Abstract
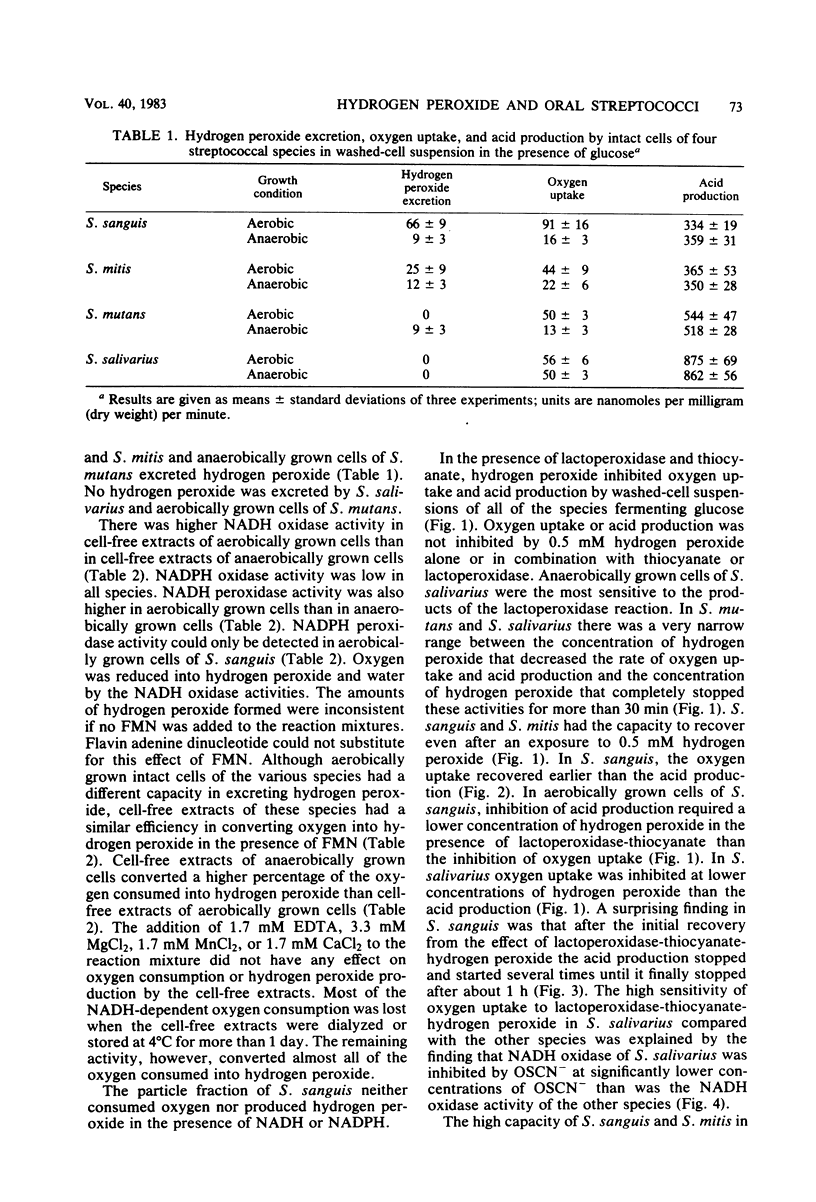
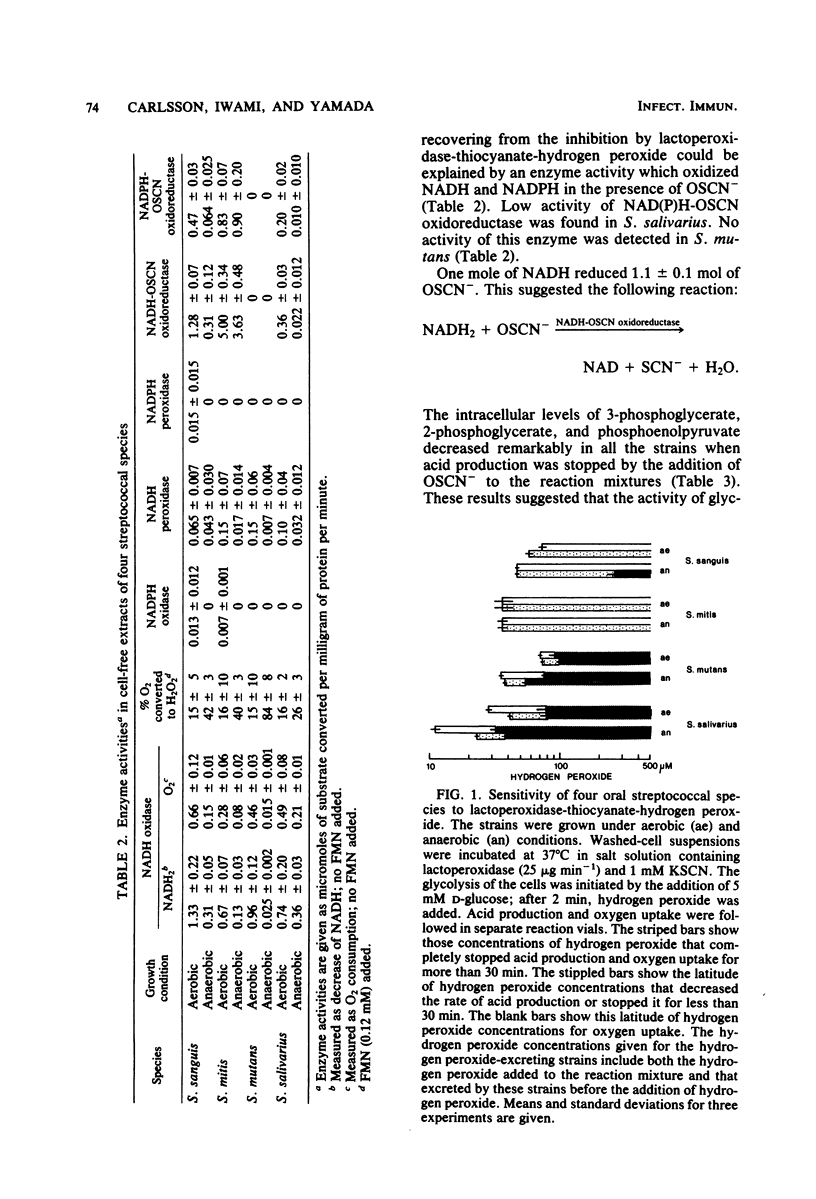
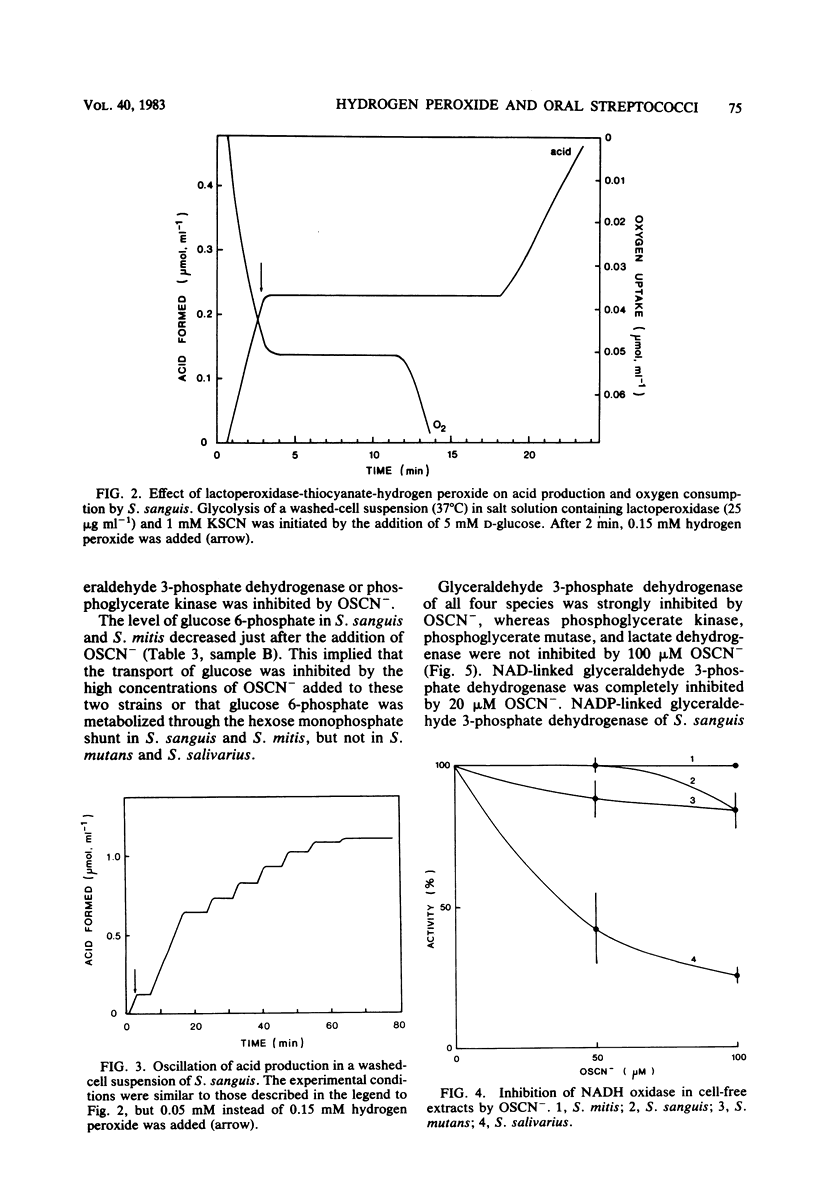
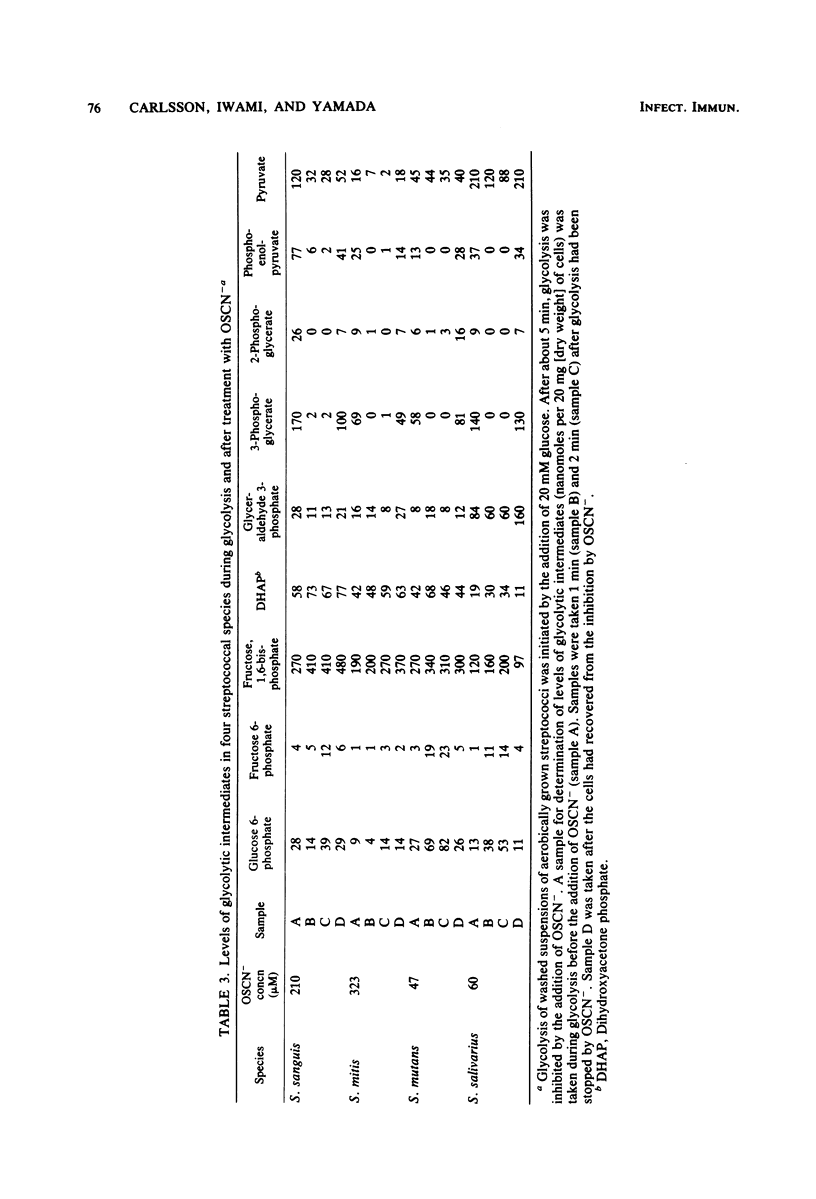
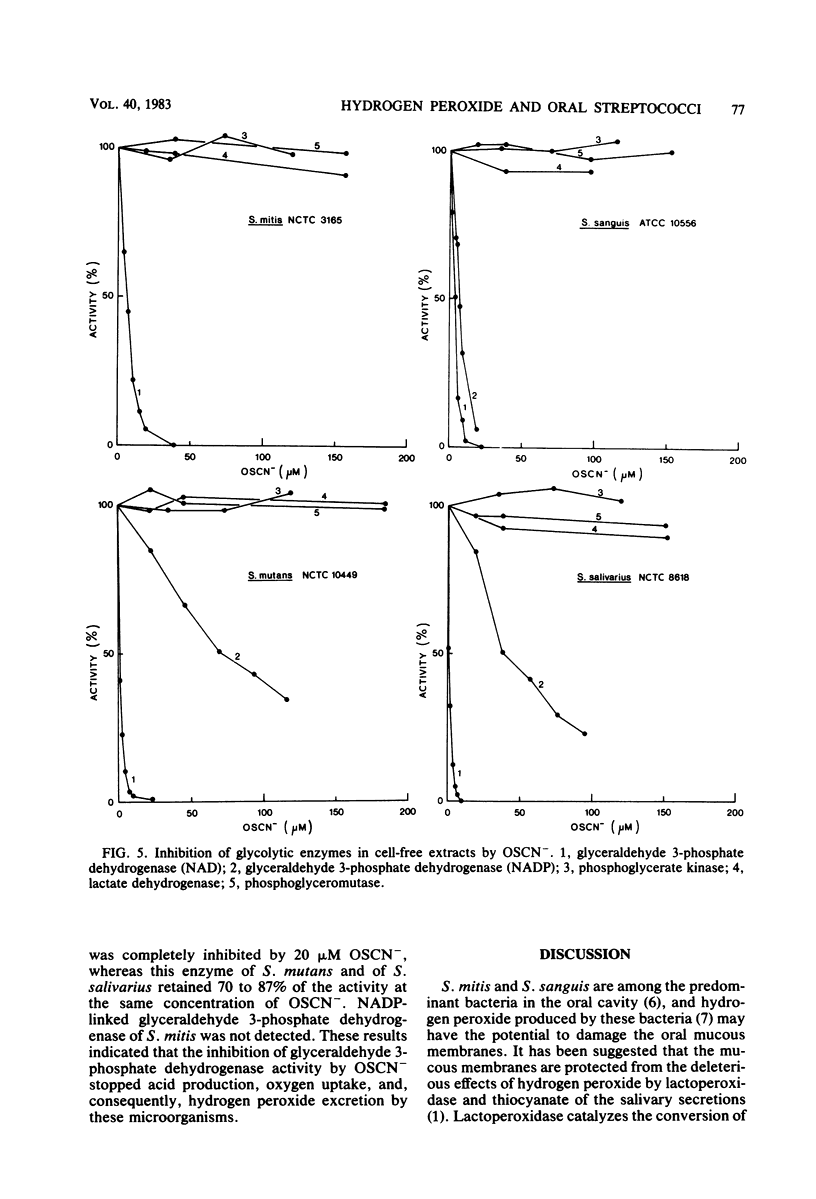
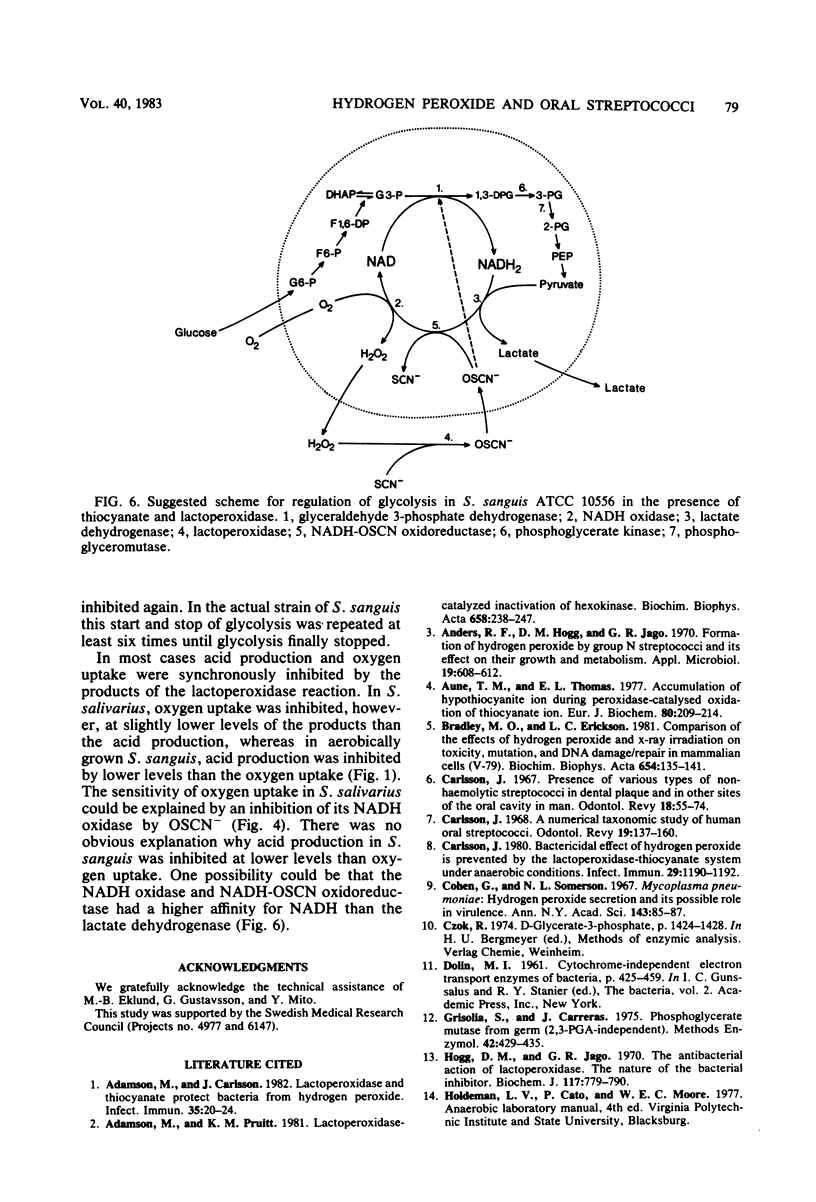
Approved type strains of Streptococcus sanguis, S. mitis, S. mutans, and S. salivarius were grown under aerobic and anaerobic conditions. The rate of hydrogen peroxide excretion, oxygen uptake, and acid production from glucose by washed-cell suspensions of these strains were studied, and the levels of enzymes in cell-free extracts which reduced oxygen, hydrogen peroxide, or hypothiocyanite (OSCN-) in the presence of NADH or NADPH were assayed. The effects of lactoperoxidase-thiocyanate-hydrogen peroxide on the rate of acid production and oxygen uptake by intact cells, the activity of glycolytic enzymes in cell-free extracts, and the levels of intracellular glycolytic intermediates were also studied. All strains consumed oxygen in the presence of glucose. S. sanguis, S. mitis, and anaerobically grown S. mutans excreted hydrogen peroxide. There was higher NADH oxidase and NADH peroxidase activity in aerobically grown cells than in anaerobically grown cells. NADPH oxidase activity was low in all species. Acid production, oxygen uptake, and, consequently, hydrogen peroxide excretion were inhibited in all the strains by lactoperoxidase-thiocyanate-hydrogen peroxide. S. sanguis and S. mitis had a higher capacity than S. mutans and S. salivarius to recover from this inhibition. Higher activity in the former strains of an NADH-OSCN oxidoreductase, which converted OSCN- into thiocyanate, explained this difference. The change in levels of intracellular glycolytic intermediates after inhibition of glycolysis by OSCN- and the actual activity of glycolytic enzymes in cell-free extracts in the presence of OSCN- indicated that the primary target of OSCN- in the glycolytic pathway was glyceraldehyde 3-phosphate dehydrogenase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson M., Carlsson J. Lactoperoxidase and thiocyanate protect bacteria from hydrogen peroxide. Infect Immun. 1982 Jan;35(1):20–24. doi: 10.1128/iai.35.1.20-24.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson M., Pruitt K. M. Lactoperoxidase-catalyzed inactivation of hexokinase. Biochim Biophys Acta. 1981 Apr 14;658(2):238–247. doi: 10.1016/0005-2744(81)90294-1. [DOI] [PubMed] [Google Scholar]
- Anders R. F., Hogg D. M., Jago G. R. Formation of hydrogen peroxide by group N streptococci and its effect on their growth and metabolism. Appl Microbiol. 1970 Apr;19(4):608–612. doi: 10.1128/am.19.4.608-612.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune T. M., Thomas E. L. Accumulation of hypothiocyanite ion during peroxidase-catalyzed oxidation of thiocyanate ion. Eur J Biochem. 1977 Oct 17;80(1):209–214. doi: 10.1111/j.1432-1033.1977.tb11873.x. [DOI] [PubMed] [Google Scholar]
- Bradley M. O., Erickson L. C. Comparison of the effects of hydrogen peroxide and x-ray irradiation on toxicity, mutation, and DNA damage/repair in mammalian cells (V-79). Biochim Biophys Acta. 1981 Jun 26;654(1):135–141. doi: 10.1016/0005-2787(81)90146-5. [DOI] [PubMed] [Google Scholar]
- Carlsson J. A numerical taxonomic study of human oral streptococci. Odontol Revy. 1968;19(2):137–160. [PubMed] [Google Scholar]
- Carlsson J. Bactericidal effect of hydrogen peroxide is prevented by the lactoperoxidase-thiocyanate system under anaerobic conditions. Infect Immun. 1980 Sep;29(3):1190–1192. doi: 10.1128/iai.29.3.1190-1192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J. Presence of various types of non-haemolytic streptococci in dental plaque and in other sites of the oral cavity in man. Odontol Revy. 1967;18(1):55–74. [PubMed] [Google Scholar]
- Cohen G., Somerson N. L. Mycoplasma pneumoniae: hydrogen peroxide secretion and its possible role in virulence. Ann N Y Acad Sci. 1967 Jul 28;143(1):85–87. doi: 10.1111/j.1749-6632.1967.tb27648.x. [DOI] [PubMed] [Google Scholar]
- Grisolia S., Carreras J. Phosphoglycerate mutase from yeast, chicken breast muscle, and kidney (2, 3-PGA-dependent). Methods Enzymol. 1975;42:435–450. doi: 10.1016/0076-6879(75)42149-8. [DOI] [PubMed] [Google Scholar]
- HOSKINS D. D., WHITELEY H. R., MACKLER B. The reduced diphosphopyridine nucleotide oxidase of Streptococcus faecalis: purification and properties. J Biol Chem. 1962 Aug;237:2647–2651. [PubMed] [Google Scholar]
- Hogg D. M., Jago G. R. The antibacterial action of lactoperoxidase. The nature of the bacterial inhibitor. Biochem J. 1970 May;117(4):779–790. doi: 10.1042/bj1170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg K., Hallander H. O. Production of bactericidal concentrations of hydrogen peroxide by Streptococcus sanguis. Arch Oral Biol. 1973 Mar;18(3):423–434. doi: 10.1016/0003-9969(73)90167-2. [DOI] [PubMed] [Google Scholar]
- Hoogendoorn H., Piessens J. P., Scholtes W., Stoddard L. A. Hypothiocyanite ion; the inhibitor formed by the system lactoperoxidase-thiocyanate-hydrogen peroxide. I. Identification of the inhibiting compound. Caries Res. 1977;11(2):77–84. doi: 10.1159/000260252. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y., Baba K., Mifuchi I. [Oxygen consumption of lactobacilli. II. relationship between NADH oxidase activity and oxygen consumption of Lactobacillus acidophilus (author's transl)]. Yakugaku Zasshi. 1979 Aug;99(8):794–799. doi: 10.1248/yakushi1947.99.8_794. [DOI] [PubMed] [Google Scholar]
- KRAUS F. W., NICKERSON J. F., PERRY W. I., WALKER A. P. Peroxide and peroxidogenic bacteria in human saliva. J Bacteriol. 1957 Jun;73(6):727–735. doi: 10.1128/jb.73.6.727-735.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Low I. E., Zimkus S. M. Reduced nicotinamide adenine dinucleotide oxidase activity and H2O2 formation of Mycoplasma pneumoniae. J Bacteriol. 1973 Oct;116(1):346–354. doi: 10.1128/jb.116.1.346-354.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson M. N. Antibacterial action of lactoperoxidase-thiocyanate-hydrogen peroxide on Streptococcus agalactiae. Appl Environ Microbiol. 1979 Nov;38(5):821–826. doi: 10.1128/aem.38.5.821-826.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson M. N. Effect of lactoperoxidase and thiocyanate on the growth of Streptococcus pyogenes and Streptococcus agalactiae in a chemically defined culture medium. J Gen Microbiol. 1966 Apr;43(1):31–43. doi: 10.1099/00221287-43-1-31. [DOI] [PubMed] [Google Scholar]
- Mickelson M. N. Glucose transport in Streptococcus agalactiae and its inhibition by lactoperoxidase-thiocyanate-hydrogen peroxide. J Bacteriol. 1977 Nov;132(2):541–548. doi: 10.1128/jb.132.2.541-548.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakami S., Suzuki C., Saito T., Yoshikawa H. Studies on erythrocyte glycolysis. I. Determination of the glycolytic intermediates in human erythrocytes. J Biochem. 1965 Dec;58(6):543–550. doi: 10.1093/oxfordjournals.jbchem.a128240. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. A convenient calibration of the Clark oxygen electrode. Anal Biochem. 1976 Feb;70(2):632–634. doi: 10.1016/0003-2697(76)90492-9. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Brukner L. H., Silverstein S. C., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. I. Pharmacologic triggering of effector cells and the release of hydrogen peroxide. J Exp Med. 1979 Jan 1;149(1):84–99. doi: 10.1084/jem.149.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The effect of the inhibitory system on susceptible and resistant strains of group N streptococci. Biochem J. 1966 Aug;100(2):373–381. doi: 10.1042/bj1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The oxidation of thiocyanate and the nature of the inhibitory compound. Biochem J. 1966 Aug;100(2):382–388. doi: 10.1042/bj1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijoan C. Secretion of hydrogen peroxide by some common pig mycoplasmas. Vet Rec. 1974 Sep 7;95(10):216–217. doi: 10.1136/vr.95.10.216. [DOI] [PubMed] [Google Scholar]
- Pruitt K. M., Adamson M., Arnold R. Lactoperoxidase binding to streptococci. Infect Immun. 1979 Jul;25(1):304–309. doi: 10.1128/iai.25.1.304-309.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt K. M., Tenovuo J. Kinetics of hypothiocyanite production during peroxidase-catalyzed oxidation of thiocyanate. Biochim Biophys Acta. 1982 Jun 4;704(2):204–214. doi: 10.1016/0167-4838(82)90147-9. [DOI] [PubMed] [Google Scholar]
- RAO D. R., OESPER P. Purification and properties of muscle phosphoglycerate kinase. Biochem J. 1961 Nov;81:405–411. doi: 10.1042/bj0810405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddles P. W., Blakeley R. L., Zerner B. Ellman's reagent: 5,5'-dithiobis(2-nitrobenzoic acid)--a reexamination. Anal Biochem. 1979 Apr 1;94(1):75–81. doi: 10.1016/0003-2697(79)90792-9. [DOI] [PubMed] [Google Scholar]
- THOMPSON R., JOHNSON A. The inhibitory action of saliva on the diphtheria bacillus: hydrogen peroxide, the inhibitory agent produced by salivary streptococci. J Infect Dis. 1951 Jan-Feb;88(1):81–85. doi: 10.1093/infdis/88.1.81. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action. Infect Immun. 1978 May;20(2):456–463. doi: 10.1128/iai.20.2.456-463.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Bates K. P., Jefferson M. M. Hypothiocyanite ion: detection of the antimicrobial agent in human saliva. J Dent Res. 1980 Sep;59(9):1466–1472. doi: 10.1177/00220345800590090201. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Phosphoenolpyruvate carboxylase and ammonium metabolism in oral streptococci. Arch Oral Biol. 1973 Jul;18(7):799–812. doi: 10.1016/0003-9969(73)90051-4. [DOI] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J Bacteriol. 1975 Oct;124(1):55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


