C) Relative abundances of dinoflagellate-specific cob PCR products shown on agarose gel.
Dinoflagellate taxa and species diversity in LIS as revealed by cob barcode sequence analysis.
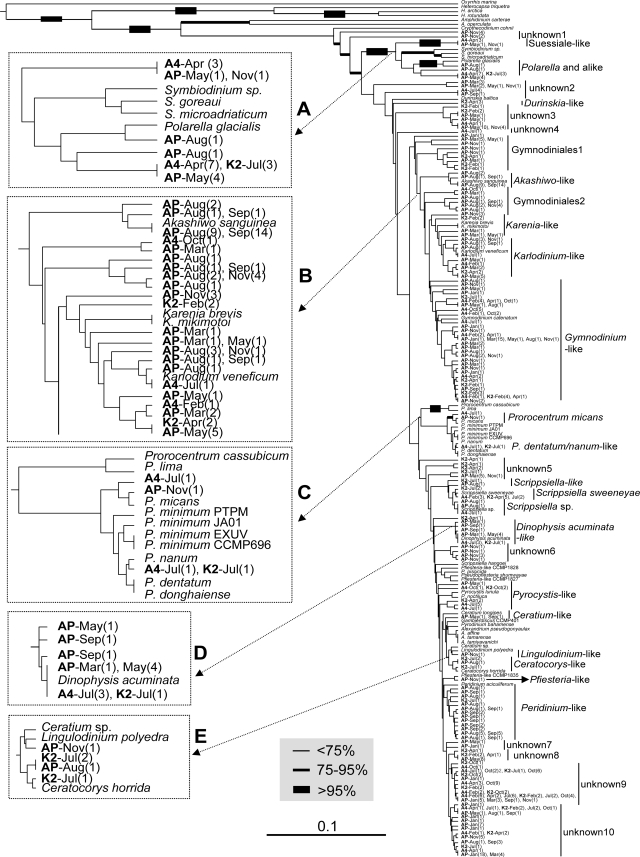
With the primer set Dinocob4F-Dinocob3R, PCR on each field-collected DNA sample consistently yielded a good DNA product of the expected size. BLAST analysis showed that all the sequences retrieved from these PCR products belonged to dinoflagellates, confirming the specificity of the primers for dinoflagellates. Alignment of the sequences with counterparts from documented species was coherent except for the two cases of indels for cultured species mentioned earlier and a 35-bp insertion in two of the station A4 July clones (the alignment file is available upon request). This 35-bp insertion was at the same location as the insertion in O. marina. Because no single universal distance threshold could be established to define species, distance-based UPGMA phylogenetic analysis was performed to facilitate identification of species on the basis of relative branch lengths between interspecific and intraspecific taxa. As a result, a high level of diversity of dinoflagellates was revealed in the samples, with many gene clones scattered throughout the tree, some closely and some distantly related to documented species (Fig. 5). Some of these clones can be assigned to the species level because their distance to a documented species was shorter than the interspecific distance within that lineage (Table 4), e.g., clones belonging to A. sanguinea (Fig. 5, insert B), Dinophysis acuminata (insert D), and Karlodinium veneficum (insert B), Prorocentrum micans (insert C), Scrippsiella sweeneyae, and Ceratocorys horrida. There were also clones that could be assigned only to a genus or an order because the cob barcode sequence was scarce in that genus or order, e.g., Peridinium, Scrippsiella, or that labeled Ceratocorys-like, Prorocentrum spp., Pfiesteria-like, Lingulodinium-like, Gymnodinium-like, Gymnodiniales1, or Gymnodiniales2 (Table 3 and Fig. 5). Among these, a clone from an Avery Point August sample was closely related to P. glacialis (97.6% nucleotide identity), which had been found only in the polar regions (22, 23), as a sister lineage to Symbiodinium. Sixteen other clones from all three sampling stations in various seasons also clustered with P. glacialis, but with longer distances, and these clones likely belonged to Polarella or a related genus. In addition, there was a large number of cob clones whose taxonomic designation remains unclear because of lack of sequence data for related taxa.
FIG. 5.
UPGMA phylogram of cob barcode sequences from cultured and LIS dinoflagellate clones. Bootstrap values, analyzed on the basis of 500 replications, are indicated for major clades by branch thickness: thickest line, >95%; second-thickest line, 75 to 95%; thin line, <75%. The scale bar indicates the substitution rate per nucleotide (0.1).
Spatial variations were observed between stations A4 and K2 for relative abundance and genetic diversity of dinoflagellates. Under the same reaction conditions, PCR using the cob barcode primer set for each DNA extract from the same amount of water sample yielded different amounts of amplicons (Fig. 4C). Since these DNA samples had been shown to be of good quality (17, 43, 46), most likely the difference in PCR product abundance was due to a greater abundance of dinoflagellates at the A4 station than at the K2 station, in agreement with the trend of dinoflagellate microscopic cell counts (Fig. 4A). Overall, more cob genotypes were detected at the K2 station than at the A4 station, although the H′ value derived from the microscopic result showed a contrary pattern (Fig. 4B). The discrepancy was likely caused by the much higher detection sensitivity of PCR than of microscopic counting and by some genotypes that could not be discriminated morphologically. At Avery Point, the overall cob genotypic diversity in the study period (2003) was similar to that in the K2 station (Table 3), consistent with their proximity.
Temporally, at the A4 and K2 stations, more cob genotypes (13 types out of 27 clones and 15 types out of 22 clones, respectively) were detected in July 2002 than in other seasons (Table 3). In October, diversity was lower (8 types out of 22 and 19 clones at A4 and K2, respectively). Taxon diversity showed the same trend, with an unknown genotype (Unknown9) predominant at both the A4 and the K2 stations in October (Table 3). The H′ value derived from the microscopic data was highest in July at station A4, whereas similar diversity levels were seen for February and July at station K2 (Fig. 4B). Microscopic results showed negligible abundance and species diversity in April 2002 for both stations when substantial cob diversity was detected (Table 3), indicating the higher detection sensitivity of the molecular method. At Avery Point, cob genotypic diversity was higher in August (29 types out of 46 clones), September (21 types out of 43 clones), and November (28 types out of 47 clones) than in other seasons (Table 3). At all three stations, Gymnodinium was predominant in the winter season (February to March), although it was commonly found throughout the study period (Table 3).
Dinoflagellate taxa and seasonal succession in Mirror Lake.
PCR with the Dinocob4F-Dinocob3R primer sets also yielded products of the expected size for samples from Mirror Lake. Sequencing of the resulting clones verified the identity of the clones as dinoflagellates. As shown in Fig. 6, some of the clones were identical or almost identical to the cob barcode sequence of a Ceratium sp. that was also detected microscopically in this study and molecularly in previous studies (18S rRNA genes [17] and cob [45]). Other clones tightly formed a separate cluster, nested between Crypthecodinium and Symbiodinium in the phylogenetic tree, whose identity could not be resolved, due to a lack of related data. The dinoflagellate assemblage in this pond exhibited a temporal succession of different lineages: from the Ceratium sp. on 1 June to both the Ceratium sp. and the unknown lineage on 28 June and then exclusively the unknown lineage on 25 August.
FIG. 6.
UPGMA phylogram of cob barcode sequences from cultured and Mirror Lake dinoflagellate clones. Bootstrap values, analyzed based on 500 replications, were indicated for major clades by branch thickness: thickest line, >95%; second-thickest line, 75 to 95%; thin line, <75%. Dinoflagellate cob clones recovered from Mirror Lake are in bold type. Note that Ceratium and a novel lineage that was affiliated between Crypthecodinium and Symbiodinium occurred and dominance shifted over time. The scale bar indicates the substitution rate per nucleotide (0.1).
DISCUSSION
This is the first attempt to explore a cob-based DNA barcoding marker for dinoflagellates and any marine phytoplankton and the first application of the barcoding marker to investigate dinoflagellate species diversity over spatial and temporal scales in marine and freshwater ecosystems. Dinoflagellate diversity and new lineages have continually been discovered, but all through random sequencing of eukaryotic 18S rRNA gene clones (e.g., references 20, 24, 30, and 42) rather than systematic dinoflagellate-specific analysis. These nonsystematic analyses can hardly retrieve the full range of dinoflagellate species diversity, especially when other plankton was dominant in the water sample. In a recent study, we developed a dinoflagellate-specific 18S rRNA gene primer set and demonstrated its utility for recovering dinoflagellate community structure (17). This primer set has been used to analyze water samples from various ecosystems, and its usefulness has been further verified (S. Lin and H. Zhang, unpublished data). However, there are some limitations for this primer set. First of all, the PCR product generated by this primer set is long (about 1.6 kb), which requires more effort to sequence and assemble in order to obtain a fully usable sequence. This becomes a problem when a large number of samples need to be analyzed. Second, the dinoflagellate 18S rRNA gene sequence is often too conserved to be useful in resolving closely related lineages (e.g., the so-called GPP complex, including Gymnodiniales, Prorocentrales, and Peridiniales). These three orders of dinoflagellates represent lineages with no theca, bivalve theca, or multiplate theca, respectively, and their evolution cannot be resolved with the single subunit rRNA gene tree, due to extremely low divergence rates (33). Recently, Litaker et al. (19) showed that ITS1/5.8/ITS2 rRNA gene sequences (the internal transcribed spacer [ITS] region) has the potential to serve as a unique species-specific DNA barcode for most dinoflagellates. However, because sequences flanking the ITS region (18S and 28S rRNA genes, respectively) are highly conserved in most of the eukaryotes and the ITS region is highly variable even within dinoflagellates, it is expected to be difficult if not impossible to design a dinoflagellate-specific primer set to amplify the ITS region for all or most of the dinoflagellate taxa. Therefore, this ITS region can be useful for identifying single cells or monospecific cultures (18), but its utility for natural dinoflagellate assemblages remains to be assessed.
Although mitochondrial cox1 is well established as a DNA barcode for animals (reference 15 and references therein) and has been shown to be suitable for species discrimination for red algae and fungi (29, 35), this gene is too conserved to be used as a barcode in higher plants (reference 26 and references therein). For phytoplankton, cox1 has been tested for determining phylogenetic relationships among Sellaphora species (diatoms), but results showed generally lower bootstrap support than for other genes examined (7). For dinoflagellates, although cox1 was once used successfully to distinguish different Symbiodinium genotypes (37), the utility of this gene as a barcode is questionable due to the repetitive failure of the primer sets to amplify all dinoflagellate species in addition to the demonstrated lower resolving power (45). In contrast, cob seems to be more promising. The Dinocob4F-Dinocob3R primer set is more universal within the dinoflagellate phylum, effectively amplifying all the dinoflagellate taxa examined (except several taxa in which the amplicons turned out to be pseudogenes and their usefulness to species recognition is uncertain). In our laboratory, we have found that this primer set is able to detect less than 0.1 cell in a PCR for A. tamarense, Pfiesteria piscicida, K. veneficum, P. minimum, and A. sanguinea. The sensitivity of this primer set is very important for field surveying, especially when dinoflagellate abundance is low. The 385-bp cob gene fragment also meets two other criteria of a DNA barcode: short length for ease for complete sequencing and adequate sequence variability for species delineation. The short length of the cob barcoding sequence makes this sequence well suited for the 454 Sequencing technology when high-throughput analysis is needed for large-scale environmental samples. Although a somewhat longer fragment, like the conventional cox1 barcode (∼650 bp), may offer higher taxon resolution, primer sets for longer fragments that we have tested failed to work for some species and were less sensitive. However, the generally higher variability of cob (45) can compensate for the short length to some extent. Similarly, use of cob also has been shown to increase resolving power for DNA barcoding in the case of rotifers (5). Furthermore, this cob primer set is specific for dinoflagellates, as indicated by the results of tests done for laboratory-grown algae as well as field-collected samples (see below). The only shortcoming recognized so far is that this gene fragment does not provide a uniform species-discriminating distance threshold across the dinoflagellate phylum. Instead, the interspecific distance based on this cob fragment varies from one lineage to the other. As a result, the overall interspecific distance (>0.008982 in most cases) and intraspecific distance (0 to 0.008982) lack a gap expected of a good DNA barcoding marker (11).
Nevertheless, the interspecific variation of the 385-bp cob fragment has allowed species discrimination for most of the lineages as long as sequence data are available for related intraspecific strains and congeneric species to establish an interspecific distance threshold. For instance, species identification can be done for Prorocentrum because the distance between Prorocentrum lima and other Prorocentrum species is 0.01497 to 0.065868 (0.0340 ± 0.0244), whereas the distance within P. minimum strains is 0.00599 to 0.00898 (0.006487 ± 0.0035) (Table 4). The utility of this gene fragment is supported by the fact that the phylogenetic tree inferred from this cob fragment for cultured dinoflagellates (Fig. 4) is congruent with trees derived from multiple genes (e.g., reference 45). Among other monophyletic lineages formed in this tree, Polarella glacialis, the suessiale species from the Antarctic and Arctic Oceans, formed a well-supported cluster with the sister group Symbiodinium, an affiliation previously suggested by morphological and rRNA gene molecular data (22, 23). It should be noted that the apparent lack of variation in the cob sequence for Alexandrium spp. is agreeable with the absence of variation in A. tamarense, Alexandrium fundyense, and Alexandrium catenella on the basis of 18S rRNA genes, ITS, and morphology (differing only by the presence or absence of a ventral pore on the apical plate), which renders these species to be treated as an A. tamarense species complex (2, 4). A recent study using morphological, genetic, and proteomic analyses showed that A. tamarense and A. catenella could not be distinguished and should be considered one species (41). A large-scale survey of rRNA gene sequences suggested a collapse of the morphospecies and use of genetic clades (16). Similarly, Karenia brevis and Karenia mikimotoi have been shown to be essentially identical with respect to 18S rRNA genes (4- or 5-nucleotide differences out of 1.8 kb, as shown in the sequences under GenBank accession numbers AF009216, AF022195, and EF492501 to EF492505) as well as the cob sequence analyzed in this study.
The potential utility of the cob fragment for barcoding particular lineages of dinoflagellates is also evident in the retrieval of dinoflagellate diversity in LIS (Fig. 5) and Mirror Lake (Fig. 6). The use of the cob fragment has revealed a high level of diversity of dinoflagellate lineages in LIS without detecting other organisms occurring in the environment. These dinoflagellate taxa can be recognized to the species level when there is a sufficient database for related species and genera. Among the species retrieved are A. sanguinea, K. veneficum, P. micans, Prorocentrum nanum, D. acuminata, Ceratocorys horrida, Pfiesteria-like taxon CCMP1835, and S. sweeneyae, most of which have been detected previously using the dinoflagellate-specific18S rRNA genes and other primers (17, 46). Interestingly, this marker also has unveiled a variety of taxa closely related to the bipolar species P. glacialis, one of which likely belongs to the same species or genus. It is also intriguing to observe, based on the 385-bp cob fragment, the predominance of two clades of dinoflagellate in Mirror Lake and the transition from one to the other in a 2-month period. In our analysis, the identities of a substantial number of the taxa in the environmental samples remain unclear, such as the great variety of gymnodinioid species, because the cob database is still limited. DNA barcoding in general requires an existing systematic framework and a large database comprising barcode sequences from all documented species to which a new barcode sequence from a question taxon can be compared. Gymnodinioid taxa are usually abundant in estuaries, and it would be of high interest to resolve the species identities of these taxa. When a broader cob database becomes available, the taxon-resolving power of this gene would certainly increase and many more environmental dinoflagellate populations can be identified. In addition, the cob sequences obtained in this and future studies will potentially be useful for developing fluorescent in situ hybridization probes for applications to microscopic identifications of unknown sequences.
Supplementary Material
Acknowledgments
We thank Sheng Liu and the crew of the Water Quality Monitoring Program in the Connecticut Department of Environmental Protection for assistance with sample collection from Mirror Lake and LIS, respectively.
This research was supported by NSF grant EF0629624 and CAS/SAFEA International Partnership Program for Creative Research Teams of the People's Republic of China grant KZCX2-YW-T001.
Footnotes
Published ahead of print on 29 December 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Anderson, D. M. 1994. Red tides. Sci. Am. 271:62-68. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, D. M., D. M. Kulis, G. J. Doucette, J. C. Gallagher, and E. Balech. 1994. Biogeography of toxic dinoflagellates in the genus Alexandrium from the northeastern United States and Canada. Mar. Biol. 120:467-478. [Google Scholar]
- 3.Avise, J. C. 1994. Molecular markers: natural history and evolution. Chapman & Hall, New York, NY.
- 4.Balech, E. 1985. The genus Alexandrium or Gonyaulax of the tamarensis group, p. 33-38. In D. M. Anderson, A. W. White, and D. G. Baden (ed.), Toxic dinoflagellates. Elsevier, New York, NY.
- 5.Birky, C. W., Jr. 2007. Workshop on barcoded DNA: application to rotifer phylogeny, evolution, and systematics. Hydrobiologia 593:175-183. [Google Scholar]
- 6.De Salas, M., C. J. S. Bolch, L. Botes, G. Nash, S. W. Wright, and G. M. Hallegraeff. 2003. Takayama gen. nov. (Gymnodiniales, Dinophyceae), a new genus of unarmored dinoflagellates with sigmoid apical grooves, including the description of two new species. J. Phycol. 39:1233-1246. [Google Scholar]
- 7.Evans, K. M., A. H Wortleya, and D. G. Manna. 2007. An assessment of potential diatom “barcode” genes (cox1, rbcL, 18S and ITS rDNA) and their effectiveness in determining relationships in Sellaphora (Bacillariophyta). Protist 158:349-364. [DOI] [PubMed] [Google Scholar]
- 8.Guindon, S., and O. Gascuel. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696-704. [DOI] [PubMed] [Google Scholar]
- 9.Hebert, P. D. N., A. Cywinska, S. L. Ball, and J. R. deWaard. 2003. Biological identifications through DNA barcodes. Proc. R. Soc. B 270:313-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hebert, P. D. N., S. Ratnasingham, and J. R. deWaard. 2003. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. B 270:S96-S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebert, P. D. N., M. Y. Stoeckle, T. S. Zemlak, and C. M. Francis. 2004. Identification of birds through DNA barcodes. PLoS Biol. 2:e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horiguchi, T., and C. Sukigara. 2005. Pyramidodinium atrofuscum gen. et sp. nov. (Dinophyceae), a new marine sand-dwelling coccoid dinoflagellate from tropical waters. Phycol. Res. 53:247-254. [Google Scholar]
- 13.Jeong, H. J., J. S. Kim, J. Y. Park, J. H. Kim, S. Him, I. Lee, S. H. Lee, J. H. Ha, and W. H. Yih. 2005. Stoeckeria algicida n. gen., n. sp. (Dinophyceae) from the coastal waters off southern Korea: morphology and small subunit ribosomal DNA gene sequence. J. Eukaryot. Microbiol. 52:382-390. [DOI] [PubMed] [Google Scholar]
- 14.Kress, J. W., K. J. Wurdack, E. A. Zimmer, L. A. Weigt, and D. H. Janzen. 2005. Use of DNA barcodes to identify flowering plants. Proc. Natl. Acad. Sci. USA 102:8369-8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lahaye, R., M. van der Bank, D. Bogarin, J. Warner, F. Pupulin, G. Gigot, O. Maurin, S. Duthoit, T. G. Barraclough, and V. Savolainen. 2008. DNA barcoding the floras of biodiversity hotspots. Proc. Natl. Acad. Sci. USA 105:2923-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lilly, E. L., K. M. Halanych, and D. M. Anderson. 2007. Species boundaries and global biogeography of the Alexandrium tamarense complex (Dinophyceae). J. Phycol. 43:1329-1338. [Google Scholar]
- 17.Lin, S., H. Zhang, Y. Hou, L. Miranda, and D. Bhattacharya. 2006. Development of a dinoflagellate-oriented PCR primer set leads to detection of picoplanktonic dinoflagellates from Long Island Sound. Appl. Environ. Microbiol. 72:5626-5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litaker, R. W., M. W. Vandersea, S. R. Kibler, K. S. Reece, N. A. Stokes, K. A. Steidinger, D. F. Millie, B. J. Bendis, R. J. Pigg, and P. A. Tester. 2003. Identification of Pfiesteria piscicida (Dinophyceae) and Pfiesteria-like organisms using internal transcribed spacer-species PCR assays. J. Phycol. 35:1379-1389. [Google Scholar]
- 19.Litaker, R. W., M. W. Vandersea, S. R. Kibler, R. Steven, K. S. Reece, S. Kimberly, N. A. Stokes, F. M. Lutzoni, B. A. Yonish, M. A. West, M. N. D. Black, and P. A. Tester. 2007. Recognizing dinoflagellate species using ITS rDNA sequences. J. Phycol. 43:344-355. [Google Scholar]
- 20.López-García, P., H. Philippe, F. Gail, and D. Moreira. 2003. Autochthonous eukaryotic diversity in hidrotermal sediment and experimental microcolonizeres at the Mid-Atlantic Ridge. Proc. Natl. Acad. Sci. USA 100:697-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDevit, D., and G. Saunders. 2007. Barcoding brown algae: the DNA barcode initiative is changing our view of the Phaeophyceae in Canada. Abstr. 61st Annu. Meet. Phycol. Soc. Am. http://www.psaalgae.org/ops/PSA-ISOP2007-Program.pdf.
- 22.Montresor, M., G. Procaccini, and D. K. Stoecker. 1999. Polarella glacialis, gen. nov., sp. nov. (Dinophyceae): Suessiaceae are still alive! J. Phycol. 35:186-197. [Google Scholar]
- 23.Montresor, M., C. Lovejoy, L. Orsini, G. Procaccini, and S. Roy. 2003. Bipolar distribution of the cyst-forming dinoflagellate Polarella glacialis. Polar Biol. 26:186-194. [Google Scholar]
- 24.Moon-van der Staay, S., R. D. Wachter, and D. Vaulot. 2001. Oceanic 18S rDNA sequences from picoplankton reveal unsuspected eukaryotic diversity. Nature 409:607-610. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura, Y. 1999. Heterotrophic dinoflagellates, cyclopoid copepods and appendicularians: their ecological importance in the energy flow of coastal ecosystem. Bull. Plankton Soc. Jpn. 46:70-77. [Google Scholar]
- 26.Newmaster, S. G., A. J. Fazekas, and S. Ragupathy. 2006. DNA barcoding in land plants: evaluation of rbcL in a multigene tiered approach. Can. J. Bot. 84:335-341. [Google Scholar]
- 27.Palmer, J. D., and L. A. Herbon. 1988. Plant mitochondrial DNA evolved rapidly in structure, but slowly in sequence. J. Mol. Evol. 28:87-97. [DOI] [PubMed] [Google Scholar]
- 28.Posada, D., and K. A. Crandall. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817-818. [DOI] [PubMed] [Google Scholar]
- 29.Robba, L., S. J., Russell, G. L. Baker, and J. Brode. 2006. Assessing the use of the mitochondrial cox1 marker for use in DNA barcoding of red algae (Rhodophyta). Am. J. Bot. 93:1101-1108. [DOI] [PubMed] [Google Scholar]
- 30.Romari, K., and D. Vaulot. 2004. Composition and temporal variability of picoeukaryote communities at a coastal site of the English Channel from 18S rDNA sequences. Limnol. Oceanogr. 49:784-798. [Google Scholar]
- 31.Rowan, R. 1998. Diversity and ecology of zooxanthallae on coral reefs. J. Phycol. 34:407-417. [Google Scholar]
- 32.Saccone, C., C. Gissi, C. Lanave, A. Larizza, G. Pesole, and A. Reyes. 2000. Evolution of the mitochondrial genetic system: an overview. Gene 261:153-159. [DOI] [PubMed] [Google Scholar]
- 33.Saldarriaga, J. F., F. J. R. Taylor, P. J. Keeling, and T. Cavalier-Smith. 2001. Dinoflagellate nuclear SSU rDNA phylogeny suggests multiple plastid losses and replacements. J. Mol. Evol. 53:204-213. [DOI] [PubMed] [Google Scholar]
- 34.Saunders, G. W. 2005. Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Philos. Trans. R. Soc. Lond. B 360:1879-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seifert, K. A., R. A. Samson, J. R. deWaard, J. Houbraken, C. A. Levesque, J. M. Moncalvo, G. Louis-Seize, and P. D. N. Hebert. 2007. Prospects for fungus identification using CO1 DNA barcodes, with Penicillium as a test case. Proc. Natl. Acad. Sci. USA 104:3901-3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steidinger, K. A., and K. Tengen. 1997. Dinoflagellates, p. 387-584. In C. R. Tomas (ed.), Identifying marine phytoplankton. Academic Press, New York, NY.
- 37.Takabayashi, M., S. R. Santos, and C. B. Cook. 2004. Mitochondrial DNA phylogeny of the symbiotic dinoflagellates (Symbiodinium, Dinophyta). J. Phycol. 40:160-164. [Google Scholar]
- 38.Tamura, M., M. Iwataki, and T. Horiguchi. 2005. Heterocapsa psammophila sp. nov. (Peridiniales, Dinophyceae), a new sand-dwelling marine dinoflagellate. Phycol. Res. 53:303-311. [Google Scholar]
- 39.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Utermöhl, H. 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt. Int. Ver. Theor. Angew. Limnol. 9:1-38. [Google Scholar]
- 41.Wang, D.-Z., L. Lin, H.-F. Gu, L. L. Chan, and H.-S. Hong. 2008. Comparative studies on morphology, ITS sequence and protein profile of Alexandrium tamarense and A. catenella isolated from the China Sea. Harmful Algae 7:106-113. [Google Scholar]
- 42.Yuan, J., M.-Y. Chen, P. Shao, H. Zhou, Y.-Q. Chen, and L.-H. Qu. 2004. Genetic diversity of small eukaryotes from the coastal waters of Nansha Islands in China. FEMS Microbiol. Lett. 240:163-170. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, H., and S. Lin. 2005. Development of a cob-18S rRNA gene real-time PCR assay for quantifying Pfiesteria shumwayae in the natural environment. Appl. Environ. Microbiol. 71:7053-7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, H., D. Bhattacharya, and S. Lin. 2005. Phylogeny of dinoflagellates based on mitochondrial cytochrome b and nuclear small subunit rDNA sequence comparisons. J. Phycol. 41:411-420. [Google Scholar]
- 45.Zhang, H., D. Bhattacharya, and S. Lin. 2007. A three-gene dinoflagellate phylogeny suggests monophyly of Prorocentrales and a basal position for Amphidinium and Heterocapsa. J. Mol. Evol. 65:463-474. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, H., W. Litaker, M. W. Vandersea, P. Tester, and S. Lin. 2008. Geographic distribution of Karlodinium veneficum in the US east coast as detected by dual-gene real-time PCR assay. J. Plankton Res. 30:905-922. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.