Abstract
Epstein-Barr virus (EBV) growth transformation of primary B lymphocytes into indefinitely proliferating lymphoblastoid cell lines (LCLs) depends on the concerted activities of a subset of viral proteins expressed during latency. EBV drives quiescent B cells into S phase, and consequently, a host response is activated that includes expression of p53 and its target genes. Since LCLs retain wild-type p53, it was of interest to determine what contribution the p53 pathway may have in controlling established LCL growth and EBV-mediated transformation of primary B cells. We found that liberation of p53 through chemical antagonism of one of its major ubiquitin ligases, MDM2, using the small-molecule Nutlin-3 led to apoptosis of established LCLs and suppressed EBV-mediated transformation of primary B cells. The activation of latent p53 induced target genes associated with apoptosis. Furthermore, MDM2 antagonism synergized with NF-κB inhibition in killing LCLs. NF-κB was important to increase steady-state MDM2 protein levels rather than in affecting p53-dependent transcription, suggesting a unique mechanism by which LCLs survive in the presence of a primed p53 pathway. Nutlin sensitivity of EBV-infected cells provides a novel system for studying the pathways that dictate LCL survival and regulate EBV transformation. Finally, MDM2 antagonists may be considered for therapeutic intervention in EBV-associated malignancies expressing wild-type p53.
Epstein-Barr virus (EBV) is an oncogenic herpesvirus capable of transforming primary B lymphocytes into indefinitely proliferating lymphoblastoid cell lines (LCLs). EBV is associated with the development of endemic Burkitt's lymphoma, some forms of Hodgkin's disease, and human immunodeficiency virus-associated lymphomas (15). The expression of latent viral proteins found in LCLs mimics that found in posttransplant lymphoproliferative disorder. EBV growth transformation in vitro requires the functions of a subset of viral latent proteins. Latent membrane protein 1 (LMP1) mimics the prosurvival functions of an activated tumor necrosis factor receptor (7), is capable of cooperating with Ras in promoting oncogenic transformation of Rat-1 fibroblasts (34), and promotes lymphomagenesis in transgenic Scid/hu mice (16). The viral nuclear proteins EBNA2, -3A, -3C, and -LP collectively modulate viral and host transcription primarily through the intracellular Notch-directed DNA binding protein RBP-Jk/CSL (12). The nuclear EBNA1 protein is required for replication and maintenance of the viral episome as well as transcriptional activation of viral and cellular promoters (2, 17, 36). The coordination of the pathways controlled by these proteins is critical for LCL growth and survival.
The latency III gene expression program drives quiescent primary B cells into the cell cycle. Initial transition from the G0 to G1 phase of the cell cycle is mediated by EBNALP- and EBNA2-induced cyclin D2 expression (27). Concomitantly, hyperphosphorylation of pRb, p107, and p130 lead to E2F family member expression and cyclinE/cdk2 complex activation, driving infected cells into S phase (8). Following S phase induction, p53 is stabilized, and its downstream targets are expressed (1, 23). While EBV provokes this initial response, the latent gene expression program does not appear to interfere with p53 function directly (1). Accordingly, DNA double-stranded break initiators, such as gamma irradiation, lead to a normal p53 response in EBV-transformed cells (20). Thus, unlike small DNA tumor viruses, such as human papillomavirus and simian virus 40, EBV does not directly interfere with the p53 protein to inhibit this innate tumor suppression pathway. However, since EBV provokes the p53 pathway and LCLs are able to proliferate indefinitely, a mechanism which protects LCLs from oncogenic stress-induced growth suppression likely exists downstream of p53.
The degradation of p53 by the ubiquitin ligase MDM2 represents a critical circuit in the regulation of p53 both in response to acute DNA damage and in its tumor suppressor functions (18). The small-molecule Nutlin-3 was recently identified as an inhibitor of the interaction between MDM2 and p53 (29). Nutlin-3 consequently stabilizes p53 and induces growth arrest or apoptosis in tumor cells with wild-type (wt) p53 (28). In fact, the growth of cell lines latently infected with the gammaherpesvirus Kaposi's sarcoma-associated herpesvirus (KSHV) is sensitive to Nutlin-3 (26). In this previous study, Nutlin-3 was able to stabilize p53 but showed little effect on the growth of EBV-transformed cells. As a means to determine whether an oncogenic-stress pathway is activated by EBV in primary B cells and LCLs that is constitutively regulated by MDM2, we assessed the effect of Nutlin-3 on EBV transformation and EBV-infected cell growth and survival. Further experiments were performed to characterize the phenotype of Nutlin-treated cells toward understanding the signals that govern the response.
MATERIALS AND METHODS
Cells and viruses.
LCLs GM05422 and GM15807 were obtained from the Coriell Cell Repository (Camden, NJ). BL41/B95-8 cells were obtained from George Mosialos (Aristotle University, Thessaloniki, Greece). MutuI and MutuIII cells were kindly provided by Jeff Cohen (NCI, Bethesda, MD). EBV-positive Akata cells and p53 wt (TK6), mutant (WTK1), and deleted (NH32) LCLs were provided by Ellen Cahir-McFarland (Harvard Medical School, Boston, MA). The p53 lines were originally produced and kindly provided by Howard Liber (Colorado State University, Fort Collins, CO) (37). Human peripheral blood mononuclear cells (PBMCs) were obtained by Ficoll purification (Sigma) of buffy coats from normal donors (Carolina Red Cross). B95-8 marmoset cells were grown in R10 (RPMI 1640 with 10% fetal bovine serum, 1 mM l-glutamine, and 100 μg/ml penicillin-streptomycin). Supernatants from B95-8 cells were filtered through a 0.45-μm filter and used to infect PBMCs for LCL outgrowth assays as described below.
Compounds.
Nutlin-3 was purchased as a racemic mixture from Calbiochem and dissolved in dimethyl sulfoxide (DMSO) at a 10 mM concentration. IKKβ inhibitor IV was purchased from Calbiochem as a 10 mM solution stock.
Flow cytometry and apoptosis assays.
Cells were harvested after Nutlin treatment, washed once with phosphate-buffered saline (PBS), and fixed with ethanol at −20°C for 20 min. Fixed cells were washed again with PBS and incubated in cell cycle analysis buffer (PBS, 0.05% Triton X-100, 200 μg/ml RNase A, and 25 μg/ml propidium iodide). Cell cycle analysis was performed using a FACSCalibur (BD Biosciences) instrument. Initial experiments characterizing apoptosis (annexin staining, mitochondrial depolymerization, and caspase activity) were assayed using the Guava Technologies PCA-96 instrument, using the manufacturer's suggested protocols (Nexin assay, MitoPotential assay, and multicaspase detection kits, respectively). Follow-up experiments assaying apoptosis by annexin V binding with ICAM1 staining or sorted cells used the annexin V-Alexa568 reagent (Roche). For ICAM1 sorting, 2.5 × 107 LCLs were washed in autoMACS running buffer (Miltenyi Biotec) and resuspended at a concentration of 106 cells/ml. For the labeling, 1 μg of purified antihuman CD54 antibody (BD Biosciences) was incubated per 106 cells for 30 min at 4°C. After one wash with autoMACS running buffer, cells were incubated with Alexa Fluor 488 goat anti-mouse immunoglobulin G (IgG) (H+L) (Invitrogen) for 20 min at 4°C. The cells were sorted after a wash in autoMACS running buffer on a FACSVantage cell sorter flow cytometer (Becton Dickinson).
LCL outgrowth experiments.
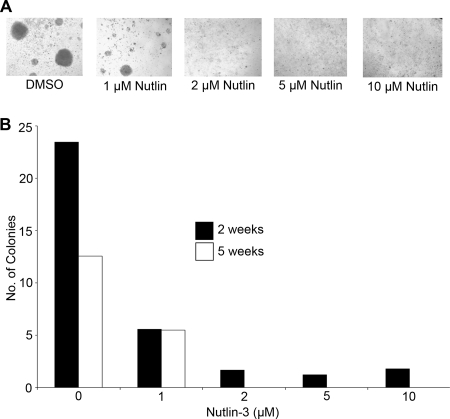
A total of 107 human PBMCs were infected with 3 ml filtered B95-8 supernatant in the presence of 0.5 μg/ml cyclosporine A (CsA) for 1 h at 37°C. Subsequently, infected cells were brought to 14.4 ml in R15 as described above with 0.5 μg/ml CsA and plated at 150 μl/well onto 96-well plates. After 1 week, cells were fed with an additional 100 μl of fresh CsA containing R15. At 2 and 5 weeks, cells were assayed using the Acumen Explorer (TTP Labtech) following incubation for 30 min with 0.5 μM calcein AM (a vital fluorescent dye). Calcein taken up into live cells in the culture enabled characterization of EBV-infected B-cell aggregates. Algorithms were set to count the number of aggregates (i.e., given volume) within an entire well of a 96-well plate based on the volume (as determined by fluorescence intensity and area) of single cells. Reported aggregates in Fig. 5 were those larger than approximately 200 cells and were not present in uninfected wells at early (2-week) and late (5-week) times.
FIG. 5.
Nutlin-3 inhibits primary B-cell growth transformation by EBV. (A) Micrographs are shown of representative wells 5 weeks following infection of adult PBMCs with EBV (B95-8) in the presence of the noted concentrations of Nutlin-3 (0, 1, 2, 5, and 10 μM). Aggregates of cells are evident in the untreated and 1 μM Nutlin-3-treated cells, the latter notably smaller, and grow out to form bona fide EBV-transformed LCLs. (B) The effect of Nutlin-3 on EBV-mediated growth transformation. Results are shown as the number of large cell aggregates present per well in a 96-well plate 2 or 5 weeks following infection.
Gene expression and immunoblotting.
TaqMan assays specific for the following gene products were purchased from Applied Biosystems: p21 (Hs00355782_m1), MDM2 (long) (Hs00234753_m1), PIG3 (Hs00153280_m1), Bfl1-A1 (Hs00187845_m1), IκBα (Hs00153283_m1), TRAF1 (Hs00194639), PUMA (Hs00248075_m1), Bax (Hs00180269_m1), A20 (Hs00560402_m1), and 18S rRNA (4319413E). MDM2 (short) was detected using the following primers and 6-carboxyfluorescein-minor groove binder-labeled probe: forward primer, 5′-CTGCTGATCCAGGCAAATGTGCAA-3′; reverse primer, 5′-TTCCGAAGCTGGAATCTGTGAGGT-3′; probe, 5′-FAM/CCAACATGTCTGTACCTACTGATG.
Assays were performed according to the manufacturer's protocol, using 50 ng total RNA per reaction. RNA was purified using the Qiagen RNeasy kit and using QIAshredder homogenizers. Reverse transcription and cDNA amplification were performed in one step using the TaqMan one-step reverse transcription-PCR (RT-PCR) master mix reagent kit. Western blot analyses were performed with 50 μg of LCL lysate per lane by using the following antibodies: p53 (1C12; Cell Signaling Technologies), MDM2 (2A9; Calbiochem), p21 (C-19; Santa Cruz Biotechnology), MDMX (BL1258; Bethyl Laboratories), β-actin (Rockland), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase; Santa Cruz Biotechnology).
RESULTS
Nutlin-3 inhibits LCL growth.
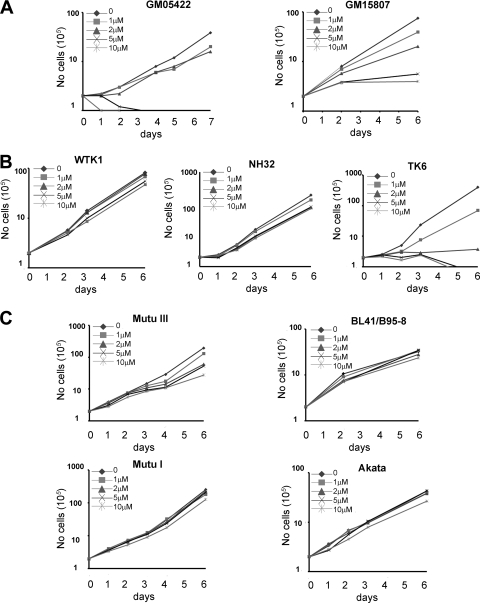
The importance of the MDM2-p53 interaction was initially assessed in two independently derived LCLs by addition of a racemic mixture of the MDM2 antagonist Nutlin-3. Dose-dependent inhibition of LCL growth was observed, where treatment of cells with 1 to 2 μM Nutlin-3 slowed proliferation and ≥5 μM Nutlin-3 completely inhibited proliferation (Fig. 1A). This effect was specific for LCLs containing wt p53, as an LCL with mutant p53 (WTK1) or with p53 deleted by homologous recombination (NH32) (37) was insensitive to doses of Nutlin-3 that suppressed growth of a wt LCL from the same donor (TK6) (Fig. 1B). Furthermore, several EBV-infected Burkitt's lymphoma cell lines with p53 mutation or deletion were insensitive to the growth-suppressive effects of Nutlin-3 (Fig. 1C). These included the infected latency III BL lines MutuIII and BL41/B95-8 and the latency I-expressing MutuI and Akata cell lines. Previous work suggested that EBV-transformed cells were insensitive to the growth-suppressive effects of Nutlin-3 (26). However, our data strongly support a key role for MDM2 antagonism of p53 activity in maintaining LCL viability.
FIG. 1.
Nutlin-3 suppresses LCL growth. wt LCL GM05422 and wt LCL GM15807 (A), WTK1 (p53 mutant), NH32 (p53 deleted), and TK6 (wt) LCLs derived from the same donor (B), and MutuIII, BL41/B95-8, MutuI, and Akata cells (C) were seeded at 2 × 105 cells/ml and treated with DMSO or 1, 2, 5, or 10 μM Nutlin-3. Cells were counted every 2 to 3 days, and cumulative average viable cell counts are reported in the graphs. When cells reached confluence, they were split to the original 2 × 105 cells/ml. These experiments were performed in triplicate, and the results shown are representative of three independent experiments.
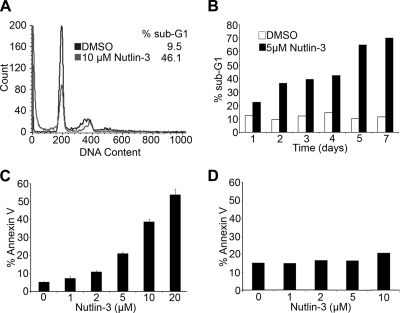
Nutlin-3 induces apoptosis of LCLs.
Nutlin-3 has been shown to induce either cell cycle arrest or apoptosis in tumor cell lines with wt p53 (28). Cell cycle analysis of LCLs treated with 10 μM Nutlin-3 for 48 h revealed an increase in a sub-G1 population of cells (Fig. 2A). The sub-G1 population was observed in Nutlin-treated LCLs as early as 24 h and, by 5 days after treatment, accounted for more than 50% of the cells (Fig. 2B). Furthermore, the increased sub-G1 population correlated with cells staining positive with annexin V, indicating that they were undergoing apoptosis (Fig. 2C). Annexin V positivity was dependent on the dose of Nutlin-3, with a response similar to that observed for growth inhibition (Fig. 2C). To control for the specificity of p53, several p53-negative cell lines were tested. The p53 mutant LCLs WTK1 and NH32 and the EBV-infected BL lines BL41/B95-8, Akata, MutuI, and MutuIII were not increased for annexin V binding after Nutlin-3 treatment (Fig. 2D; also see Fig. S1 in the supplemental material).
FIG. 2.
Nutlin-3 induces apoptosis in LCLs. (A) LCL GM05422 cells were treated with DMSO or 10 μM Nutlin-3 for 48 h. Cells were stained with propidium iodide, and cell cycle analysis was performed. The histogram plot shows DMSO-treated DNA content, in black, overlaid with Nutlin-treated DNA content, in gray. The percentage of cells with sub-G1 DNA content is shown. (B) As in panel A, sub-G1 DNA content in DMSO- versus Nutlin-treated (5 μM) LCL is plotted as a function of time (in days). The results displayed are the averages of the results for two independent experiments. (C) LCL GM05422 cells were treated with increasing concentrations of Nutlin-3 (0, 1, 2, 5, 10, and 20 μM). After 48 h, cells were harvested and stained with fluorescein isothiocyanate-labeled annexin V and 7-amino-actinomycin D. Total percentages of annexin V-positive cells are plotted versus Nutlin concentrations. (D) BL41/B95-8 cells were treated with increasing concentrations of Nutlin-3, as in panel C, and the percentages of annexin V-positive cells are plotted. The lack of Nutlin-induced increased annexin staining in BL41/B95-8 cells is consistent with the lack of effect on growth (Fig. 1C).
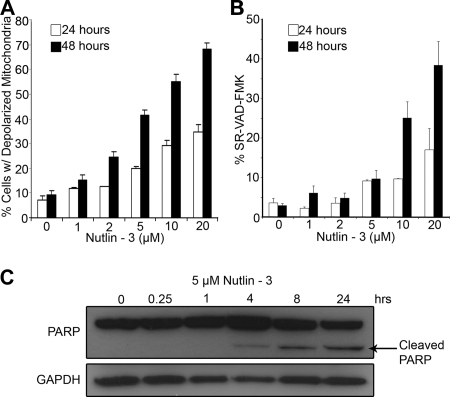
To better characterize the apoptotic cell death induced by Nutlin-3 in LCLs, several apoptotic markers were assayed for their activation. LCLs exhibited a loss of mitochondrial membrane potential in a dose-dependent manner following Nutlin-3 treatment (Fig. 3A). Approximately 40% of LCLs treated with 5 μM Nutlin-3 contained depolarized mitochondria at 48 h after treatment, compared to 8% of DMSO-treated cells. Nutlin-mediated apoptosis was also characterized by increased caspase-3 and -7 activity assayed by sulforhodaminyl-l-valylalanylaspartyl fluoromethyl ketone (SR-VAD) peptide cleavage and endogenous poly(ADP) ribose polymerase (PARP) cleavage (Fig. 3B and C).
FIG. 3.
Nutlin-3 induces mitochondrial depolarization and caspase activity in LCLs. (A) LCL GM05422 cells were treated with increasing concentrations of Nutlin-3 as in Fig. 2 and stained with JC-1, a dye that fluoresces differentially as an oligomer when present in intact mitochondria versus as a monomer, indicating depolymerization (25). At 24 and 48 h, mitochondrial depolymerization was assessed and reported here as a function of Nutlin concentration. Results shown are representative of two independent experiments, each performed in triplicate. (B) LCL GM05422 cells were treated as in panel A and assayed for caspase activity by using the fluorescent covalent caspase inhibitor, SR-VAD-FMK. At 24 and 48 h, caspase activity was assessed using Guava PCA-96 and reported as the percentage of SR-VAD-FMK-positive cells as a function of Nutlin concentration. Results shown are representative of two independent experiments, each performed in triplicate. (C) LCL GM05422 cells were treated with 5 μM Nutlin-3, and cell lysates were immune-blotted for PARP. The appearance of cleaved PARP over time following Nutlin treatment is indicative of induced caspase activity.
Nutlin-3 induces p53-dependent gene expression.
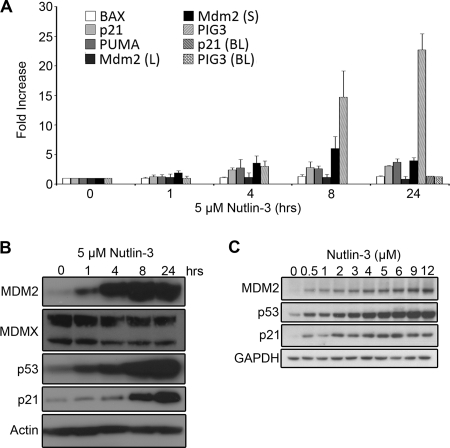
Given the observation that BL41/B95-8 cells, which express a p53 mutant incapable of binding DNA, were not affected by Nutlin-3 treatment, the activation of apoptosis in LCLs following MDM2 antagonism likely followed p53-mediated gene expression. Accordingly, Nutlin-3 treatment of LCLs upregulated a canonical set of p53 target genes (Fig. 4A). MDM2 is activated by p53 through an internal promoter, P2 (14), and this short mRNA isoform was upregulated following Nutlin treatment of LCLs, while the long form was unchanged. In addition, mRNAs encoding p21 and the proapoptotic PUMA, BAX, and PIG3 proteins were upregulated following Nutlin treatment. However, p53-responsive genes were not uniformly upregulated under these conditions, as there were no changes in the mRNA levels of NOXA and BAK1 after Nutlin-3 treatment (data not shown).
FIG. 4.
Nutlin-3 stabilizes p53 and leads to p53-dependent gene expression. (A) LCL GM05422 cells were treated with 5 μM Nutlin-3 for 0, 1, 4, 8, 12, and 24 h. Total RNA was harvested, and cDNA was reverse transcribed and amplified in one step, using TaqMan probe sets for the following mRNAs: BAX, p21, PUMA, MDM2 (long), MDM2 (short), and PIG3. Similarly, RNA was harvested, and cDNA was reverse transcribed and amplified from BL41/B95-8 (BL) cells harvested at 24 h following Nutlin-3 treatment with probes for p21 and PIG3. Induction (n-fold) relative to untreated cells is shown as a function of time after Nutlin treatment. Average values from the results of three independent experiments are shown. (B) Immunoblot analysis was performed on 5 μM Nutlin-treated LCL at the indicated time points, as in panel A. Stabilization of p53 and p53-mediated induction of target proteins are shown. The following antibodies were used: MDM2 (2A9; Calbiochem); MDMX (BL1258; Bethyl Laboratories); p53 (1C12; Cell Signaling Technologies); p21 (C-20; Santa Cruz); and GAPDH (Santa Cruz). (C) Immunoblot analysis of LCL GM05422 cells treated with increasing doses of Nutlin-3 at 24 h shows dose-dependent responsiveness to MDM2 antagonism. Antibodies used were as described for panel B.
Nutlin treatment led to a concomitant increase in the protein levels of several of these p53 targets, and as anticipated based on the results of Sarek et al. (26), p53 levels were stabilized (Fig. 4B). Nutlin-induced gene induction was also dose dependent, where as little as 0.5 μM Nutlin-3 was able to activate MDM2 and p21 expression (Fig. 4C). As a negative control, BL41/B95-8 cells did not show any change in p53 target gene expression upon 5 μM Nutlin-3 treatment (Fig. 4A and data not shown). Finally, we observed that the Nutlin treatment of LCLs induced a modest downregulation of MDMX protein levels (Fig. 4B). The loss of MDMX has been described as a critical determinant of Nutlin sensitivity in other cell types and is thought to result from increased MDM2 expression and consequent ubiquitin ligase activity toward MDMX (13, 21, 30).
Nutlin-3 inhibits EBV-mediated growth transformation of primary B cells.
The effects of Nutlin-3 on LCL survival lead us to ask whether the sensitization to p53-mediated apoptosis occurred early after infection or was only acquired later in passage. To address this, we infected PBMCs with EBV-containing B95-8 supernatants in the absence or presence of increasing concentrations of Nutlin-3. We assessed both short- and long-term effects of Nutlin-3 on LCL outgrowth. At 2 weeks after infection, an average of 23 cellular aggregates greater than 200 cells was observed per well of a 96-well plate, and all wells contained aggregates. At this early time point, EBV-induced cell proliferation was suppressed in a dose-dependent manner in the presence of Nutlin-3 (Fig. 5A). After 5 weeks, the true outgrowth of LCLs was similarly inhibited by Nutlin-3. While an average of 13 large cell aggregates grew out of the untreated, EBV-infected PBMC cultures, <50% of outgrowth was observed in the presence of 1 μM Nutlin-3, and no outgrowth was observed in the presence of >2 μM Nutlin-3 (Fig. 5B). Thus, MDM2-dependent p53 inhibition was required for the efficient outgrowth of EBV-transformed B cells.
NF-κB modulates the response of LCLs to Nutlin-mediated apoptosis.
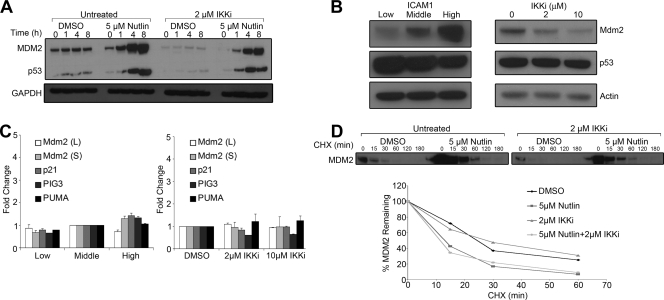
NF-κB activity is critical for LCL survival (6) and is known to antagonize DNA damage-induced p53-mediated apoptosis (33). Furthermore, LMP1 has been shown to antagonize p53-mediated apoptosis (10, 19). This prompted us to ask whether NF-κB activity in LCLs may play a role in modulating their sensitivity to Nutlin-mediated apoptosis. In the first set of experiments, an inhibitor of the canonical NF-κB-activating IKKβ kinase (IKKi IV; Calbiochem) was assayed for its ability to enhance the sensitivity of LCLs to Nutlin-mediated apoptosis. Inhibition of NF-κB consensus oligonucleotide DNA binding was verified by an electromobility shift assay (see Fig. S2A in the supplemental material), and activation of NF-κB-dependent target genes was verified by quantitative RT-PCR of mRNAs encoding Bfl1-A1, TRAF1, and IκBα following IKKi treatment of LCLs (see Fig. S2B in the supplemental material). As anticipated based on previous observations with small-molecule and dominant-negative IκBα mutant-based antagonism of the NF-κB pathway in LCLs (6), IKK inhibition lead to apoptosis. Low-dose treatment of LCLs with NF-κB inhibitors did not lead to appreciable annexin V staining; however, higher dose treatment did result in increased annexin V signal (see Fig. S2C in the supplemental material). Therefore, in these experiments, low concentrations of IKKi were used such that NF-κB activity was decreased but cells remained viable and only weakly annexin V positive (15% at 2 μM IKKi, compared to 10% with DMSO). IKKi treatment lead to an enhanced sensitivity of LCLs to Nutlin-3-induced apoptosis (Fig. 6A). LCLs treated with 5 μM Nutlin-3 alone were 35% annexin V positive after 48 h, while the addition of 2 μM IKKi to the Nutlin-3-treated cells yielded 65% annexin V-positive cells.
FIG. 6.
Nutlin-3 and NF-κB inhibition synergize in LCL killing. (A) LCL GM05422 cells were treated with either DMSO (white) or 5 μM Nutlin-3 (black) plus increasing concentrations of IKKβ inhibitor IV (0, 0.2, 1, and 2 μM). At 48 h, cells were harvested, and apoptosis was assayed by annexin V binding. Results of a representative of three independent experiments are shown. (B) LCLs were treated with DMSO or 5 μM or 10 μM Nutlin-3 and, 48 h later, subjected to fluorescence-activated cell sorter analysis with antibody to ICAM1 and annexin V-Alexa568. ICAM1 staining is shown on the x axis, and annexin V binding is shown on the y axis. The percentages of each quadrant are noted. Cells with lower levels of ICAM1 staining are more sensitive to Nutlin-induced annexin V binding. (C) Sorted LCLs based on ICAM1 levels (low, middle, high; see Fig. S2 in the supplemental material) were treated with DMSO or 5 μM or 10 μM Nutlin-3. Annexin V positivity is shown for each sample, where low-level ICAM1-expressing cells are considerably more sensitive to Nutlin-induced annexin V binding than are high-level ICAM1-expressing cells. (D) The annexin V-negative cells from panel C after sorting and Nutlin-3 treatment were analyzed for ICAM1 levels. ICAM1 expression consistently increased after Nutlin-3 treatment, suggesting selection for higher NF-κB activity.
Next, we took a complementary approach to assess the role of NF-κB activity in determining Nutlin sensitivity. Here, the NF-κB-regulated gene product ICAM1 was used as an endogenous reporter of NF-κB activity in LCLs. First, we treated cells with Nutlin-3 and, 48 h later, determined ICAM1 expression levels relative to annexin V positivity. As shown in Fig. 6B, Nutlin-treated LCLs staining positive with annexin V expressed low levels of ICAM1. This result could have been either due to increased Nutlin sensitivity of LCLs expressing low levels of ICAM1 or due to the decreased expression or detection of ICAM1 on cells undergoing apoptosis. To rule out the latter concern, we first sorted cells based on ICAM1 expression levels (see Fig. S3A in the supplemental material) and assessed the Nutlin sensitivity of each population. LCLs with low, intermediate, or high levels of cell surface ICAM1 expressed corresponding levels of three NF-κB-dependent transcriptional targets (see Fig. S3B in the supplemental material), thereby validating our approach. Sorted cells were treated with 0, 5, or 10 μM Nutlin-3 and assayed for annexin V positivity 48 h later. As shown in Fig. 6C, cells expressing low levels of surface ICAM1 were substantially more sensitive to Nutlin-3-induced apoptosis than were cells with high-level ICAM1 expression. These experiments collectively implicate NF-κB activity as a key determinant of Nutlin sensitivity in LCLs.
A corollary to these experiments emerged based on the analysis of ICAM1 expression level in the sorted populations after Nutlin treatment. In all subpopulations, 48 h of Nutlin-3 treatment led to the selection of cells with an increased ICAM1 expression level (Fig. 6D). For example, in untreated cells with low levels of ICAM1, cells retained a low ICAM1 mean fluorescence intensity of 459, while those treated with 5 μM Nutlin had an ICAM1 mean fluorescence intensity of 1,184. Importantly, short-term Nutlin treatment of LCLs did not result in an increase in ICAM1 level (see Fig. S3C in the supplemental material). This suggests that Nutlin treatment selects for a population of LCLs with higher NF-κB activity. Collectively, the ICAM1-sorting experiments corroborate those using the IKK inhibitor in demonstrating that NF-κB activity in LCLs dictates Nutlin sensitivity.
NF-κB activity in LCLs controls MDM2 protein levels independent of p53 target gene transcription.
We were interested to determine the mechanism by which NF-κB signaling modulates Nutlin-mediated apoptosis in LCLs. We first assayed MDM2 and p53 protein expression levels following Nutlin treatment in cells preexposed to the IKK inhibitor. IKK inhibition reduced the Nutlin-induced level of MDM2 protein by approximately threefold and modestly reduced p53 levels (Fig. 7A, compare lane labeled Untreated/Nutlin/8 h to that labeled IKKi/Nutlin/8 h). Interestingly, IKK inhibition also suppressed steady-state MDM2 protein levels in the absence of Nutlin-3 treatment (Fig. 7A, compare lanes labeled Untreated/DMSO to those labeled IKKi/DMSO). This result prompted us to assess the steady-state levels of MDM2 in ICAM1-sorted and IKK-inhibited LCLs. In both settings, MDM2 protein levels were positively correlated with the NF-κB activity state of the cells (Fig. 7B).
FIG. 7.
NF-κB activity modulates MDM2 protein levels in LCLs. (A) LCLs were treated with DMSO or 2 μM IKKβ inhibitor IV overnight. Then, cells were treated with either DMSO or 5 μM Nutlin-3 for the indicated amounts of time. Cells were lysed and blotted for MDM2, p53, and GAPDH. Cells preexposed to the IKK inhibitor overnight expressed lower levels of MDM2. (B) LCLs sorted as in Fig. 6C were lysed and immune-blotted for MDM2, p53, and actin. Similar to what is shown in Fig. 7A, MDM2 protein levels positively correlated with NF-κB activity. (C) LCLs sorted as in Fig. 6C and those treated with 2 μM or 10 μM IKK inhibitor for 24 h were subjected to quantitative RT-PCR for the following mRNAs: MDM2 (long), MDM2 (short), p21, PIG3, and PUMA. Values were normalized to 18S rRNA levels and are represented as either change relative to middle ICAM1 level (left) or change relative to DMSO-treated cells (right). (D) LCLs were treated with DMSO or 2 μM IKK inhibitor overnight, as in panel A, followed by DMSO or 5 μM Nutlin-3 treatment for 4 h. Cells were then washed, and fresh medium containing 75 μg/ml of cycloheximide was added to inhibit new protein synthesis. Protein samples were taken at the time points shown and immune-blotted for MDM2. Densitometry of the MDM2 band was performed using a Bio-Rad ChemiDoc gel documentation system and is plotted below the blot as the percentage of total MDM2 remaining over time.
We next asked whether NF-κB activity controlled MDM2 protein levels through p53-dependent transcription. In other systems NF-κB- and p53-dependent transcription has been shown to be reciprocally antagonistic (9, 24, 31, 35). However, IKK inhibition in LCLs did not increase the steady-state mRNA levels of the p53 targets MDM2, PUMA, p21, and PIG3 (Fig. 7C). In fact, PIG3 mRNA levels were both mildly sensitive to IKK inhibition and also positively correlated with ICAM1 expression levels, suggesting that PIG3 is also an NF-κB target gene in LCLs. Importantly, levels of canonical NF-κB target genes did not decrease upon Nutlin treatment (see Fig. S4 in the supplemental material). Therefore, direct reciprocal repression of transcriptional targets does not appear to be the mechanism by which the inhibition of NF-κB activity sensitizes LCLs to Nutlin-mediated apoptosis.
We further asked whether NF-κB may alter the translation of MDM2 mRNA through enrichment in the polysomal RNA fraction. We tested whether IKK inhibition decreased the fraction of polysome-associated MDM2 mRNA (both short and long) relative to actin transcripts. We observed no significant decrease in polysomal MDM2 mRNAs in IKKi-treated cells (data not shown). As a positive control, the short, P2-derived MDM2 mRNA was significantly enriched in the polysomal fraction following Nutlin treatment (data not shown).
Finally, experiments were performed to assess the stability of MDM2 protein in IKKi- and Nutlin-treated LCLs. Cycloheximide-sensitive decay of MDM2 did not differ in LCLs pretreated with IKKi in the presence or absence of Nutlin-3 (Fig. 7D). Therefore, NF-κB increased steady-state MDM2 protein levels independent of mRNA levels or protein stability.
DISCUSSION
The ability of Nutlin-3 to inhibit growth and induce apoptosis in EBV-transformed LCLs indicates that these cells require MDM2-dependent regulation of p53 activity for their survival. Furthermore, the suppression of EBV-mediated transformation of primary B lymphocytes by Nutlin-3 provides evidence for an early requirement of p53 suppression in the growth transformation process. In LCLs, Nutlin-stabilized p53 induced the expression of genes important in the activation of the apoptotic process, which lead to mitochondrial outer membrane permeabilization, caspase activity, and ultimately, cell death. The effects of Nutlins on LCL survival were enhanced by inhibition of NF-κB signaling. Importantly, NF-κB activity controlled the steady-state protein level of MDM2 and thus dictated the ratio of MDM2 to p53 in LCLs. These data collectively suggest that EBV induces a constitutively active pathway in B cells that primes their sensitivity to MDM2 antagonists and, furthermore, that NF-κB levels dictate the degree of sensitivity.
Recently, Sarek et al. reported that KSHV-infected cells were sensitive to Nutlin-3-mediated growth suppression, whereas EBV-transformed LCLs were not (26). Our interpretation of their data in the context of our findings suggests rather that both KSHV- and EBV-infected cells are sensitive to MDM2 antagonists. While we consistently observed apoptosis downstream of Nutlin treatment in LCLs, Sarek et al. observed an inhibition of cell cycle progression and modest apoptosis induction. Both studies showed the biochemical stabilization of p53 and the activation of p53 target genes in LCLs. Our study extends these findings to characterize Nutlin-mediated apoptosis, demonstrate a requirement for MDM2 suppression of p53 for EBV transformation of primary B cells, and identify an unexpected role for NF-κB in controlling MDM2 protein levels.
Studies of DNA damage responses in LCLs have indicated that the p53 response is functional (20). However, the specific type of DNA damage determines the cellular outcome. Double-stranded breaks induced by ionizing radiation lead to p53 stabilization and cell cycle arrest, whereas alkylating agents that generate bulky DNA adducts transmit p53 signals which are inhibited downstream through degradation of the cyclin-dependent kinase inhibitor p21 (20). In our experiments, p53 was liberated from MDM2 regulation in the absence of exogenous genotoxic stress. The apoptotic effects observed after Nutlin treatment therefore indicate that an endogenous constitutively active pathway exists in LCLs, which sensitizes them to p53-mediated cell death.
Recently, a set of genes was identified in a screen for sensitizers to Nutlin-mediated antiproliferative effects (4). Several of these genes were involved in the recognition and checkpoint signaling pathways downstream of DNA double-stranded breaks. These data are consistent with recent findings that tumor cells and early neoplastic lesions harbor activation markers of the DNA damage response (3, 11). Consistent with these findings, we have preliminary evidence of DNA damage foci early after EBV infection of primary B cells, suggesting that a low-level persistent oncogenic stress signal may sensitize these cells to the effects of Nutlins (data not shown). In fact, recently, KSHV-infected cells were shown to harbor such increased DNA damage signals as well as sensitivity to Nutlins (22, 26). Testing of this hypothesis by using small-molecule and genetic approaches is under way to identify the critical functional mediators of such a signal which may suppress EBV transformation.
What are the signals that dictate Nutlin sensitivity? Our data support a model whereby activation of NF-κB by LMP1 and possibly other viral and cellular proteins in EBV-transformed cells suppress p53-mediated apoptosis. Indeed, NF-κB activation through the heterologous expression of LMP1 is known to directly inhibit p53-mediated apoptosis (10, 19). In LCLs, this effect was not due to direct effects on p53-dependent transcription because IKK inhibition did not lead to an appreciable increase in p53-responsive genes. Two mechanisms are likely responsible for the heightened responsiveness of LCLs to Nutlin-mediated apoptosis in the low NF-κB state. First, several NF-κB targets, such as Bfl1-A1, A20, and c-IAPs, are well-characterized antiapoptotic molecules (5, 10, 32, 33). Second, our data provide evidence for NF-κB-dependent positive regulation of steady-state MDM2 protein levels. As several translation factors are NF-κB targets in LCLs (5), this increase may be due to enhanced translation of an MDM2 mRNA species. However, we have not ruled out the possibility that an as-yet-undetected MDM2 mRNA isoform is increased transcriptionally or that the stability of an MDM2 mRNA isoform is increased with NF-κB activity.
Another important aspect of this work derives from a comparison of the molecular phenotypes of different cell types in response to Nutlin-3 treatment. In a recent study, Nutlin-3 was shown to inhibit the protein expression of a subset of NF-κB-dependent gene products, including ICAM1, induced by proinflammatory cytokines in lung cancer cell lines (9). It was suggested that Nutlin-3 would thereby act as a bitargeted anticancer agent, simultaneously inducing p53-dependent antiproliferative effects while inhibiting promoter-specific NF-κB responses. While this may be the case in lung cancer cells and other cell types, EBV-transformed cells with constitutive NF-κB activity were sensitive to Nutlin-3, independent of effects on NF-κB-regulated gene expression. In particular, Nutlin-3 did not negatively affect constitutive NF-κB-regulated ICAM1 protein levels in LCLs. Thus, while our results differ mechanistically with those in lung cancer cells, the concept of using p53 activation and NF-κB inhibition to simultaneously and synergistically kill tumor cells is consistent. Therefore, the characterization of Nutlin-induced phenotypes in diverse wt p53 containing tumor cell types as well as in vivo using animal models will be critical toward the development of MDM2 antagonist therapies and, importantly, tumor-specific prognostic and diagnostic biomarkers.
Collectively, our data demonstrating that modest inhibition of NF-κB signaling can synergize with Nutlins support the notion that LCL survival depends on the subtle balance between antiapoptotic NF-κB signals and oncogenic stress-derived p53-dependent apoptotic signals. Furthermore, as many tumors display constitutively active NF-κB activation, this balance may be critical in determining their sensitivity to Nutlin-mediated antiproliferative effects. Specifically in the case of EBV, simultaneous inhibition of IKK signaling and liberation of p53 may provide a unique therapeutic window in targeting malignancies such as posttransplant lymphoproliferative disorder and AIDS-associated central nervous system lymphomas.
Supplementary Material
Acknowledgments
We thank Andrea Carfi, Giuseppe Roscilli, and Bruno Cadot for their help with this work. We greatly appreciate the assistance of David Tainter in preparing the manuscript and Ellen Cahir-McFarland for critically reading the manuscript and sending reagents. We acknowledge the help of Shelton Bradrick and Matthias Gromeier with the polysome fractionation experiments and Hal Bogerd with EMSAs.
This work was supported by a Duke CFAR developmental grant awarded to M.A.L.
Footnotes
Published ahead of print on 14 January 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Allday, M. J., A. Sinclair, G. Parker, D. H. Crawford, and P. J. Farrell. 1995. Epstein-Barr virus efficiently immortalizes human B cells without neutralizing the function of p53. EMBO J. 141382-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altmann, M., D. Pich, R. Ruiss, J. Wang, B. Sugden, and W. Hammerschmidt. 2006. Transcriptional activation by EBV nuclear antigen 1 is essential for the expression of EBV's transforming genes. Proc. Natl. Acad. Sci. USA 10314188-14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartkova, J., Z. Horejsi, K. Koed, A. Kramer, F. Tort, K. Zieger, P. Guldberg, M. Sehested, J. M. Nesland, C. Lukas, T. Orntoft, J. Lukas, and J. Bartek. 2005. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434864-870. [DOI] [PubMed] [Google Scholar]
- 4.Brummelkamp, T. R., A. W. Fabius, J. Mullenders, M. Madiredjo, A. Velds, R. M. Kerkhoven, R. Bernards, and R. L. Beijersbergen. 2006. An shRNA barcode screen provides insight into cancer cell vulnerability to MDM2 inhibitors. Nat. Chem. Biol. 2202-206. [DOI] [PubMed] [Google Scholar]
- 5.Cahir-McFarland, E. D., K. Carter, A. Rosenwald, J. M. Giltnane, S. E. Henrickson, L. M. Staudt, and E. Kieff. 2004. Role of NF-κB in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J. Virol. 784108-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahir-McFarland, E. D., D. M. Davidson, S. L. Schauer, J. Duong, and E. Kieff. 2000. NF-κB inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 976055-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahir McFarland, E. D., K. M. Izumi, and G. Mosialos. 1999. Epstein-Barr virus transformation: involvement of latent membrane protein 1-mediated activation of NF-κB. Oncogene 186959-6964. [DOI] [PubMed] [Google Scholar]
- 8.Cannell, E. J., P. J. Farrell, and A. J. Sinclair. 1996. Epstein-Barr virus exploits the normal cell pathway to regulate Rb activity during the immortalisation of primary B-cells. Oncogene 131413-1421. [PubMed] [Google Scholar]
- 9.Dey, A., E. T. Wong, P. Bist, V. Tergaonkar, and D. P. Lane. 2007. Nutlin-3 inhibits the NFkappaB pathway in a p53-dependent manner: implications in lung cancer therapy. Cell Cycle 62178-2185. [DOI] [PubMed] [Google Scholar]
- 10.Fries, K. L., W. E. Miller, and N. Raab-Traub. 1996. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J. Virol. 708653-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorgoulis, V. G., L. V. Vassiliou, P. Karakaidos, P. Zacharatos, A. Kotsinas, T. Liloglou, M. Venere, R. A. Ditullio, Jr., N. G. Kastrinakis, B. Levy, D. Kletsas, A. Yoneta, M. Herlyn, C. Kittas, and T. D. Halazonetis. 2005. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434907-913. [DOI] [PubMed] [Google Scholar]
- 12.Hayward, S. D. 2004. Viral interactions with the Notch pathway. Semin. Cancer Biol. 14387-396. [DOI] [PubMed] [Google Scholar]
- 13.Hu, B., D. M. Gilkes, B. Farooqi, S. M. Sebti, and J. Chen. 2006. MDMX overexpression prevents p53 activation by the MDM2 inhibitor Nutlin. J. Biol. Chem. 28133030-33035. [DOI] [PubMed] [Google Scholar]
- 14.Juven, T., Y. Barak, A. Zauberman, D. L. George, and M. Oren. 1993. Wild type p53 can mediate sequence-specific transactivation of an internal promoter within the mdm2 gene. Oncogene 83411-3416. [PubMed] [Google Scholar]
- 15.Kieff, E., and A. Rickinson. 2006. Epstein-Barr virus and its replication, p. 2603-2654. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 5th ed. Lippincott, Williams, and Wilkins, Philadelphia, PA.
- 16.Kulwichit, W., R. H. Edwards, E. M. Davenport, J. F. Baskar, V. Godfrey, and N. Raab-Traub. 1998. Expression of the Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc. Natl. Acad. Sci. USA 9511963-11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, M. A., M. E. Diamond, and J. L. Yates. 1999. Genetic evidence that EBNA-1 is needed for efficient, stable latent infection by Epstein-Barr virus. J. Virol. 732974-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meek, D. W. 2004. The p53 response to DNA damage. DNA Repair (Amsterdam) 31049-1056. [DOI] [PubMed] [Google Scholar]
- 19.Okan, I., Y. Wang, F. Chen, L. F. Hu, S. Imreh, G. Klein, and K. G. Wiman. 1995. The EBV-encoded LMP1 protein inhibits p53-triggered apoptosis but not growth arrest. Oncogene 111027-1031. [PubMed] [Google Scholar]
- 20.O'Nions, J., and M. J. Allday. 2003. Epstein-Barr virus can inhibit genotoxin-induced G1 arrest downstream of p53 by preventing the inactivation of CDK2. Oncogene 227181-7191. [DOI] [PubMed] [Google Scholar]
- 21.Patton, J. T., L. D. Mayo, A. D. Singhi, A. V. Gudkov, G. R. Stark, and M. W. Jackson. 2006. Levels of HdmX expression dictate the sensitivity of normal and transformed cells to Nutlin-3. Cancer Res. 663169-3176. [DOI] [PubMed] [Google Scholar]
- 22.Petre, C. E., S. H. Sin, and D. P. Dittmer. 2007. Functional p53 signaling in Kaposi's sarcoma-associated herpesvirus lymphomas: implications for therapy. J. Virol. 811912-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pokrovskaja, K., I. Okan, E. Kashuba, M. Lowbeer, G. Klein, and L. Szekely. 1999. Epstein-Barr virus infection and mitogen stimulation of normal B cells induces wild-type p53 without subsequent growth arrest or apoptosis. J. Gen. Virol. 80987-995. [DOI] [PubMed] [Google Scholar]
- 24.Ravi, R., B. Mookerjee, Y. van Hensbergen, G. C. Bedi, A. Giordano, W. S. El-Deiry, E. J. Fuchs, and A. Bedi. 1998. p53-mediated repression of nuclear factor-kappaB RelA via the transcriptional integrator p300. Cancer Res. 584531-4536. [PubMed] [Google Scholar]
- 25.Reers, M., S. T. Smiley, C. Mottola-Hartshorn, A. Chen, M. Lin, and L. B. Chen. 1995. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 260406-417. [DOI] [PubMed] [Google Scholar]
- 26.Sarek, G., S. Kurki, J. Enback, G. Iotzova, J. Haas, P. Laakkonen, M. Laiho, and P. M. Ojala. 2007. Reactivation of the p53 pathway as a treatment modality for KSHV-induced lymphomas. J. Clin. Investig. 1171019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinclair, A. J., I. Palmero, G. Peters, and P. J. Farrell. 1994. EBNA-2 and EBNA-LP cooperate to cause G0 to G1 transition during immortalization of resting human B lymphocytes by Epstein-Barr virus. EMBO J. 133321-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tovar, C., J. Rosinski, Z. Filipovic, B. Higgins, K. Kolinsky, H. Hilton, X. Zhao, B. T. Vu, W. Qing, K. Packman, O. Myklebost, D. C. Heimbrook, and L. T. Vassilev. 2006. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc. Natl. Acad. Sci. USA 1031888-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vassilev, L. T., B. T. Vu, B. Graves, D. Carvajal, F. Podlaski, Z. Filipovic, N. Kong, U. Kammlott, C. Lukacs, C. Klein, N. Fotouhi, and E. A. Liu. 2004. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303844-848. [DOI] [PubMed] [Google Scholar]
- 30.Wade, M., E. T. Wong, M. Tang, J. M. Stommel, and G. M. Wahl. 2006. Hdmx modulates the outcome of p53 activation in human tumor cells. J. Biol. Chem. 28133036-33044. [DOI] [PubMed] [Google Scholar]
- 31.Wadgaonkar, R., K. M. Phelps, Z. Haque, A. J. Williams, E. S. Silverman, and T. Collins. 1999. CREB-binding protein is a nuclear integrator of nuclear factor-kappaB and p53 signaling. J. Biol. Chem. 2741879-1882. [DOI] [PubMed] [Google Scholar]
- 32.Wang, C. Y., D. C. Guttridge, M. W. Mayo, and A. S. Baldwin, Jr. 1999. NF-κB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol. Cell. Biol. 195923-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 2811680-1683. [DOI] [PubMed] [Google Scholar]
- 34.Wang, D., D. Liebowitz, and E. Kieff. 1985. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 43831-840. [DOI] [PubMed] [Google Scholar]
- 35.Webster, G. A., and N. D. Perkins. 1999. Transcriptional cross talk between NF-κB and p53. Mol. Cell. Biol. 193485-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yates, J. L., N. Warren, and B. Sugden. 1985. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature 313812-815. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, Q., Y. Liu, J. Zhou, W. Chen, Y. Zhang, and H. L. Liber. 2007. Wild-type p53 reduces radiation hypermutability in p53-mutated human lymphoblast cells. Mutagenesis 22329-334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.