Abstract
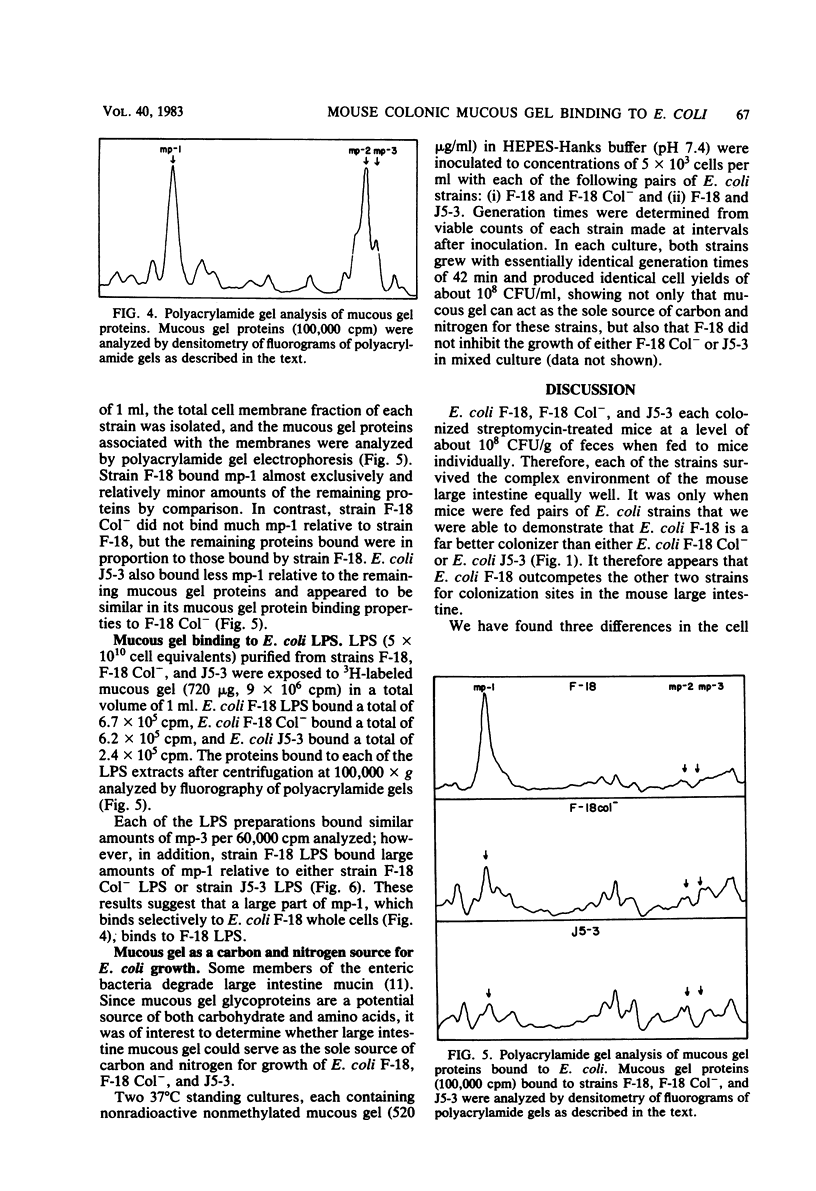
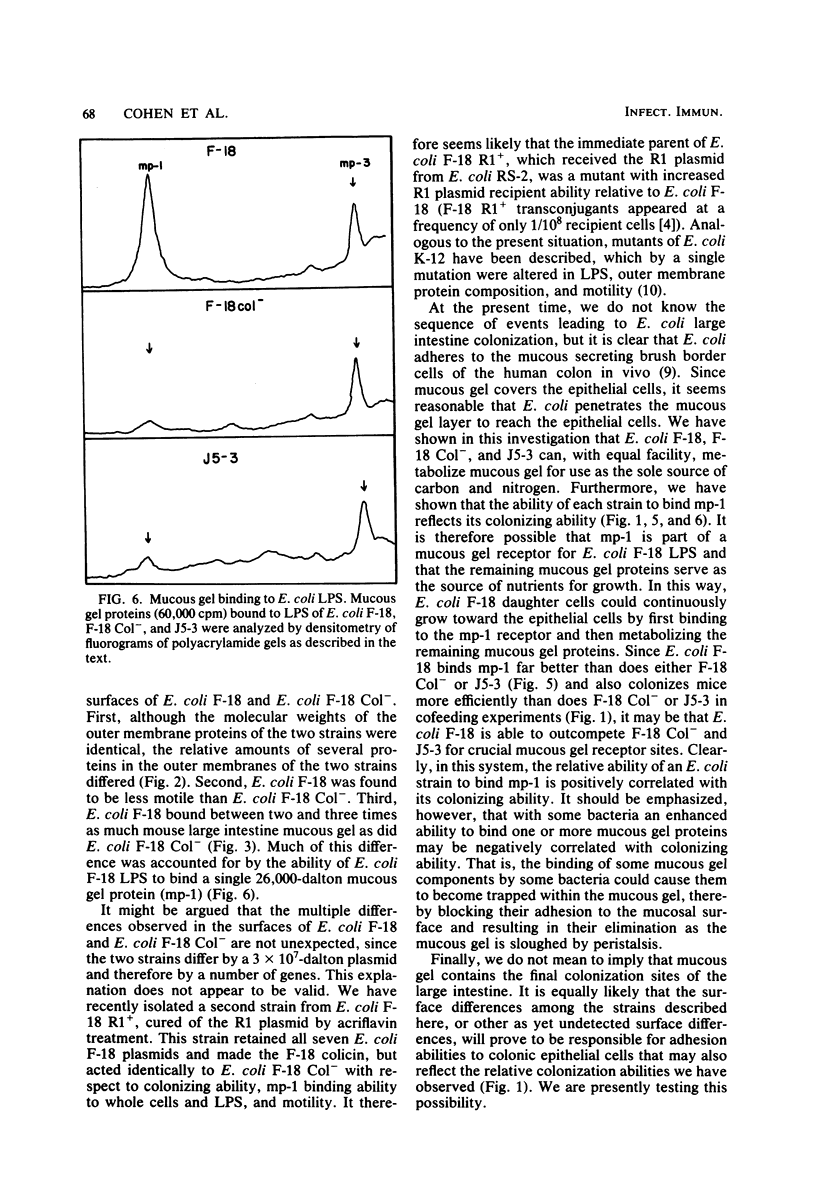
Escherichia coli F-18, isolated from the feces of a healthy human, is an excellent colonizer of the large intestines of streptomycin-treated CD-1 mice. E. coli F-18 Col-, a poor mouse colonizer relative to F-18, lacks a 3 X 10(7)-dalton plasmid present in E. coli F-18. Both strains are human type A erythrocyte hemagglutination negative, have identical surface hydrophobicities, contain the same number of lipopolysaccharide molecules with the same O-side chain length, and have identical amounts of capsule. Differences between the two strains were observed. The relative amounts of specific outer membrane proteins differed, and E. coli F-18 was less motile than E. coli F-18 Col-. The abilities of the two strains to bind mouse large intestine mucous gel was also examined. Although each strain was able to use mucous gel as the sole source of carbon and nitrogen with equal ability, E. coli F-18 bound between two and three times more mucous gel than did E. coli F-18 Col-. Most of the difference in mucous gel binding ability of the two strains was accounted for by the greater ability of E. coli F-18 lipopolysaccharide to bind a single 26,000-dalton mucous gel protein. E. coli J5-3, a typical K-12 strain that is also a poor colonizer relative to E. coli F-18, was identical to E. coli F-18 Col- with respect to mucous gel binding ability.
Full text
PDF

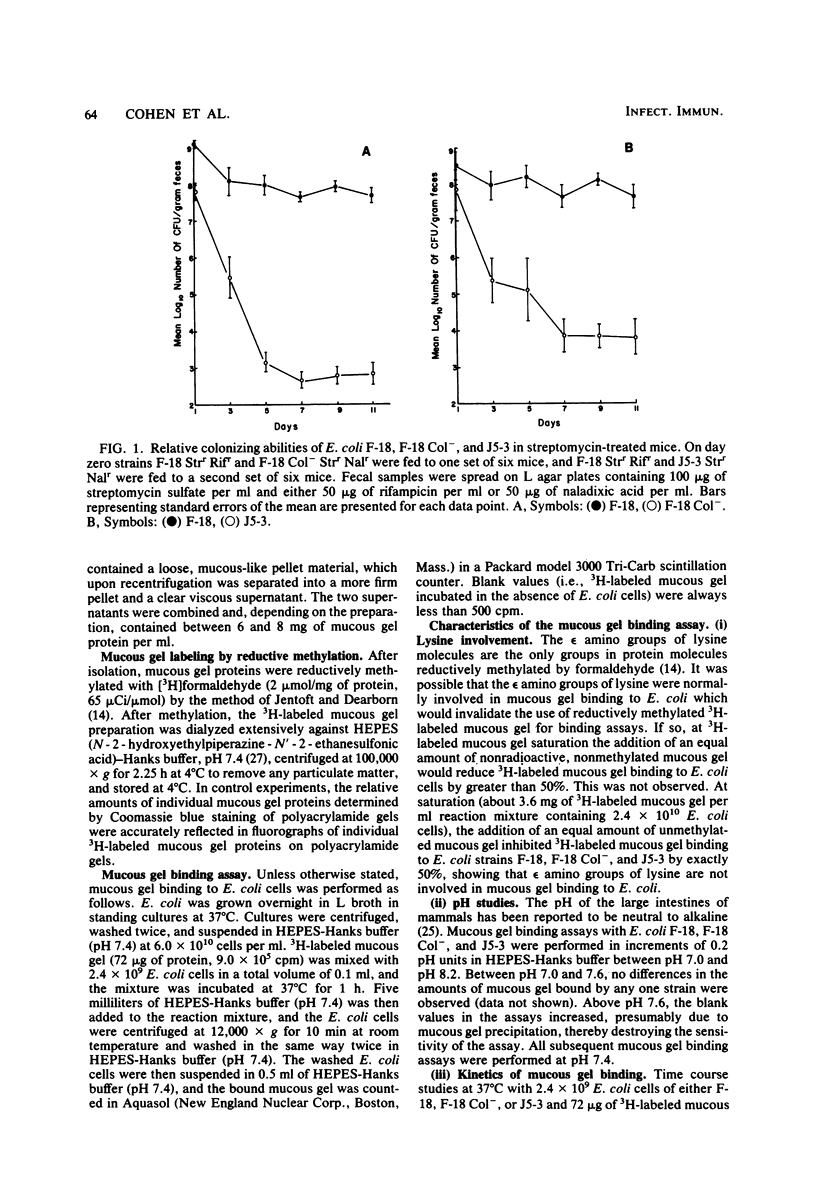
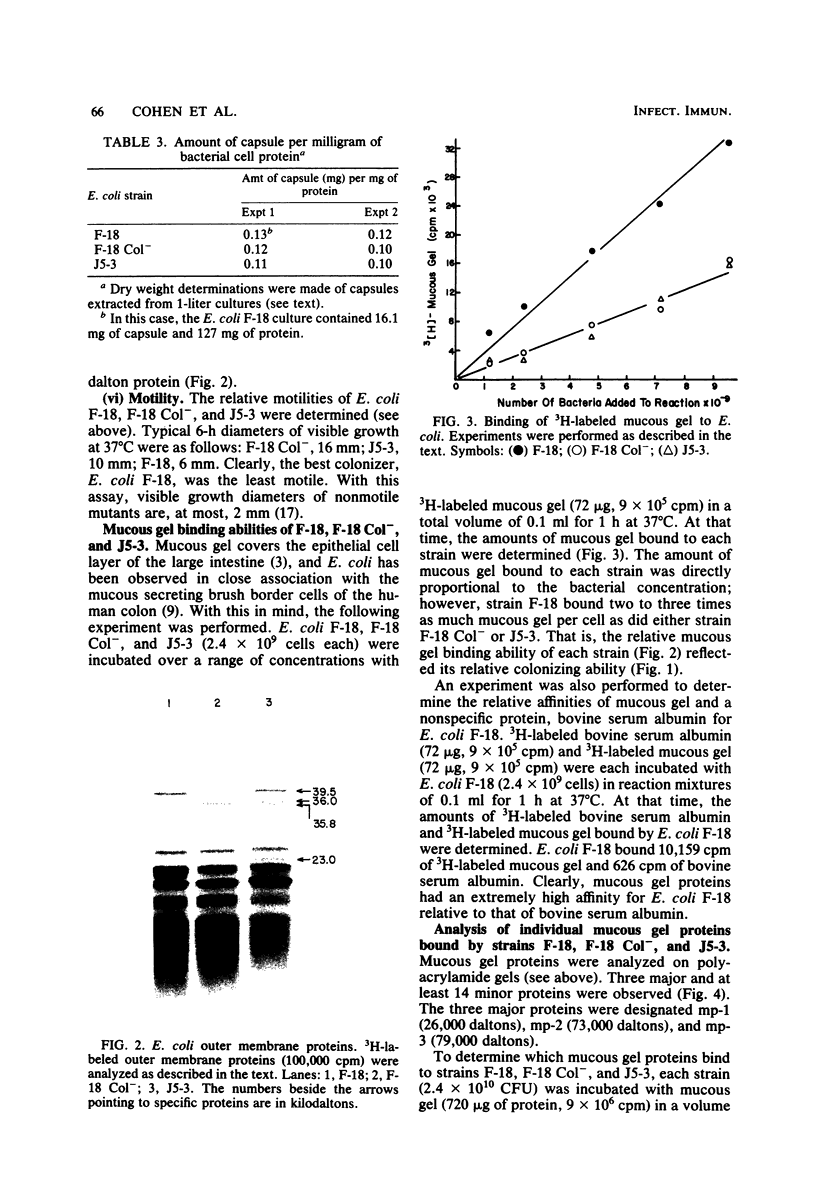

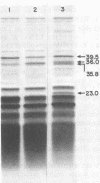
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Snary D. The structure and function of gastric mucus. Gut. 1972 Aug;13(8):666–672. doi: 10.1136/gut.13.8.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp J. R., Fraser G., Read A. E. Study of the carbohydrate content of mucus glycoproteins from normal and diseased colons. Clin Sci (Lond) 1981 Aug;61(2):229–234. doi: 10.1042/cs0610229. [DOI] [PubMed] [Google Scholar]
- Corliss T. L., Cohen P. S., Cabelli V. J. R-Plasmid Transfer to and from Escherichia coli Strains Isolated from Human Fecal Samples. Appl Environ Microbiol. 1981 Apr;41(4):959–966. doi: 10.1128/aem.41.4.959-966.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corliss T. L., Cohen P. S., Cabelli V. J. R-Plasmid Transfer to and from Escherichia coli Strains Isolated from Human Fecal Samples. Appl Environ Microbiol. 1981 Apr;41(4):959–966. doi: 10.1128/aem.41.4.959-966.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr New surface-associated heat-labile colonization factor antigen (CFA/II) produced by enterotoxigenic Escherichia coli of serogroups O6 and O8. Infect Immun. 1978 Aug;21(2):638–647. doi: 10.1128/iai.21.2.638-647.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley C. L., Neumann C. S., Richmond M. H. Adhesion of commensal bacteria to the large intestine wall in humans. Infect Immun. 1979 Jan;23(1):128–132. doi: 10.1128/iai.23.1.128-132.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C., Boulding E. T. Mucin degradation in human colon ecosystems. Evidence for the existence and role of bacterial subpopulations producing glycosidases as extracellular enzymes. J Clin Invest. 1981 Jan;67(1):163–172. doi: 10.1172/JCI110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Nagy B., Moon H. W. Colonization of porcine small intestine by Escherichia coli: colonization and adhesion factors of pig enteropathogens that lack K88. J Infect Dis. 1977 Apr;135(4):531–539. doi: 10.1093/infdis/135.4.531. [DOI] [PubMed] [Google Scholar]
- Jann K., Schmidt G., Blumenstock E., Vosbeck K. Escherichia coli adhesion to Saccharomyces cerevisiae and mammalian cells: role of piliation and surface hydrophobicity. Infect Immun. 1981 May;32(2):484–489. doi: 10.1128/iai.32.2.484-489.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. The association of K88 antigen with haemagglutinating activity in porcine strains of Escherichia coli. J Gen Microbiol. 1974 Sep;84(1):135–144. doi: 10.1099/00221287-84-1-135. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Komeda Y., Icho T., Iino T. Effects of galU mutation on flagellar formation in Escherichia coli. J Bacteriol. 1977 Feb;129(2):908–915. doi: 10.1128/jb.129.2.908-915.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lynch K. R., Pennica D., Ennis H. L., Cohen P. S. Separation and purification of the mRNAs for vesicular stomatitis virus NS and M proteins. Virology. 1979 Oct 15;98(1):251–254. doi: 10.1016/0042-6822(79)90543-9. [DOI] [PubMed] [Google Scholar]
- Myhal M. L., Laux D. C., Cohen P. S. Relative colonizing abilities of human fecal and K 12 strains of Escherichia coli in the large intestines of streptomycin-treated mice. Eur J Clin Microbiol. 1982 Jun;1(3):186–192. doi: 10.1007/BF02019621. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Smith H. W., Sojka W. J. The establishment of K99, a thermolabile, transmissible escherichia coli K antigen, previously called "Kco", possessed by calf and lamb enteropathogenic strains. Acta Pathol Microbiol Scand B. 1975 Feb;83(1):31–36. doi: 10.1111/j.1699-0463.1975.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Revel H. R. Restriction of nonglucosylated T-even bacteriophage: properties of permissive mutants of Escherichia coli B and K12. Virology. 1967 Apr;31(4):688–701. doi: 10.1016/0042-6822(67)90197-3. [DOI] [PubMed] [Google Scholar]
- Savage D. C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosbeck K., Handschin H., Menge E. B., Zak O. Effects of subminimal inhibitory concentrations of antibiotics on adhesiveness of Escherichia coli in vitro. Rev Infect Dis. 1979 Sep-Oct;1(5):845–851. doi: 10.1093/clinids/1.5.845. [DOI] [PubMed] [Google Scholar]