Abstract
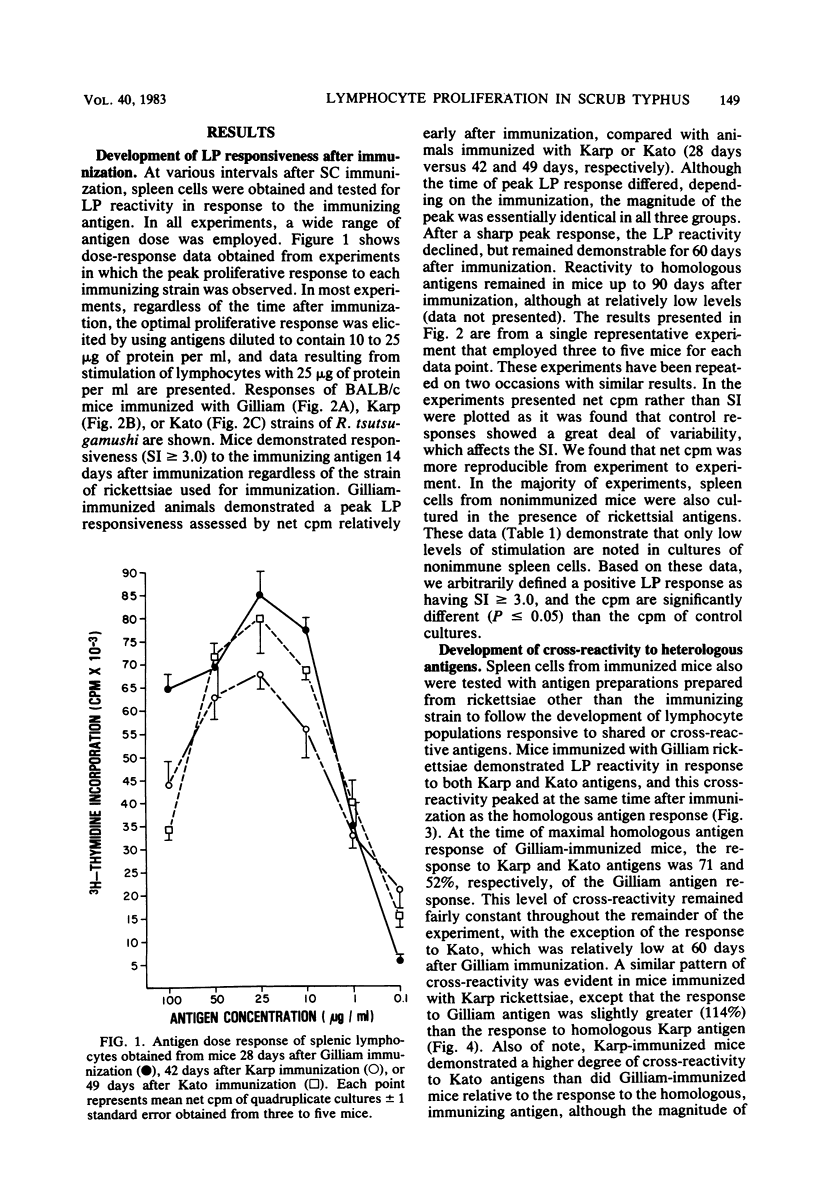
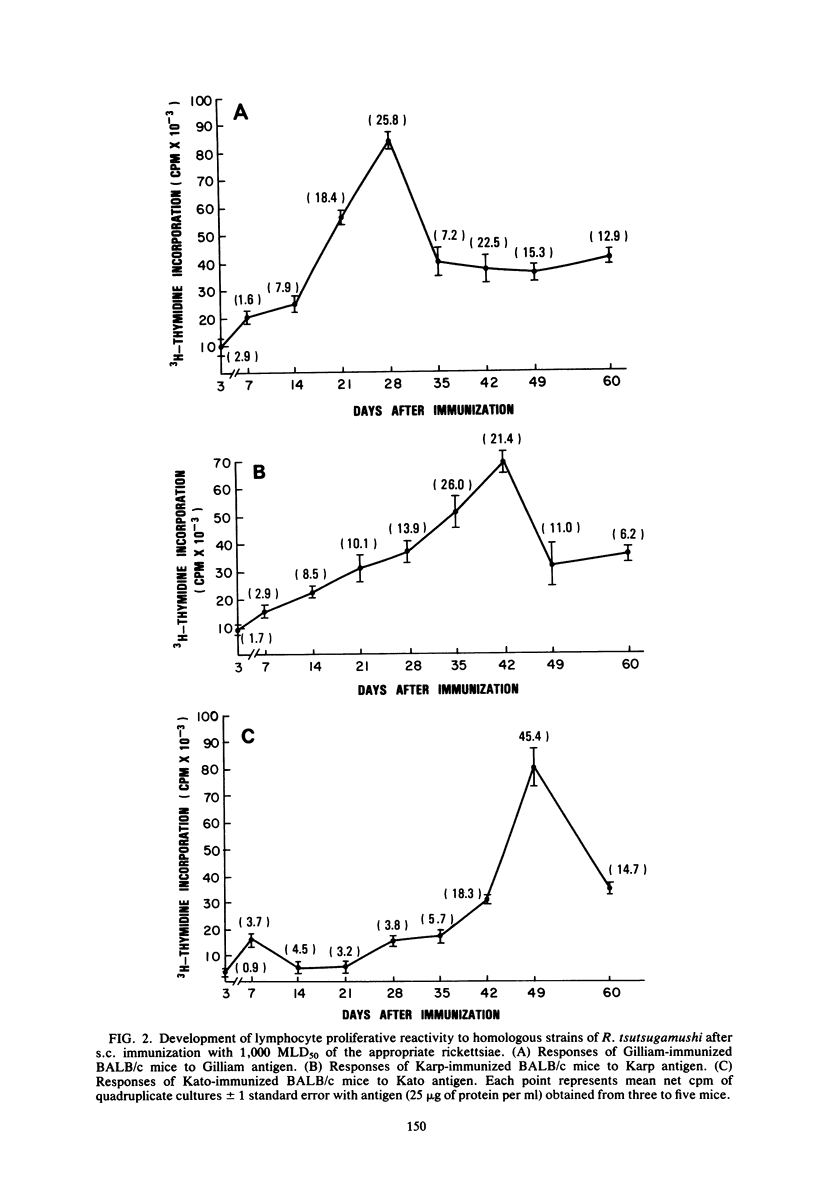
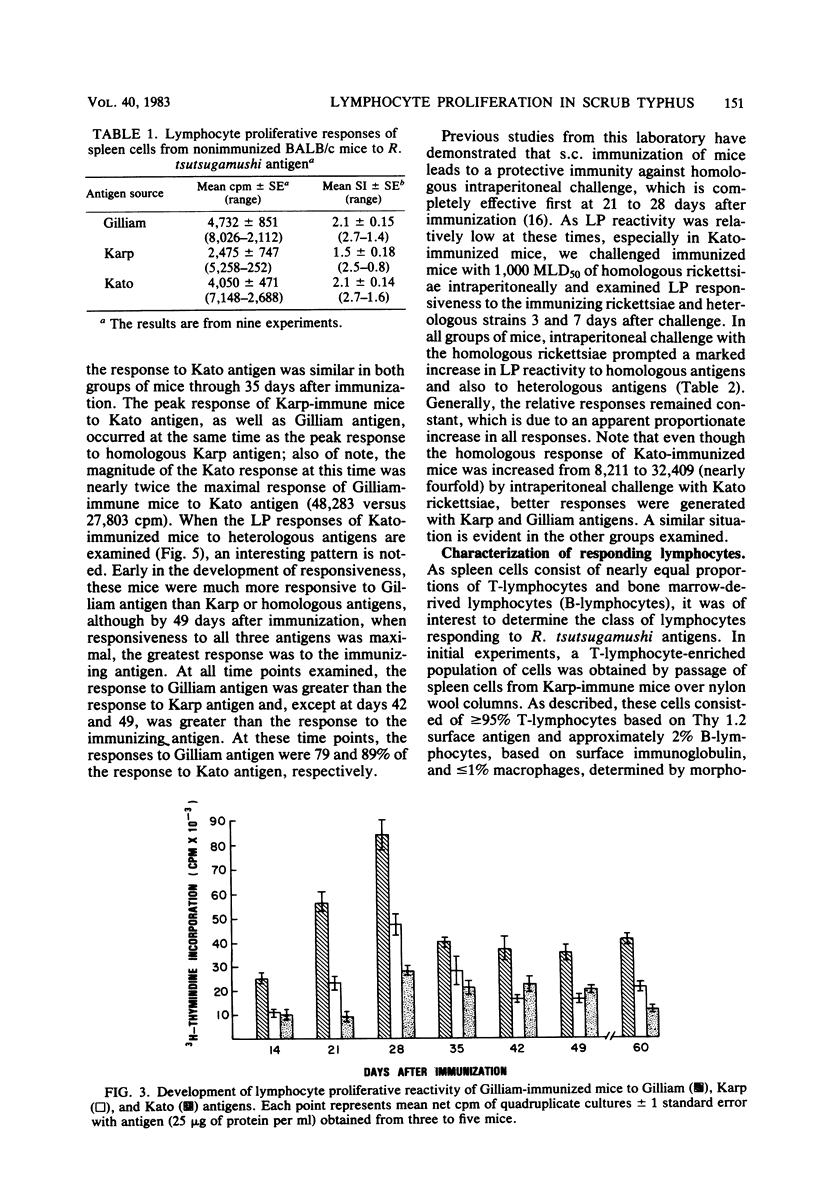
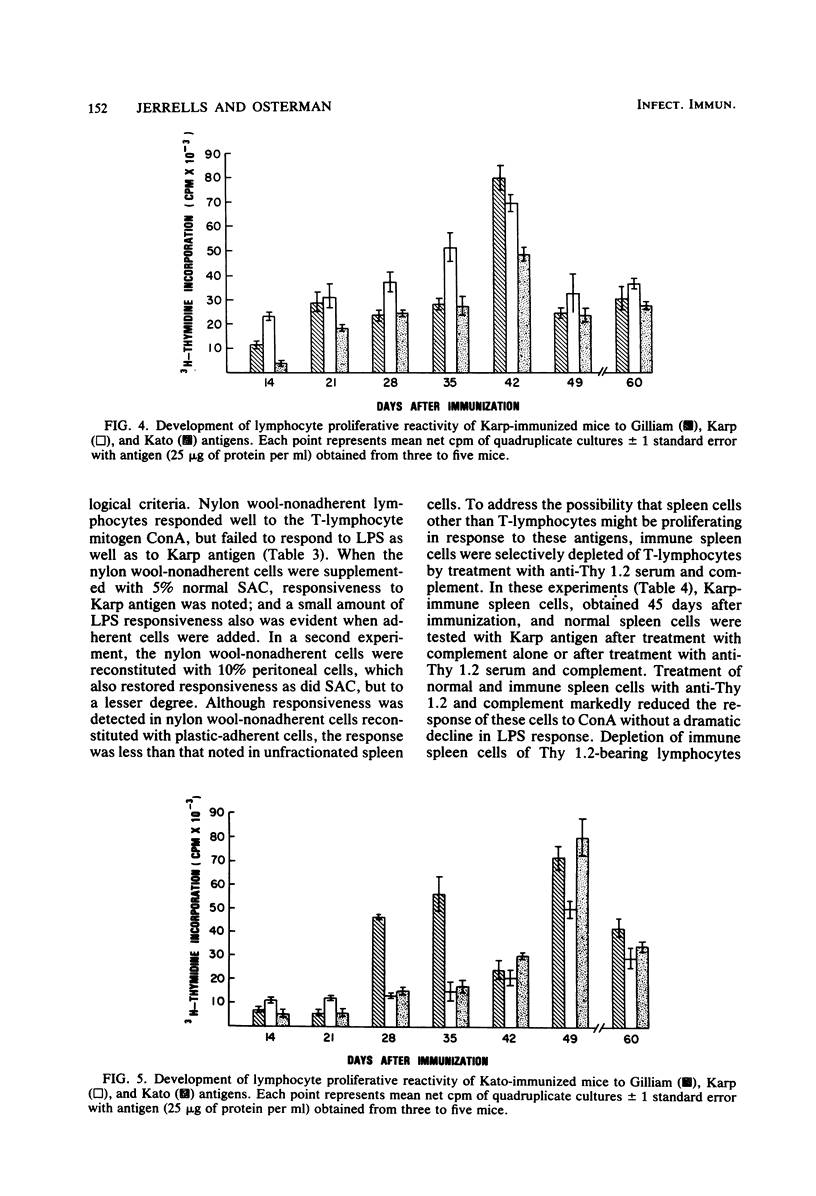
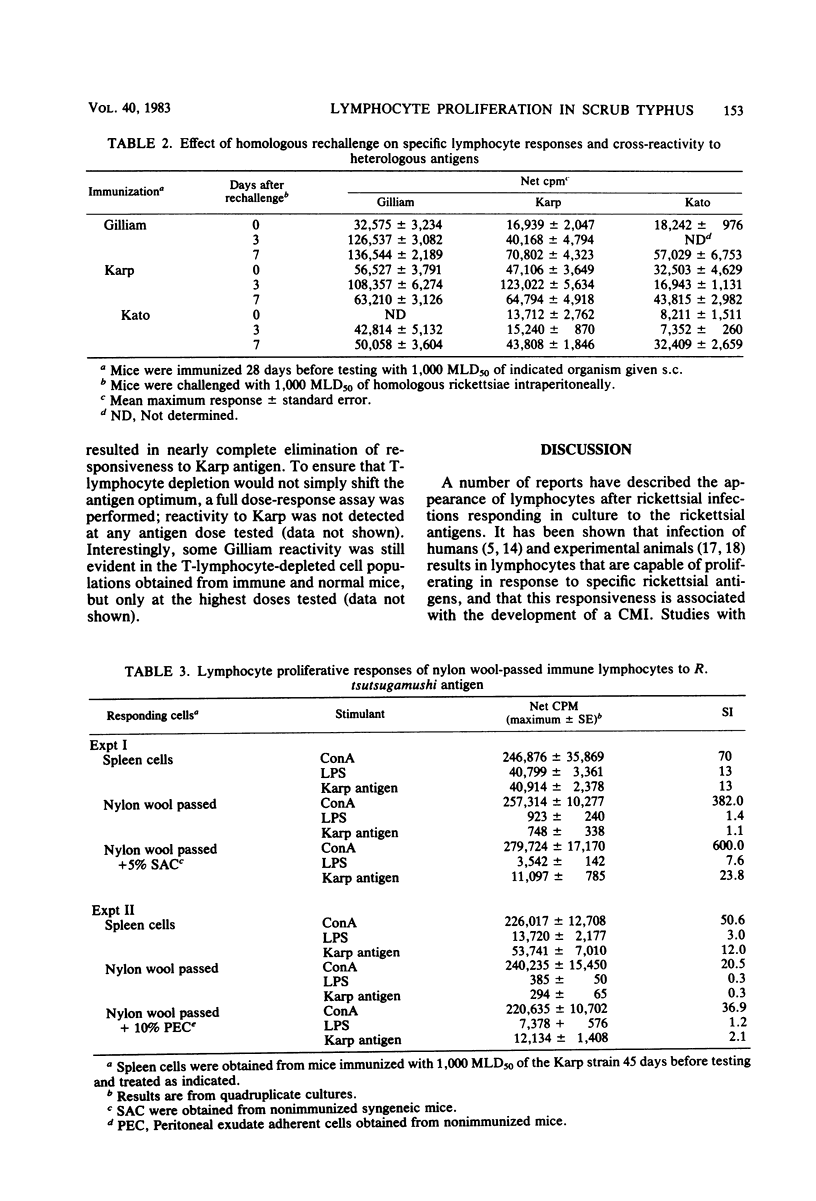
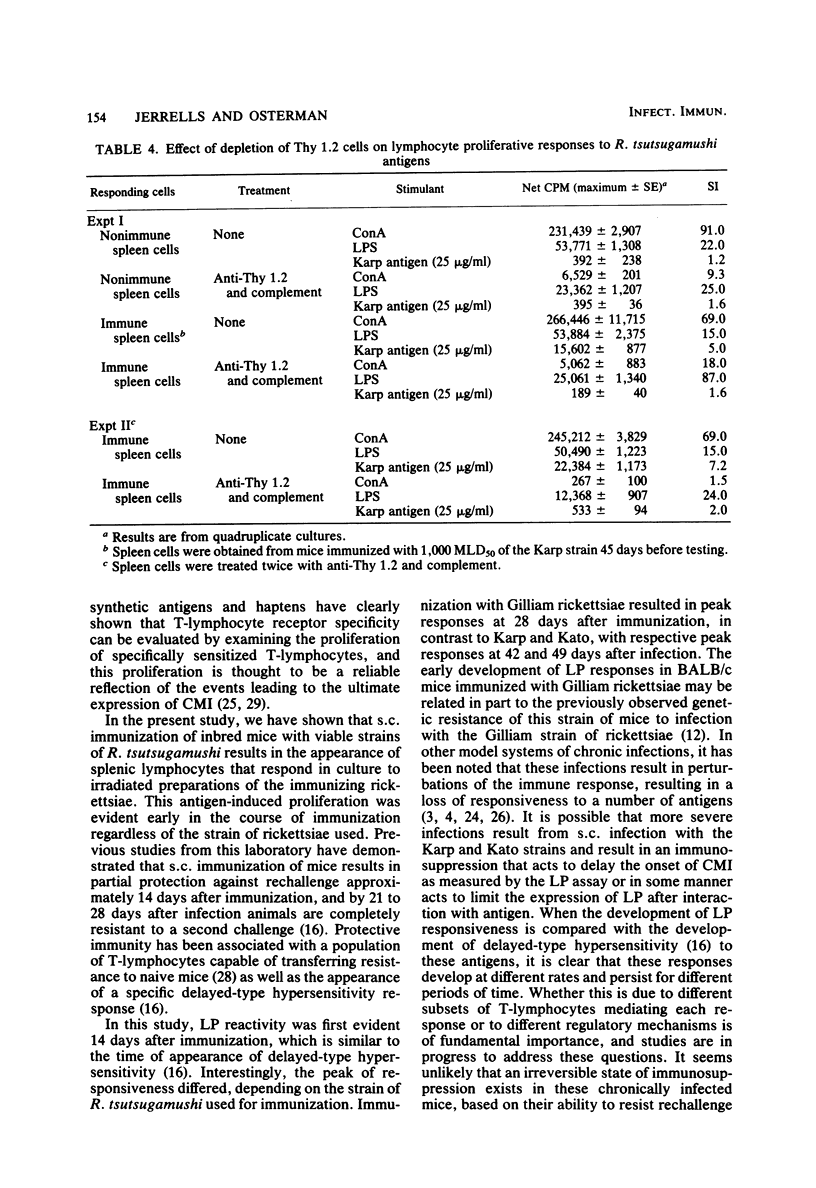
The development of antigen-responsive lymphocytes was followed in mice immunized with the Gilliam, Karp, or Kato strains of Rickettsia tsutsugamushi by utilizing an in vitro lymphocyte proliferation assay. Subcutaneous immunization with viable rickettsiae of all three strains resulted in the appearance of lymphocytes in the spleen responding to irradiated tissue culture-grown rickettsiae used as stimulating antigens. Although all animals demonstrated antigen-induced proliferation elicited by homologous antigen by 14 days after immunization, the time of peak responsiveness varied, depending on the strain of rickettsiae used for immunization. In all cases, peak proliferative responses occurred at a time after immunization that was after the previously reported time after immunization at which resistance to rechallenge was observed. Reactivity to heterologous strains of R tsutsugamushi developed roughly in parallel with homologous reactivity in Karp- and Gilliam-immunized mice, with a marked degree of heterologous reactivity evident. Kato-immunized mice demonstrated greater reactivity to heterologous antigens early in the development of antigen reactivity and demonstrated a somewhat greater degree of cross-reactivity, relative to homologous responses, than the other groups. It was found that nylon wool-nonadherent immune cells, if cultured with antigen and adherent cells obtained from normal spleens or peritoneal exudates, responded in culture. The thymus-derived lymphocyte nature of the responding cell was further suggested when treatment of immune spleen cells with anti-Thy 1.2 serum and complement eliminated antigen response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker L. F., Patt J. K., Hopps H. E. Titration and neutralization of Rickettsia tsutsugamushi in tissue culture. J Immunol. 1968 Apr;100(4):825–830. [PubMed] [Google Scholar]
- Cheers C., Pavlov H., Riglar C., Madraso E. Macrophage activation during experimental murine brucellosis. III. Do macrophages exert feedback control during brucellosis? Cell Immunol. 1980 Jan;49(1):168–177. doi: 10.1016/0008-8749(80)90066-0. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Watson S. R. Suppressor T-cells in BCG-infected mice. Infect Immun. 1979 Aug;25(2):491–496. doi: 10.1128/iai.25.2.491-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod J. D., Shepard C. C. Lymphocyte transformation in rickettsioses. J Immunol. 1971 Jan;106(1):209–216. [PubMed] [Google Scholar]
- Eisemann C. S., Osterman J. V. Proteins of typhus and spotted fever group rickettsiae. Infect Immun. 1976 Jul;14(1):155–162. doi: 10.1128/iai.14.1.155-162.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg G. H., Jr, Osterman J. V. Experimental scrub typhus immunogens: gamma-irradiated and formalinized rickettsiae. Infect Immun. 1977 Jan;15(1):124–131. doi: 10.1128/iai.15.1.124-131.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfenbein G. J., Shevach E. M., Green I. Proliferation by bone marrow-derived lymphocytes in response to antigenic stimulation in vitro. J Immunol. 1972 Oct;109(4):870–874. [PubMed] [Google Scholar]
- Ewing E. P., Jr, Takeuchi A., Shirai A., Osterman J. V. Experimental infection of mouse peritoneal mesothelium with scrub typhus rickettsiae: an ultrastructural study. Infect Immun. 1978 Mar;19(3):1068–1075. doi: 10.1128/iai.19.3.1068-1075.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves M. G., Osterman J. V. Host defenses in experimental scrub typhus: genetics of natural resistance to infection. Infect Immun. 1978 Feb;19(2):583–588. doi: 10.1128/iai.19.2.583-588.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphres R. C., Hinrichs D. J. Role of antibody in Coxiella burnetii infection. Infect Immun. 1981 Feb;31(2):641–645. doi: 10.1128/iai.31.2.641-645.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Mallavia L. P., Hinrichs D. J. Detection of long-term cellular immunity to Coxiella burneti as assayed by lymphocyte transformation. Infect Immun. 1975 Feb;11(2):280–286. doi: 10.1128/iai.11.2.280-286.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Host defenses in experimental scrub typhus: delayed-type hypersensitivity responses of inbred mice. Infect Immun. 1982 Jan;35(1):117–123. doi: 10.1128/iai.35.1.117-123.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Host defenses in experimental scrub typhus: inflammatory response of congenic C3H mice differing at the Ric gene. Infect Immun. 1981 Mar;31(3):1014–1022. doi: 10.1128/iai.31.3.1014-1022.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon R. H., Ascher M. S., Kishimoto R. A., Pedersen C. E., Jr In vitro guinea pig leukocyte reactions to Rickettsia rickettsii. Infect Immun. 1977 Dec;18(3):840–846. doi: 10.1128/iai.18.3.840-846.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto R. A., Johnson J. W., Kenyon R. H., Ascher M. S., Larson E. W., Pedersen C. E., Jr Cell-mediated immune responses of guinea pigs to an inactivated phase I Coxiella burnetii vaccine. Infect Immun. 1978 Jan;19(1):194–198. doi: 10.1128/iai.19.1.194-198.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Rosenthal A. S. The induction and regulation of guinea pig B-lymphocyte proliferation in vitro. J Immunol. 1976 Nov;117(5 Pt 1):1594–1602. [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S. Macrophages in resistance to rickettsial infection: macrophage activation in vitro for killing of Rickettsia tsutsugamushi. J Immunol. 1979 Dec;123(6):2544–2549. [PubMed] [Google Scholar]
- Nacy C. A., Osterman J. V. Host defenses in experimental scrub typhus: role of normal and activated macrophages. Infect Immun. 1979 Nov;26(2):744–750. doi: 10.1128/iai.26.2.744-750.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks S. C., Jr, Hetrick F. M., Osterman J. V. A plaque reduction assay for studying antigenic relationships among strains of Rickettsia tsutsugamushi. Am J Trop Med Hyg. 1980 Sep;29(5):998–1006. doi: 10.4269/ajtmh.1980.29.998. [DOI] [PubMed] [Google Scholar]
- Oaks S. C., Jr, Osterman J. V., Hetrick F. M. Plaque assay and cloning of scrub typhus rickettsiae in irradiated L-929 cells. J Clin Microbiol. 1977 Jul;6(1):76–80. doi: 10.1128/jcm.6.1.76-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riglar C., Cheers C. Macrophage activation during experimental murine brucellosis. II. Inhibition of in vitro lymphocyte proliferation by brucella-activated macrophages. Cell Immunol. 1980 Jan;49(1):154–167. doi: 10.1016/0008-8749(80)90065-9. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H., Horton C. L., Paul W. E. T-lymphocyte-enriched murine peritoneal exudate cells. IV. Genetic control of cross-stimulation at the T-cell level. J Exp Med. 1977 Feb 1;145(2):327–343. doi: 10.1084/jem.145.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. T. Delayed hypersensitivity to Trypanosoma cruzi in mice: specific suppressor cells in chronic infection. Immunology. 1981 Oct;44(2):409–417. [PMC free article] [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Eisenberg G. H., Jr, Osterman J. V. Host defenses in experimental scrub typhus: effect of chloramphenicol. Infect Immun. 1977 Nov;18(2):324–329. doi: 10.1128/iai.18.2.324-329.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Phillips S. M., Osterman J. V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976 Jul;14(1):39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinger A. M., Schwartz R. H. The T lymphocyte proliferative response to poly-L-Glu-poly-D,L-Ala--poly-L-Lys. J Immunol. 1980 May;124(5):2485–2490. [PubMed] [Google Scholar]
- Tse H. Y., Mond J. J., Paul W. E. T lymphocyte-dependent B lymphocyte proliferative response to antigen. I Genetic restriction of the stimulation of B lymphocyte proliferation. J Exp Med. 1981 Apr 1;153(4):871–882. doi: 10.1084/jem.153.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Peenen P. F., Ho C. M., Bourgeois A. L. Indirect immunofluorescence antibodies in natural and acquired Rickettsia tsutsugamushi infections of Philippine rodents. Infect Immun. 1977 Mar;15(3):813–816. doi: 10.1128/iai.15.3.813-816.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981 Nov;127(5):1869–1875. [PubMed] [Google Scholar]


