Abstract
The tegument protein pp65 of human cytomegalovirus (HCMV) represents the major component of mature virus particles. Nevertheless, deletion of pp65 has been shown to have no effects on virus replication and morphogenesis in fibroblasts in vitro. We have studied the HCMV virion composition in the absence of pp65 and viral growth of a pp65 stop mutant in different cell types, including monocyte-derived macrophages. Two stop codons at amino acids 11 and 12 of pp65 were introduced by bacterial artificial chromosome mutagenesis into the endotheliotropic strain TB40/E. Clear changes of the tegument composition could be observed in purified mutant virus particles, where the amount of tegument protein pUL25 was drastically reduced. In addition, pUL69 and the virally encoded protein kinase UL97 were undetectable in the pp65 stop mutant. Expression of pUL69 in infected cells was unaltered while pUL25 accumulated in the absence of pp65, thus demonstrating that only incorporation into virus particles is dependent on pp65. Coimmunoprecipitation experiments using lysates of infected cells revealed an interaction between pUL69 and pp65. This interaction was verified in pull-down experiments using transfected cells, which showed that pp65 and pUL69 do not require the presence of other viral proteins for their interaction. We conclude that pp65 is required for the incorporation of other viral proteins into the virus particle and thus is involved in the protein-protein interaction network leading to normal tegument formation. When studying growth kinetics of the pp65 stop mutant in different cell types, we found a severe impairment of viral growth in monocyte-derived macrophages, showing for the first time a strong cell-specific role of pp65 in viral growth.
Human cytomegalovirus (HCMV), a member of the Betaherpesvirinae subfamily, is a threatening pathogen for immunocompromised patients, such as transplant recipients, AIDS patients, and conatally infected infants (15). HCMV infection of individuals with a compromised immune system causes considerable morbidity and mortality after primary infection or reactivation from latency.
Mature HCMV virions comprise four distinct structures: core, capsid, tegument, and envelope. The nucleocapsid consists of the core containing the approximately 240-kb linear double-stranded DNA genome, which is embedded in an icosahedral capsid. Between the envelope, a cellularly derived lipid membrane containing viral glycoproteins, and the nucleocapsid, a protein layer called tegument (26), is located. The tegument of HCMV is composed of at least 25 viral proteins. Tegument proteins have been proposed to act in several processes, such as immune evasion (reviewed in reference 30), release of viral DNA into the nucleus (6), and initiation and regulation of the viral replication cycle (3, 7, 16, 31, 41). However, for many of the tegument proteins, the morphogenetic or regulatory functions are unknown. An increasing number of host cell proteins, e.g., cytoskeletal proteins such as α- and β-actin, have also been detected in HCMV particles (4, 39). In addition to infectious virions, HCMV-infected cells generate two types of aberrant particles: noninfectious enveloped particles (NIEPs) and dense bodies (DBs) (18). The protein composition and morphology of NIEPs are nearly identical to those of mature virions; however, their lack of an electron-dense DNA-containing core allows discrimination of NIEPs from infectious virions by electron microscopy (18). DBs are fusion-competent enveloped particles lacking a nucleocapsid. They are composed primarily of the tegument protein pp65 (ppUL83) (4, 18, 39).
For a long time, the herpesvirus tegument has been considered to be unstructured. Data mainly from alphaherpesviruses indicate that morphogenesis depends on an intricate network of tegument protein-protein interactions (reviewed in reference 23). Interestingly, for most tegument proteins of alphaherpesviruses relevant for primary tegumentation and envelopment, homologues have been found in HCMV, whereas there is much less homology between the proteins involved in secondary tegumentation and envelopment. Cryoelectron microscopic analyses of herpesvirus particles, including HCMV, provide evidence for an icosahedral symmetry and protein-protein complexes forming substructures, at least for the innermost part of the tegument (11).
Remarkably, the most abundant tegument protein and major constituent of extracellular virions, pp65, is not essential for virus replication in fibroblasts in vitro. Deletion of pp65 in HCMV strain AD169 causes a complete loss of DB formation, while production of infectious virus in fibroblasts appears to be unaffected (34). Wild-type virus particle-associated pp65 is rapidly translocated to the nuclei of infected cells after penetration of the incoming virus (4, 33). Newly synthesized pp65 accumulates in both nucleus and cytoplasm at later stages of infection. In all, the precise function of pp65 during infection is not clear.
During HCMV infection, pp65 is a major antigen for cellular immune responses. Besides its function as a structural component of the virus, pp65 seems to be involved in manipulation of the host's immune system. Recent reports provide evidence that pp65 is involved in subverting the host immune response by mediating a decreased expression of major histocompatibility complex class II molecules (27). Microarray studies demonstrating an increase in the cellular antiviral cytokine response during infection with a pp65 deletion mutant suggested that pp65 is involved in downmodulation of beta interferon and of a number of chemokines (1, 8). However, most recent work demonstrates that not pp65 but the immediate-early 2 (IE2) gene product IE86 is responsible for the block of beta interferon induction during HCMV infection and that IE86 expression is delayed in the pp65 deletion mutant due to a decreased expression of pp71 (36). It has also been shown that pp65 can directly interact with NKp30, the natural killer (NK) cell-activating receptor, and that this interaction leads to a general inhibition of the killing ability of NK cells (2). Because of the requirement of cell-free pp65, the relevance of this interaction during HCMV infection in vivo is not entirely clear and needs to be investigated in more detail.
Another feature of pp65 is the ability to interact with cellular as well as viral proteins. The interaction of pp65 with the cellular Polo-like kinase 1 (Plk1) results in an incorporation of Plk1 into virus particles. Plk1 is able to phosphorylate pp65 in vitro (14). Recently, it has been shown that pp65 interacts directly with the viral protein kinase pUL97 (20). pUL97 seems to be required for normal intranuclear distribution of pp65. Inhibition of the pUL97 kinase activity with maribavir or mutation of an essential amino acid in the kinase domain results in accumulation of pp65 in characteristic inclusions in the nuclei of infected as well as transfected cells (28).
To extend our knowledge about pp65 and its function, we investigated the composition of endotheliotropic HCMV particles in the absence of the most abundant tegument protein, pp65. We hypothesized that other viral or cellular proteins might compensate for the lack of pp65 in virus particles, as described for tegument mutants of pseudorabies virus (25). The results presented here, using a pp65 stop codon mutant of the endotheliotropic HCMV strain TB40/E, demonstrate that in contrast to our hypothesis, incorporation of at least three other HCMV tegument proteins, pUL25, pUL69, and pUL97, is severely impaired when pp65 is lacking. For pUL69, a direct interaction with pp65 could be shown in infected as well as transfected cells. These results show that pp65 interacts with other viral tegument proteins during infection, which in turn is important for the incorporation of these proteins into mature virus particles. Finally, for the first time, we could show a cell-specific biological relevance of pp65 for growth of HCMV in monocyte-derived macrophages (MDM).
MATERIALS AND METHODS
Viruses.
A recombinant HCMV virus unable to express UL83-encoded pp65 was generated by introduction of two stop codons at amino acid positions 11 and 12 of pp65 using a markerless two-step RED-GAM recombination protocol (37). The primers used for this protocol were 65stop-for (5′-CGGCTTTCAGCACGTGCCCCGAAATGGGACCCAGTACGGATCACTATTCGGGACAACGGCGACCAACCAATTAACCAATTCTGATTAG-3′) and 65stop-rev (5′-CGCAGGCAGCATGGAGTCGCGCGGTCGCCGTTGTCCCGAATAGTGATCCGTACTGGGTCCCATAGGATGACGACGATAAGTAGGG-3′). The primers are homologous to the UL83 gene both upstream and downstream of the sequence to be mutated and are homologous to the pEPkan-S template plasmid (underlined) (37). Recombination was used to modify an infectious clone of the endotheliotropic HCMV strain TB40/E (kindly provided by G. Hahn, Ingolstadt, Germany). The infectious clone used in this work, referred to below as the wild type, differs only slightly from the sequenced infectious clone TB40-BAC4 (35) in that the unique short region seems to be inverted (unpublished data). By an insertion of two stop codons in UL83, a recombinant infectious clone, 65stop, was generated. The mutations were confirmed by sequencing. To generate a revertant virus of the pp65 stop mutant, RED-GAM mutagenesis was performed using recombinant bacmid clone 65stop. The primers used for the generation of the revertant were 65resc-for (5′-CGGCTTTCAGCACGTGCCCCGAAATGGGACCCAGTACGGATATCATTTCGGGACAACGGCGACCAACCAATTAACCA TTCTGATTAG-3′) and 65resc-rev (5′-CGCAGGCAGCATGGAGTCGCGCGGTCGCCGTTGTCCCGAAATGATATCCGTACTGGGTCCCATA GGATGACGACGATAAGTAGGG-3′). The resulting recombinant infectious clone, 65rev, was confirmed by sequencing. Bacmid DNAs of the wild type, 65stop, and 65rev were electroporated into MRC-5 cells, which resulted in reconstitution of wild-type virus, the pp65 stop mutant v65stop, and the revertant virus termed v65rev.
Rate-velocity centrifugation, used for purification of virus particles, was performed essentially as previously described (29) with a few modifications. Human foreskin fibroblasts (HFF) were infected with the different viruses at a multiplicity of infection (MOI) of 1 PFU per cell and incubated for 5 to 9 days. Supernatants from at least three 175-cm2 flasks were collected, and cellular debris was removed by low-speed centrifugation (6,000 rpm, 4°C, 15 min). The virus particles were pelleted by ultracentrifugation for 1 h at 4°C and 23,000 rpm in an SW-28 rotor (Beckman). Pelleted virus particles were resuspended in 2 ml of 0.04 M sodium phosphate buffer (pH 7.4) and layered onto glycerol-tartrate gradients formed in 0.04 M sodium phosphate. Virus particles were then separated by centrifugation for 90 min at 6°C and 23,000 rpm in an SW-41 rotor. NIEPs, virions, and DBs were visualized with incandescent light and were collected from the gradient. Particles extracted from the gradient were diluted with the sodium phosphate buffer and again centrifuged at 23,000 rpm for 1 h using the SW-41 rotor. To obtain higher purities of virion and DB fractions, virus particles were purified twice on a glycerol-tartrate gradient. After the second purification, pelleted virus particles were resuspended in 200 μl of 0.04 M sodium phosphate buffer and stored at −80°C.
Expression plasmids.
The entire open reading frame of UL83 was amplified with primers 65amp-for (5′-TAGGATCCGTGCCACCATGGAGTCGCGCGGTCG-3′) and 65amp-rev (5′-GATCGAATTCGAGTCGTCTTAAGCGCGTGC-3′). The resulting PCR product was cloned into eukaryotic expression vector pEF1/Myc-His C (Invitrogen, Karlsruhe, Germany) by using BamHI and EcoRI restriction sites (underlined), resulting in pEF-pp65. In order to fuse pp65 with a Myc tag, the coding sequence of pp65 was amplified with primers 65amp-for (see above) and 65co1-rev (5′-GATGAATTCCACCTCGATGCTTTTTGGGCG-3′). Cloning of the resulting PCR product into pEF1/Myc-His C by using BamHI and EcoRI restriction sites (underlined) resulted in plasmid pEF-pp65Myc, in which pp65 is fused to the Myc-His tag at the C terminus. The pp150 expression plasmid pc-pp150 was kindly provided by M. Winkler, University Schleswig-Holstein, Kiel. The UL69 expression plasmid pEF-UL69 was generated by subcloning of UL69 from plasmid pHM160 (kindly provided by M. Winkler [41]) using BamHI and EcoRI restriction sites.
Cells, transfection, and infection.
HFF were maintained in minimal essential medium (Gibco/Invitrogen, Karlsruhe, Germany) and used before passage 20. Human MRC-5 embryonic lung fibroblasts (European Collection of Cell Cultures) and U373-MG (human astrocytoma glioblastoma) cells as well as 293T (human embryonic kidney) cells obtained from the American Type Culture Collection (Manassas, VA) were maintained in Dulbecco's modified Eagle medium (Gibco/BRL). All media were supplemented with 10% fetal calf serum, 2 mM l-glutamine (Biochrom AG, Berlin, Germany), 100 U of penicillin and 100 μg of streptomycin (Gibco/BRL) per ml, and 1× nonessential amino acids (Biochrom AG). Human umbilical cord endothelial cells (HUVEC) were purchased from Clonetics (BioWhittaker, Walkersville, MA) and cultured in endothelial cell basal medium supplemented with 5% fetal calf serum and growth factors (EGM-MV SingleQuots; BioWhittaker). The MDM used in this study were produced from at least three different blood donors. Briefly, monocytes were isolated from buffy coats of HCMV-seronegative blood donors by negative selection with magnetic microbeads according to the manufacturer's instructions (monocyte isolation kit II; Miltenyi Biotec, Bergisch Gladbach, Germany). A total of 3 × 106 monocytes/ml were then cultured for 7 days in RPMI 1640 supplemented with 10% fetal calf serum, 2 mM glutamine, antibiotics, and 100 ng/ml macrophage colony-stimulating factor (R&D System, Minneapolis, MN) on hydrophobic Lumox dishes (Greiner Bio-One GmbH, Frickenhausen, Germany). The differentiation of MDM occurred over a period of 7 days and was evaluated by morphological criteria, phagocytosis, and flow cytometric analysis.
MRC-5 cells were used for virus reconstitution after electroporation of bacmid DNA isolated from Escherichia coli using Nucleobond AX columns (Machery & Nagel). For electroporation, MRC-5 cells from one 75-cm2 flask were trypsinized, washed one time with phosphate-buffered saline (PBS), and resuspended in 250 μl Dulbecco modified Eagle medium without antibiotics. Two to 3 micrograms of bacmid DNA together with 1 μg of pp71 expression plasmid pCMV71 (32) in a total volume of 40 μl distilled water were mixed with the cells, transferred to an electroporation cuvette (4 mm), and electroporated at 260 V and 1,050 μF. HFF were used for further viral propagation, including study of growth kinetics and virus titrations. To determine viral growth characteristics, HFF were infected at an MOI of 10 (high multiplicity) or 0.01 (low multiplicity) PFU/cell. After 2 h of incubation, the inoculum was removed and cells were washed three times with PBS to remove remaining virus before addition of complete medium. Samples were taken once a day for 7 days (high-multiplicity infection) or once every 3 days for 3 weeks (low-multiplicity infection), and titers were determined by plaque assay on HFF.
Antibodies.
HCMV-encoded proteins were detected with monoclonal antibodies (MAbs) or polyclonal rabbit antiserum in Western blot analyses or coimmunoprecipitation experiments. The MAbs used in this study included those directed against the major capsid protein (MCP) (UL86, MAb 28-4), pUL69 (UL69, MAb 69-66), pp28 (UL99, MAb 41-28), gB (UL55, MAb 27-287), and pp65 (UL83, MAb 65-33) (all kindly provided by W. Britt, University of Alabama, Birmingham, AL); pp150 (UL32, XP-1) (19); and pUL48 (UL48, 5GI) (kindly provided by T. Shenk, Princeton University, Princeton, NJ) (6). Generation of the polyclonal mouse antibody directed against pUL25 has been previously described (5). Generation of the rabbit antiserum specifically recognizing pUL69 (UL69) has been previously described (41). The rabbit antiserum directed against pUL97 was generated and described previously (20). The MAb reactive with actin was purchased from Sigma (St. Louis, MO), while MAb 9E10 (13) was used to detect the Myc tag.
Western blot analyses.
For Western blot analyses, HFF were infected using an MOI of 1, and cell lysates were prepared at 5 days postinfection (p.i.). Gradient-purified virions or lysates from infected cells were mixed with sodium dodecyl sulfate (SDS) sample buffer and boiled for 5 min prior to electrophoresis using 10 to 12% SDS-polyacrylamide gels. After separation, proteins were either silver stained using a standard staining procedure or transferred to Immobilon-P polyvinylidene difluoride membranes (Millipore) by the semidry method (21). Free binding sites on the sheets were blocked by addition of 5% skim milk in PBS containing 0.3% Tween 20 before the antibodies (suspended in PBS-0.3% Tween 20 with 0.5% skim milk) were added. Bound antibodies were detected with goat anti-mouse or goat anti-rabbit horseradish peroxidase-conjugated antibodies (Pierce) and visualized by enhanced chemiluminescence (Immobilon Western HRP substrate; Millipore). Protein bands on the blots were quantified using Quantity One version 4.3.1 quantification software (Bio-Rad).
Coimmunoprecipitation.
For coimmunoprecipitation, HFF were infected with an MOI of 1. Infected cells for each virus were collected from at least three 175-cm2 flasks at 96 h p.i. and washed two times with PBS before lysis in 5 ml NP-40 lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 5 mM EDTA, 0.5% NP-40, 1× Complete protease inhibitor mix [Roche, Mannheim]). After incubation on ice for 20 min, the lysate was cleared by centrifugation for 10 min at 6,000 rpm. Virus particles were removed from the cleared lysate by ultracentrifugation at 23,000 rpm for 1 h. A preclearing was then performed using 400 μl of protein A-Sepharose CL-4B (30 mg/ml; Amersham Biosciences), followed by an incubation of 1 h at 4°C under constant shaking. After centrifugation at 6,000 rpm for 10 min to pellet the protein A-Sepharose, a 150-μl aliquot was taken as a lysate control. For the immunoprecipitation, 500-μl aliquots of the precleared lysate were taken, added to 300 μl of lysate buffer with antibody, and incubated for 1 h at 4°C. Protein A-Sepharose (40 μl) was then added, followed by additional shaking for 1 h at 4°C. Bound protein complexes were collected (5,000 rpm, 5 min, 4°C) and washed three times with 700 μl lysis buffer. The remaining liquid was completely removed after a final centrifugation at 13,000 rpm. The pellet was resuspended in 2× SDS buffer (80 mM Tris-HCl [pH 8.8], 2 mM EDTA, 400 mM sucrose, 0.4 mM dithiothreitol, 0.4% SDS, 0.1% bromphenol blue) before loading onto polyacrylamide gels to separate precipitated proteins.
For coimmunoprecipitation from transfected cells, 293T cells were seeded in six-well plates at 70% confluence the day before transfection. The next day, the cells were transfected using Lipofectamine LTX (Invitrogen) with 1.25 μg of each plasmid according to the manufacturer's protocol. After an incubation of 4 to 12 h, cells were washed one time with PBS to remove the liposome DNA mixture and incubated with fresh medium. At 48 h posttransfection, cells were collected and washed two times with PBS. Cell lysis was performed as described above using 500 μl of lysis buffer. The subsequent steps were identical to those for infected cells except that no ultracentrifugation was needed. The cell lysate was also precleared with 40 μl protein A-Sepharose before collection of the lysate control and addition of the antibodies.
Electron microscopy.
For electron microscopy, MRC-5 cells as well as HUVEC were grown on carbon-coated sapphire discs (3-mm diameter; Engineering Office M. Wohlwend GmbH, Switzerland) and infected with an MOI of 1 PFU/cell. High-pressure freezing was performed at 5 days p.i. with the HPF 01 freezing apparatus (Engineering Office M. Wohlwend GmbH, Switzerland). Freeze-substitution (40) and embedding were performed as described previously (9, 10). After thin sectioning, samples were imaged with a Zeiss EM10 or a Philips 400 transmission electron microscope at an acceleration voltage of 80 kV.
Infectivity assay.
MDM from three different donors or HFF were seeded in μ-Slide eight-well plates (Ibidi, Martinsried, Germany) and infected with wild-type, v65stop, or v65rev virus at an MOI of 1. The amount of virus in the inoculum was controlled by titration on HFF. After 24 h p.i., cells were fixed, permeabilized, and stained for HCMV IE1/2 (Argene) and DAPI (4′,6′-diamidino-2-phenylindole) (Sigma-Aldrich). Fluorescence microscopy was performed with a Zeiss Observer Z1 microscope, and the percentage of IE-positive cells was determined by counting at least 10,000 cells from three independent experiments.
RESULTS
Construction and propagation of a pp65 stop codon mutant.
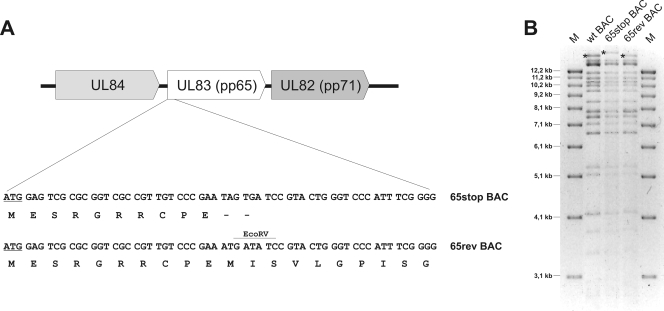
Previous reports on pp65 (UL83) deletion mutants based on the nonendotheliotropic laboratory HCMV strain AD169 suggested that deletion of UL83 from the viral genome in some way alters the expression of the downstream-located UL82, encoding pp71. We could overcome these unwanted effects by generating a UL83 stop mutant (36). To explore the role of the UL83-encoded protein pp65 as a structural component of the virus particle and to test a pp65 mutant in cells other than fibroblasts, we generated a pp65 stop codon mutant using an infectious clone of the highly endotheliotropic HCMV strain TB40/E. As depicted in Fig. 1A, two stop codons were introduced in the open reading frame of UL83 30 bp downstream of the UL83 start codon. The resulting recombinant HCMV bacmid was termed 65stop. In addition, a revertant bacmid, 65rev, in which the two stop codons of 65stop were restored to the wild-type sequence (M12 and I13) was generated.
FIG. 1.
(A) Generation of the HCMV pp65 stop codon mutant. The bacmid (BAC) for the pp65 stop codon mutant (65stop) was generated by introducing two stop codons at amino acid positions 11 and 12 of UL83. Both stop codons were then reverted to the original codons in a revertant bacmid (65rev). (B) Restriction enzyme analysis of wild-type (wt) bacmid and bacmids 65stop and 65rev with EcoRV. The destruction of an EcoRV restriction site due to the insertion of the stop codons in 65stop is mirrored by an additional DNA fragment of 16,980 bp, compared to 15,956 bp for the wild-type and 65rev viruses (asterisks). The three bacmids were used to reconstitute the respective viruses by transfecting human fibroblasts. M, molecular markers. Sizes are shown in the left margin.
Both bacmids, 65stop and 65rev, were compared with the wild-type bacmid by restriction enzyme analysis (Fig. 1B). The insertion of the two stop codons in 65stop eliminated an EcoRV restriction enzyme site of UL83, leading to the fusion of the two UL83-containing EcoRV fragments of 15,956 and 1,021 bp in size. The resulting new 16,980-bp EcoRV fragment reflected the alteration of the UL83 sequence in bacmid 65stop. The loss of the 16,980-bp EcoRV fragment and reappearance of the 15,956-bp EcoRV fragment confirmed the restoration of the EcoRV restriction enzyme site in the revertant bacmid 65rev (Fig. 1B). Additional digests with BglII and EcoRI showed that spurious rearrangements had not occurred during the recombination procedures (data not shown). Finally, proper mutation as well as restoration and sequence integrity of both the 65stop and the 65rev bacmids were further confirmed by DNA sequencing of the region that was mutated. These results showed the successful cloning of a pp65 stop codon mutant and its respective revertant bacmid in the backbone of the endotheliotropic HCMV strain TB40/E.
Wild-type virus, mutant virus v65stop, and revertant virus v65rev were reconstituted in MRC-5 cells and further analyzed.
The lack of pp65 causes changes in the overall virion composition.
It had been previously reported that the compositions of a UL83 deletion mutant and wild-type virions were indistinguishable (34). This is surprising in view of the fact that the major constituent of the tegument is missing in particles of the UL83 deletion mutant. Therefore, we examined the composition of gradient-purified virions of v65stop in comparison to wild-type and revertant viruses in greater detail. Extracellular particles of wild-type, v65stop, and v65rev viruses were gradient purified twice using glycerol-tartrate gradients. As described previously for a UL83 deletion mutant (34), gradients of v65stop showed two bands corresponding to NIEPs and virions but were devoid of DBs (data not shown). To ensure a proper comparison of v65stop with wild-type and v65rev virions, gradient-purified viruses were examined by electron microscopy before biochemical analysis to exclude DB contamination and to confirm the purity of the virion fractions. The negatively stained virion preparations showed a fairly homogeneous mixture of particles of the size, shape, and typical morphology expected for HCMV virions (data not shown).
As shown in Fig. 2A, comparable amounts of purified wild-type, v65stop, and v65rev virions were separated by SDS gel electrophoresis and bands were visualized by silver staining. Similar signal intensities of the protein bands corresponding to the large tegument protein pUL48, MCP, and the UL32-encoded pp150 were verified by Western blot analysis (data not shown), confirming equal loading onto the polyacrylamide gel. Apart from the absence of the band corresponding to pp65 in v65stop virions, the majority of virion proteins appeared to be present in approximately equal quantities compared to wild-type and revertant virions. This leads to the conclusion that other tegument proteins do not compensate for the lack of pp65 in virions by increased incorporation. To our surprise, we noticed that some proteins migrating at a molecular mass of between 75 and 100 kDa were missing in v65stop virions.
FIG. 2.
Analyses of gradient-purified virions and cytoplasmic extracts of cells infected with wild-type, v65stop, and v65rev viruses. Virions were purified by glycerol-tartrate gradient centrifugation. (A) The solubilized proteins of purified virions were separated on a 10% one-dimensional SDS-polyacrylamide gel. After electrophoretic separation, the gel was fixed and silver stained. M, molecular mass markers. Sizes are shown in the left margin. (B) Western blot analysis of gradient-purified virions using antibodies against the indicated viral proteins. (C) Cell lysates from HCMV-infected and mock-infected HFF were analyzed by Western blotting in parallel. Cells were harvested at 5 days p.i., and an antibody against β-actin was used in addition as a loading control.
These results suggested that in the absence of pp65, the virion composition is indeed altered such that several other proteins are lacking or are less efficiently incorporated into virus particles.
Incorporation of pUL69, pUL97, and pUL25 into virus particles depends on the presence of pp65.
We hypothesized that the proteins with a molecular mass of between 75 and 100 kDa that were missing in v65stop particles might be tegument proteins. Therefore, we examined the tegument composition of v65stop virions by Western blot analyses using various antibodies raised against HCMV tegument proteins (Fig. 2B; Table 1). Equal loading of wild-type, v65stop, and v65rev virions was controlled by using the MCP signal as a reference. Furthermore, pUL48 and the very abundant tegument protein pUL32 showed comparable signal intensities, confirming our previous results (Fig. 2A), namely, that the levels of pUL48 and pUL32 do not seem to be altered in particles of the pp65 stop mutant. In contrast, probing with a pUL69-specific polyclonal antibody revealed that pUL69 was not present in virions of the v65stop mutant, although it was clearly detectable in wild-type and revertant virions (39). Similar results could be obtained with the pp65 deletion mutant RVAd65 (data not shown), based on HCMV strain AD169 (34). We further tested for pUL25, an abundant HCMV tegument protein with a molecular mass of approximately 87 kDa that is present in both virions and DBs, using a polyclonal antibody against pUL25. As shown in Fig. 2B and Table 1, pUL25 levels were significantly reduced in v65stop particles compared to wild-type or revertant virus particles. Recent reports have shown a direct interaction of pp65 with pUL97 (20), which in turn might lead to an altered incorporation of pUL97 into v65stop particles. Therefore, we tested the presence of pUL97 in virus particles using a previously described polyclonal antibody against pUL97 (20). Figure 2B shows that pUL97, a tegument protein with low abundance, could be detected in wild-type as well as v65rev virions but not in v65stop virions. These data suggested that the interaction of pp65 and pUL97 could be important for pUL97 incorporation into virus particles.
TABLE 1.
Relative intensities of Western blot signals from Fig. 2B and C
| Protein | Avg virion abundance (%)a | Relative signal intensityb
|
|||||
|---|---|---|---|---|---|---|---|
| Purified virions
|
Cell lysates
|
||||||
| Wild type | v65stop | v65rev | Wild type | v65stop | v65rev | ||
| MCP | 6.0 | 1.0 | 1.3 | 1.2 | 1.0 | 1.1 | 1.0 |
| UL83 | 15.4 | 1.0 | 0.0 | 1.0 | 1.0 | 0.0 | 1.4 |
| UL69 | <0.1 | 1.0 | 0.0 | 1.5 | 1.0 | 1.3 | 0.9 |
| UL32 | 9.1 | 1.0 | 0.9 | 1.0 | 1.0 | 1.4 | 1.3 |
| UL25 | 2.2 | 1.0 | 0.1 | 1.3 | 1.0 | 11.2 | 1.4 |
| UL99 | <0.1 | 1.0 | 0.8 | 1.4 | 1.0 | 0.9 | 1.0 |
| UL97 | 0.1 | 1.0 | 0.0 | 1.7 | 1.0 | 0.6 | 1.0 |
| UL48 | 12.6 | 1.0 | 0.7 | 0.8 | 1.0 | 1.4 | 0.8 |
| Actin | ND | ND | ND | 1.0 | 1.0 | 1.5 | |
From reference 39.
Values were normalized relative to those for the wild type, which were set at 1.0. Differences in signal intensity of more than twofold relative to the wild-type signal are in bold. ND, not determined.
Given the noticeable reduction in the levels of pUL25 and the absence of pUL69 and pUL97 in v65stop virions, we investigated whether changes of the intracellular expression levels of these proteins in v65stop-infected HFF could be responsible for the observed incorporation defects. As shown in Fig. 2C, most tested proteins, including pUL69, were expressed in similar amounts in cells infected with wild-type, v65stop, and v65rev viruses except for the considerably higher protein levels of pUL25 in lysates of v65stop-infected cells (Fig. 2C; Table 1). These data suggest that incorporation of pUL69 into virus particles is abolished in the absence of pp65 irrespective of unaltered expression levels of pUL69 in v65stop-infected cells. In contrast to pUL69, pUL25 was still detectable, although drastically reduced, in particles of v65stop, indicating that pp65 is required but not essential for the incorporation of pUL25 into virus particles. In turn, the diminished virion incorporation of pUL25 is accompanied by an accumulation of pUL25 in v65stop-infected cells.
The intracellular expression of pUL97, including the various phosphorylation forms of pUL97, seem to be slightly reduced in v65stop-infected cells (Fig. 2C; Table 1). Interestingly, some of the phosphorylation forms of pUL97 which are present in wild-type- and v65rev-infected cells could not be detected in cells infected with the pp65 stop mutant.
Direct interaction of pp65 and pUL69.
In order to elucidate why pUL69 was not incorporated into v65stop virions, we investigated whether there was a direct interaction of pp65 with pUL69. Lysates of mock-, wild-type-, or v65stop-infected cells were subjected to immunoprecipitation with antibodies specific for pp65 or pUL69 and then analyzed by Western blotting. As shown in Fig. 3A (left panels), pUL69 was specifically detected in immunoprecipitates from wild-type virus-infected HFF cell lysates using the anti-pp65 antibody but not in those from mock- or v65stop-infected cell lysates, providing evidence for the specificity of that interaction. The experiment was repeated by immunoprecipitation with an antibody specific for pUL69 (Fig. 3A, right panels), and again pp65 was coprecipitated from lysates of wild-type virus-infected cells, confirming the interaction. Western blot analysis of the lysates using the same antibodies for detection of the specific proteins is shown in Fig. 3B. From these results, we concluded that pp65 and pUL69 interact to form a complex during HCMV infection. However, it could not be excluded that other proteins are involved in the complex formation.
FIG. 3.
Coimmunoprecipitation from virus-infected cell lysates. HFF were infected with wild-type (wt) virus or v65stop (MOI of 1) and harvested at 96 h p.i. Immunoprecipitation was performed using antibodies against pp65 (MAb 65-33) or pUL69 (MAb 69-66). Immunoprecipitated proteins were detected by Western blot analyses using the antibody against pp65 (MAb 65-33) and the polyclonal antibody against pUL69. IP, immunoprecipitation; WB: Western blotting. M, molecular mass markers; sizes are shown in the left margin.
Therefore, we performed a pull-down experiment with lysates of cells transfected with expression plasmids for pUL69 and pp65 (Fig. 4). Figure 4A shows that pUL69 was specifically coprecipitated with pp65 from lysates of pp65/pUL69-cotransfected 293T cells using an antibody specific for pp65. Reciprocally, we were able to detect pp65 in immunoprecipitates of pp65/pUL69-cotransfected cells using an antibody directed against pUL69 (Fig. 4A, lower panel). In immunoprecipitates of singly transfected cells or in the Sepharose A bead controls, no specific signals were detected, proving that the detected signals for pUL69 or pp65 were not caused by an unspecific binding of the antibody or binding to the Sepharose A beads used for the pull-down assay. These results were reproduced by using a plasmid expressing a Myc epitope-tagged pp65 and an antibody specific for the Myc epitope (data not shown). It is generally believed that pp65 is a very “sticky” protein that easily binds to other viral or cellular proteins. However, in coimmunoprecipitation experiments with an expression plasmid for the tegument protein pp150, no interaction with pp65 could be observed (Fig. 4B). This provided further evidence for the specificity of the pUL69-pp65 interaction. From these experiments we concluded that pp65 and pUL69 directly interact during HCMV infection and that this interaction does not require the presence of other viral proteins.
FIG. 4.
Coimmunoprecipitation from transfected cell lysates. 293T cells were transfected with different combinations of expression plasmids for pp65 (pEF-pp65), pUL69 (pEF-69), and pp150 (pc-pp150) (indicated by + and −) and harvested at 48 h posttransfection. (A) Coimmunoprecipitation using lysates of cells cotransfected with pp65 and pUL69 expression plasmids. (B) Coimmunoprecipitation using lysates of cells cotransfected with pp65 and pp150 expression plasmids. Lysate, lysate control; SephA, negative control using only Sepharose-A beads without antibodies for precipitation; IP: immunoprecipitation; WB: Western blotting. M, molecular mass markers; sizes are shown in the left margin.
Electron microscopy of human fibroblasts infected with wild-type and v65stop viruses.
We wanted to characterize by electron microscopy the morphological phenotype of our pp65 stop codon mutant in human fibroblasts and also endothelial cells by using high-pressure freezing and freeze-substitution. Fibroblasts and HUVEC were grown on sapphire disks, infected with an MOI of 1 PFU/cell, and fixed by high-pressure freezing on day 5 p.i. Different stages of virus morphogenesis of the pp65 stop codon mutant were indistinguishable from those of wild-type-infected human fibroblasts (Fig. 5A to D) and HUVEC (data not shown) except for the complete absence of DBs in v65stop-infected cells. Even when many virus particles were examined at a higher magnification, the thicknesses and structures of the teguments of v65stop and wild-type particles appeared overall to be indistinguishable (Fig. 5C to F). From these results we concluded that in accordance with published results, morphogenesis and tegumentation of virions in human fibroblasts and endothelial cells are not affected in the absence of pp65.
FIG. 5.
Electron microscopy of human fibroblasts infected with wild-type virus and v65stop. High-pressure freezing and freeze-substitution were performed at 5 days p.i. Shown are representative images of MRC-5 cells infected with wild-type (A, C, and E) and v65stop (B, D, and F) viruses. An overview of sections of infected cells with nuclear stages and cytoplasmic stages of HCMV morphogenesis at the assembly complex is shown (A and B), illustrating the lack of DBs in v65stop-infected cells (B). The structures and thicknesses of the teguments of wild-type and v65stop viruses appeared to be similar in higher magnifications of intracellular enveloped virus particles (C and D) and extracellular virus particles (E and F). Intravesicular, enveloped virions are indicated by black arrows; virions in the process of budding by stippled arrows; and DBs (present only in wild-type-infected cells) by black arrowheads. Ncl, nucleus; Mtoc, microtubule organization center. Scale bar, 500 nm in panels A and B, 200 nm in panels C and D, and 100 nm in panels E and F.
Growth characteristics in HFF, HUVEC, and macrophages.
We first compared the kinetics of replication for wild-type, v65stop, and v65rev viruses in HFF at two different MOIs. Irrespective of whether we used a high MOI of 10 PFU/cell (Fig. 6A) or a low MOI of 0.01 PFU/cell (Fig. 6B), the pp65 stop codon mutant replicated to titers virtually identical to those of the wild-type or revertant virus. There was also no detectable difference in the onset or the kinetics of virus production between the wild-type, v65stop, and v65rev viruses. These results were in agreement with those of earlier studies of an UL83 deletion mutant of HCMV strain AD169 (34). We similarly evaluated the growth properties of the pp65 stop codon mutant in endothelial cells. This had not been possible with the previously generated UL83 deletion mutant based on the nonendotheliotropic strain AD169. As for HFF, the replication kinetics of v65stop were identical to those of the wild-type and v65rev viruses (data not shown). These results show that pp65 does not play a major role in virus production and release from infected fibroblasts or endothelial cells in vitro.
FIG. 6.
Growth kinetics of the reconstituted HCMV on HFF wild type (▪), v65stop (▴), and v65rev (▾) were used. HFF were infected at an MOI of 10 (A) or 0.01 (B) PFU/cell. Samples of the supernatants were taken at the indicated time points after infection and titrated on HFF. Shown are the means and standard errors of the extracellular titers for each virus from three independent experiments.
Finally we compared viral growth of the wild type, v65stop, and v65rev in MDM using an MOI of 1 PFU/cell. This approach was chosen since macrophages play an essential role both in the host immunity and in HCMV infection. The v65stop mutant replicated to titers 20- to 100-fold lower than those for wild-type or revertant viruses in MDM (Fig. 7A). The mutant also displayed a delay in production of infectious particles compared to wild-type and revertant viruses, with the peak production at around day 7 p.i. Surprisingly, virus yields of v65stop declined starting at day 7 p.i. until no infectious virus could be detected, while virus yields of wild-type and revertant viruses remained high until the end of the experiment. The wild-type and revertant viruses exhibited nearly identical growth kinetics, indicating that the mutation was successfully repaired and that the phenotype of v65stop in MDM was due to the substitution mutation and not to another potential mutation in the genome.
FIG. 7.
Growth kinetics and infectivity assay of v65stop on macrophages. (A) MDM were infected at an MOI of 1 PFU/cell with wild-type (▪), v65stop (▴), or v65rev (▾) virus. Samples of the supernatants were taken at the indicated time points after infection and titrated on HFF. Shown are means and standard errors of the extracellular titers for each virus from four independent experiments with macrophages obtained from three different donors. (B) MDM and HFF were infected with wild type, v65stop, or v65rev virus, using an MOI of 1 PFU/cell. At 24 h p.i., cells were fixed and stained for HCMV IE1/2 and DAPI. The mean percentages of IE-positive cells and standard errors were determined by counting at least 10,000 cells from three independent experiments.
To determine whether the delay in virus production of v65stop in MDM was caused by a decreased infectivity of that mutant, we infected MDM with the wild type, v65stop, and v65rev at an MOI of 1 PFU/cell and determined the percentage of IE-positive cells at 24 h p.i. (Fig. 7B). Interestingly, the percentage of IE-positive cells was up to fivefold lower in the case of v65stop compared to the wild type and v65rev viruses. An infectivity defect of v65stop as in MDM was never observed in HFF, showing that the delayed and decreased production of infectious particles of v65stop was at least in part caused by a decreased ability of the pp65 stop mutant to infect MDM.
DISCUSSION
The HCMV tegument was long regarded as an unstructured depository for different proteins. It has since become evident that some of the more than 25 viral tegument proteins are involved in virion formation, virus transport, immunomodulation, and transactivation (26). The existence of a complex network of protein-protein interactions relevant for virus morphogenesis and virus egress is generally accepted but has been only partially proven, mostly with alphaherpesviruses (reviewed in reference 23). Analysis of virus mutants unable to express individual tegument proteins is a potent tool to determine the function of those proteins in virus infection, replication, and morphogenesis. Interestingly, some of the tegument proteins have been shown to be essential for virus growth in cell culture, while others are dispensable or lead to altered growth kinetics (12, 42). Furthermore, recent investigations of pseudorabies virus mutants suggested that deletion of single tegument proteins can lead to a global alteration of the virion composition (24, 25).
Concerning HCMV, it is still unclear how the lack of individual tegument proteins is structurally and functionally compensated for and how the resulting structure of the tegument is altered. In our present study, we could show that when the major tegument protein pp65 of HCMV is not expressed, incorporation of other HCMV tegument proteins is inhibited or altered. In the absence of pp65, we found drastically reduced levels of the abundant tegument protein pUL25 in virus particles and, more strikingly, a complete abolishment of the incorporation of minor viral proteins pUL69 and pUL97 into virions. The altered virion incorporation of pUL69 and pUL25 was not caused by reduced expression levels of those proteins in infected cells, which suggested an either direct or indirect interaction with pp65. In fact, a direct interaction of pp65 and pUL69 could be shown for both infected and transfected cells. We further could show evidence that the previously found interaction of pp65 and pUL97 (20) seems to be important for proper intracellular expression of pUL97, in addition to the finding that pp65 is crucial for the incorporation of pUL97 into the virion. These results lead us to conclude that the incorporation of pUL69 and pUL97 is dependent on the presence of and interaction with pp65.
It had been shown by other authors that pUL69 forms a complex with the tegument proteins UL47, UL48, and MCP in infected cells (6). Since pp65 was not included in those investigations, we hypothesize in view of their and our results that pp65 might be a part of that complex. It is also not clear from previous investigations which of the proteins of that complex interact directly with each other. Our results, however, clearly demonstrate that pUL69 and pp65 interact directly with each other without the need for other viral proteins. The specificity of this interaction was verified by including the tegument protein pp150 in our investigations, which had been shown not to be a component of the pUL69/UL47/UL48 complex (6). More investigations are certainly needed to fully elucidate the complexity and specificity of the protein-protein interactions required for the correct formation of this complex and finally for the incorporation of these proteins into virus particles.
It has also been reported that pp65 directly interacts with the viral protein kinase pUL97 (20) which is also a minor constituent of the viral tegument (38). These data together with our data suggest that pp65, pUL69, and pUL97 form a complex in infected cells. Similarly to that of pUL69, incorporation of pUL97 into virus particles was dependent on the presence of pp65 (this report), thus suggesting that the direct interaction with pp65 is needed for the uptake of pUL97 into virions. In infected cells pUL97 is present in several phosphorylation forms (17, 38). However, only one species seems to be incorporated into virions (reference 38 and this report). Our data indicate that in the absence of pp65, phosphorylation of pUL97 seems to be altered. Some of the phosphorylation forms of pUL97 detected in wild-type-infected cells were less present in v65stop-infected cells. It could be now hypothesized that either the autophosphorylation of pUL97 or phosphorylation by cellular kinases such as the Polo-like kinase 1, which is known to interact with pp65, is affected (14, 17). In contrast to the case for pUL69 and pUL25, intracellular pUL97 levels seem to be slightly reduced in v65stop-infected cells compared to wild-type- and revertant virus-infected cells. It is possible that the stability of pUL97 is affected in the absence of pp65. The stability of pUL97 could be increased through the direct interaction with pp65 or a different pUL97 phosphorylation status. The effects of pp65 on pUL97 are very interesting, and work is in progress to identify the mechanisms involved.
The function of pUL25 during HCMV infection is still not understood. It is a nonessential tegument protein that localizes at sites of the HCMV assembly complex late during infection and also accumulates in granular formations particularly condensed near the nucleus (5, 43). The absence of pp65 was also found to have an impact on the efficiency of incorporation of pUL25 into viral particles, while pUL25 accumulated to higher levels in cells infected with v65stop compared to wild-type and revertant viruses (Fig. 3). This result is in line with investigations of the viral protein kinase pUL97, providing evidence for an interaction of pp65 and pUL25. Blocking of the kinase function of pUL97 led to formation of nuclear inclusions in infected cells, which are composed mainly of pp65 and pUL25 (28). The formation of such inclusions was dependent on pp65, because they were not formed in the absence of pp65 (28). However, the evidence for a potential interaction of pp65 and pUL25 has to be verified by colocalization and coimmunoprecipitation studies.
Our observation that pUL25 can still be detected in virus particles of the pp65 stop mutant suggested that the incorporation of pUL25 into virions is not entirely dependent on the presence of pp65 and that other, so-far-unknown mechanisms or interactions promote pUL25 incorporation. It has been reported that pUL25 species of different molecular weights are expressed during infection, while only one form of pUL25 can be found in virus particles (5). Together with published results showing that pUL25 is phosphorylated (5), this favors the hypothesis that the phosphorylation status of pUL25 may be important for the incorporation into viral particles. Interaction of pp65 with the viral kinase UL97 (20) and also with the cellular Polo-like kinase 1 (14) might bring those kinases into close contact with other tegument proteins and thereby could explain why tegument proteins such as pp28 are hypophosphorylated in virus particles of the previously generated pp65 deletion mutant (34).
It has been shown previously that the deletion of pUL69 causes a delay in viral growth. This delay could be overcome by infection with mutant virus particles produced by infection of a complementing cell line and thus carrying pUL69 within the infecting particle (16). These data indicated a function of pUL69 in the onset of viral replication, by inducing a cell cycle arrest in the G1 phase and/or by transactivating the IE gene promoter (22, 41). We found a slightly (up to twofold) reduced transactivation of the IE promoter by purified particles of our pp65 stop mutant in an IE transactivation assay (data not shown). However, this transactivation defect did not have an impact on viral growth, since a delayed replication as previously shown for a UL69 deletion mutant was not found (Fig. 6) (16). In further Western blot analysis, no difference in IE levels could be found (data not shown), suggesting that pUL69 presence in the infecting particles is not required for an efficient onset of IE gene expression, at least in cell culture. Taking into account that newly synthesized pUL69 can be detected as early as 7 h postinfection (41), it is very reasonable to assume that de novo-synthesized pUL69 complements for the lack of pUL69 in particles of the pp65 stop codon mutant and thereby compensates a biological effect of the lack of pUL69 in the infecting particle.
The complete absence of DBs in HFF as well as in endothelial cells infected with the pp65 stop mutant (Fig. 5) is consistent with the phenotype of the previously published pp65 deletion mutant of HCMV strain AD169 in fibroblasts (34). Interestingly, a thorough examination of the morphology of virus particles of the v65stop mutant using the new electron microscopy methodology of high-pressure freezing and freeze-substitution did also not reveal any significant difference in the appearance of the viral tegument compared to that of wild-type virus. This is astonishing considering the fact that the major tegument component is missing in these viral particles. From our data we cannot entirely exclude that other viral proteins compensate for the missing proteins in v65stop virions; however, our analysis did not reveal a substantial increase of a single or several viral proteins in the silver-stained SDS gel.
Our finding of the severe growth defect of v65stop in MDM was very surprising, since this mutant exhibited no defect in all other cell types tested so far. The delayed and reduced production of virus particles in MDM was at least in part caused by a reduced ability of v65stop to infect MDM. The question still remains as to the involvement of pp65, pUL69, pUL97, or pUL25 in the early stages of infection, including entry into MDM. Work is in progress to investigate that in more detail.
Finally, it is noteworthy that production of infectious particles of v65stop declined after day 7 p.i., while wild-type and revertant virus-infected MDM produced constant yields of infectious particles. These data cannot be entirely explained by the reduced infectivity of v65stop of MDM. Therefore, it is feasible that pp65 might also play a role in the late stages of infection, at least in macrophages.
In summary, we could show for the first time that pp65 is important for efficient infection and obviously for the maintenance of infection in biologically relevant cell types such as primary macrophages. Furthermore, we could show that incorporation of the tegument proteins pUL69 and pUL97 is dependent, and incorporation of pUL25 is partially dependent, on the presence of pp65. This again proves the relevance of specific interactions between different tegument proteins, which we could show for pp65 and pUL69. Further studies are needed to elucidate the obviously complex and multiple interactions existing between tegument proteins with regard to the functions of these interactions in the infected cell, during morphogenesis, and for the morphological integrity of extracellular virions.
Acknowledgments
We thank Paul Walther for fruitful discussions and for carefully reading the paper. The technical assistance of Jutta Hegler, Monika Dürre, and Nina Blankenhorn is gratefully acknowledged, and we thank William Britt, Tom Shenk, Jeremy P. Kamil, Donald M. Coen, and Michael Winkler for providing us with reagents.
Meike Chevillotte is a participant in the International Graduate School in Molecular Medicine Ulm, funded by the Excellence Initiative of the German federal and state governments. This work was supported by the Deutsche Forschungsgesellschaft through SPP1175 (grant ME 1740/2-1).
Footnotes
Published ahead of print on 30 December 2008.
REFERENCES
- 1.Abate, D. A., S. Watanabe, and E. S. Mocarski. 2004. Major human cytomegalovirus structural protein pp65 (ppUL83) prevents interferon response factor 3 activation in the interferon response. J. Virol. 7810995-11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnon, T. I., H. Achdout, O. Levi, G. Markel, N. Saleh, G. Katz, R. Gazit, T. Gonen-Gross, J. Hanna, E. Nahari, A. Porgador, A. Honigman, B. Plachter, D. Mevorach, D. G. Wolf, and O. Mandelboim. 2005. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat. Immunol. 6515-523. [DOI] [PubMed] [Google Scholar]
- 3.Baldick, C. J., Jr., A. Marchini, C. E. Patterson, and T. Shenk. 1997. Human cytomegalovirus tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. J. Virol. 714400-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldick, C. J., Jr., and T. Shenk. 1996. Proteins associated with purified human cytomegalovirus particles. J. Virol. 706097-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battista, M. C., G. Bergamini, M. C. Boccuni, F. Campanini, A. Ripalti, and M. P. Landini. 1999. Expression and characterization of a novel structural protein of human cytomegalovirus, pUL25. J. Virol. 733800-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bechtel, J. T., and T. Shenk. 2002. Human cytomegalovirus UL47 tegument protein functions after entry and before immediate-early gene expression. J. Virol. 761043-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bresnahan, W. A., and T. E. Shenk. 2000. UL82 virion protein activates expression of immediate early viral genes in human cytomegalovirus-infected cells. Proc. Natl. Acad. Sci. USA 9714506-14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browne, E. P., and T. Shenk. 2003. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc. Natl. Acad. Sci. USA 10011439-11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buser, C., F. Fleischer, T. Mertens, D. Michel, V. Schmidt, and P. Walther. 2007. Quantitative investigation of murine cytomegalovirus nucleocapsid interaction. J. Microsc. 22878-87. [DOI] [PubMed] [Google Scholar]
- 10.Buser, C., P. Walther, T. Mertens, and D. Michel. 2007. Cytomegalovirus primary envelopment occurs at large infoldings of the inner nuclear membrane. J. Virol. 813042-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, D. H., H. Jiang, M. Lee, F. Liu, and Z. H. Zhou. 1999. Three-dimensional visualization of tegument/capsid interactions in the intact human cytomegalovirus. Virology 26010-16. [DOI] [PubMed] [Google Scholar]
- 12.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 10014223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evan, G. I., G. K. Lewis, G. Ramsay, and J. M. Bishop. 1985. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 53610-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallina, A., L. Simoncini, S. Garbelli, E. Percivalle, G. Pedrali-Noy, K. S. Lee, R. L. Erikson, B. Plachter, G. Gerna, and G. Milanesi. 1999. Polo-like kinase 1 as a target for human cytomegalovirus pp65 lower matrix protein. J. Virol. 731468-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerna, G., F. Baldanti, and M. G. Revello. 2004. Pathogenesis of human cytomegalovirus infection and cellular targets. Hum. Immunol. 65381-386. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi, M. L., C. Blankenship, and T. Shenk. 2000. Human cytomegalovirus UL69 protein is required for efficient accumulation of infected cells in the G1 phase of the cell cycle. Proc. Natl. Acad. Sci. USA 972692-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, Z., Y. S. He, Y. Kim, L. Chu, C. Ohmstede, K. K. Biron, and D. M. Coen. 1997. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J. Virol. 71405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irmiere, A., and W. Gibson. 1983. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology 130118-133. [DOI] [PubMed] [Google Scholar]
- 19.Jahn, G., H. P. Harthus, M. Broker, B. Borisch, B. Platzer, and B. Plachter. 1990. Generation and application of a monoclonal antibody raised against a recombinant cytomegalovirus-specific polypeptide. Klin. Wochenschr. 681003-1007. [DOI] [PubMed] [Google Scholar]
- 20.Kamil, J. P., and D. M. Coen. 2007. Human cytomegalovirus protein kinase UL97 forms a complex with the tegument phosphoprotein pp65. J. Virol. 8110659-10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyhse-Andersen, J. 1984. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J. Biochem. Biophys. Methods 10203-209. [DOI] [PubMed] [Google Scholar]
- 22.Lu, M., and T. Shenk. 1999. Human cytomegalovirus UL69 protein induces cells to accumulate in G1 phase of the cell cycle. J. Virol. 73676-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mettenleiter, T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Research. 106167-180. [DOI] [PubMed] [Google Scholar]
- 24.Michael, K., S. Bottcher, B. G. Klupp, A. Karger, and T. C. Mettenleiter. 2006. Pseudorabies virus particles lacking tegument proteins pUL11 or pUL16 incorporate less full-length pUL36 than wild-type virus, but specifically accumulate a pUL36 N-terminal fragment. J. Gen. Virol. 873503-3507. [DOI] [PubMed] [Google Scholar]
- 25.Michael, K., B. G. Klupp, T. C. Mettenleiter, and A. Karger. 2006. Composition of pseudorabies virus particles lacking tegument protein US3, UL47, or UL49 or envelope glycoprotein E. J. Virol. 801332-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegalovirus and their replication, p. 2629-2673. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Strauss (ed.), Fields virology. Lippincott-Raven, Philadelphia, PA.
- 27.Odeberg, J., B. Plachter, L. Branden, and C. Soderberg-Naucler. 2003. Human cytomegalovirus protein pp65 mediates accumulation of HLA-DR in lysosomes and destruction of the HLA-DR alpha-chain. Blood 1014870-4877. [DOI] [PubMed] [Google Scholar]
- 28.Prichard, M. N., W. J. Britt, S. L. Daily, C. B. Hartline, and E. R. Kern. 2005. Human cytomegalovirus UL97 kinase is required for the normal intranuclear distribution of pp65 and virion morphogenesis. J. Virol. 7915494-15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampaio, K. L., Y. Cavignac, Y. D. Stierhof, and C. Sinzger. 2005. Human cytomegalovirus labeled with green fluorescent protein for live analysis of intracellular particle movements. J. Virol. 792754-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scalzo, A. A., A. J. Corbett, W. D. Rawlinson, G. M. Scott, and M. A. Gli-Esposti. 2007. The interplay between host and viral factors in shaping the outcome of cytomegalovirus infection. Immunol. Cell Biol. 8546-54. [DOI] [PubMed] [Google Scholar]
- 31.Schierling, K., C. Buser, T. Mertens, and M. Winkler. 2005. Human cytomegalovirus tegument protein ppUL35 is important for viral replication and particle formation. J. Virol. 793084-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schierling, K., T. Stamminger, T. Mertens, and M. Winkler. 2004. Human cytomegalovirus tegument proteins ppUL82 (pp71) and ppUL35 interact and cooperatively activate the major immediate-early enhancer. J. Virol. 789512-9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmolke, S., P. Drescher, G. Jahn, and B. Plachter. 1995. Nuclear targeting of the tegument protein pp65 (UL83) of human cytomegalovirus: an unusual bipartite nuclear localization signal functions with other portions of the protein to mediate its efficient nuclear transport. J. Virol. 691071-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmolke, S., H. F. Kern, P. Drescher, G. Jahn, and B. Plachter. 1995. The dominant phosphoprotein pp65 (UL83) of human cytomegalovirus is dispensable for growth in cell culture. J. Virol. 695959-5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinzger, C., G. Hahn, M. Digel, R. Katona, K. L. Sampaio, M. Messerle, H. Hengel, U. Koszinowski, W. Brune, and B. Adler. 2008. Cloning and sequencing of a highly productive, endotheliotropic virus strain derived from human cytomegalovirus TB40/E. J. Gen. Virol. 89359-368. [DOI] [PubMed] [Google Scholar]
- 36.Taylor, R. T., and W. A. Bresnahan. 2006. Human cytomegalovirus immediate-early 2 protein IE86 blocks virus-induced chemokine expression. J. Virol. 80920-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tischer, B. K., J. von Einem, B. Kaufer, and N. Osterrieder. 2006. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. BioTechniques 40191-197. [DOI] [PubMed] [Google Scholar]
- 38.van Zeijl, M., J. Fairhurst, E. Z. Baum, L. Sun, and T. R. Jones. 1997. The human cytomegalovirus UL97 protein is phosphorylated and a component of virions. Virology 23172-80. [DOI] [PubMed] [Google Scholar]
- 39.Varnum, S. M., D. N. Streblow, M. E. Monroe, P. Smith, K. J. Auberry, L. Pasa-Tolic, D. Wang, D. G. Camp, I. I., K. Rodland, S. Wiley, W. Britt, T. Shenk, R. D. Smith, and J. A. Nelson. 2004. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 7810960-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walther, P., and A. Ziegler. 2002. Freeze substitution of high-pressure frozen samples: the visibility of biological membranes is improved when the substitution medium contains water. J. Microsc. 2083-10. [DOI] [PubMed] [Google Scholar]
- 41.Winkler, M., S. A. Rice, and T. Stamminger. 1994. UL69 of human cytomegalovirus, an open reading frame with homology to ICP27 of herpes simplex virus, encodes a transactivator of gene expression. J. Virol. 683943-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu, D., M. C. Silva, and T. Shenk. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. USA 10012396-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zini, N., M. C. Battista, S. Santi, M. Riccio, G. Bergamini, M. P. Landini, and N. M. Maraldi. 1999. The novel structural protein of human cytomegalovirus, pUL25, is localized in the viral tegument. J. Virol. 736073-6075. [DOI] [PMC free article] [PubMed] [Google Scholar]