Abstract
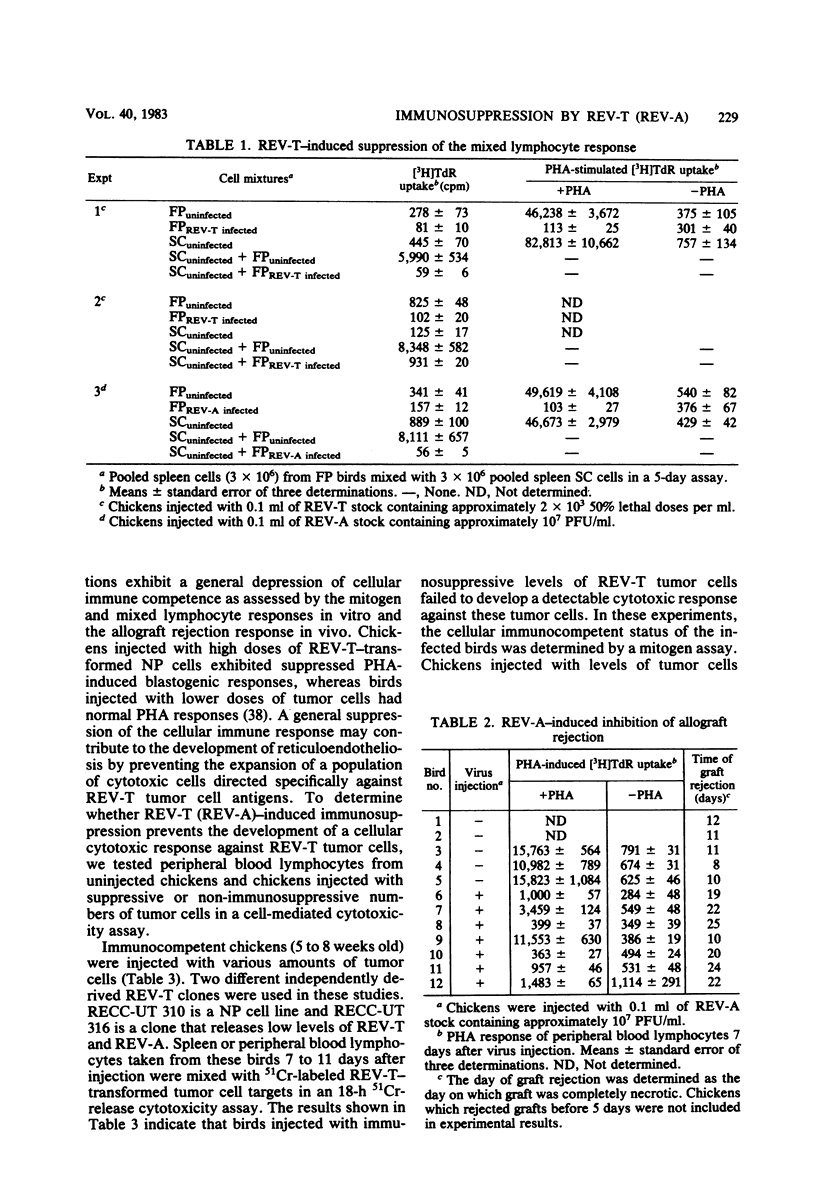
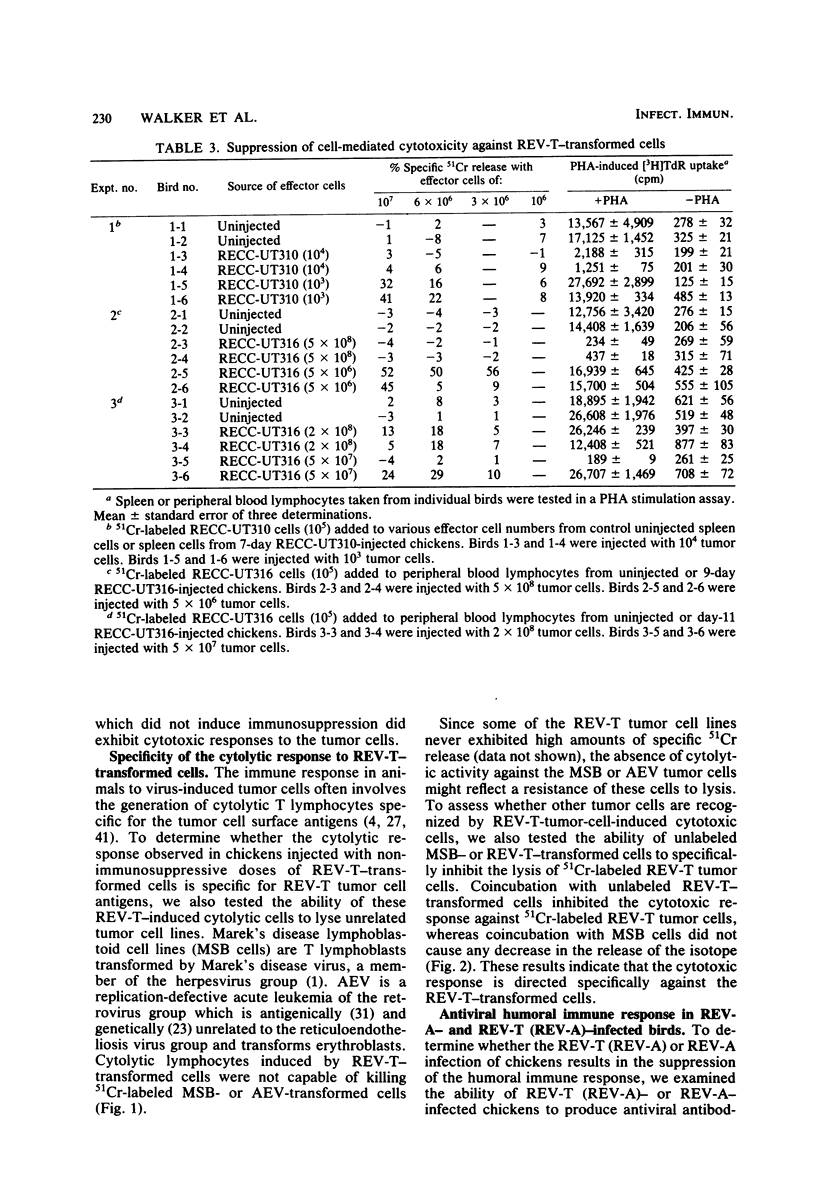
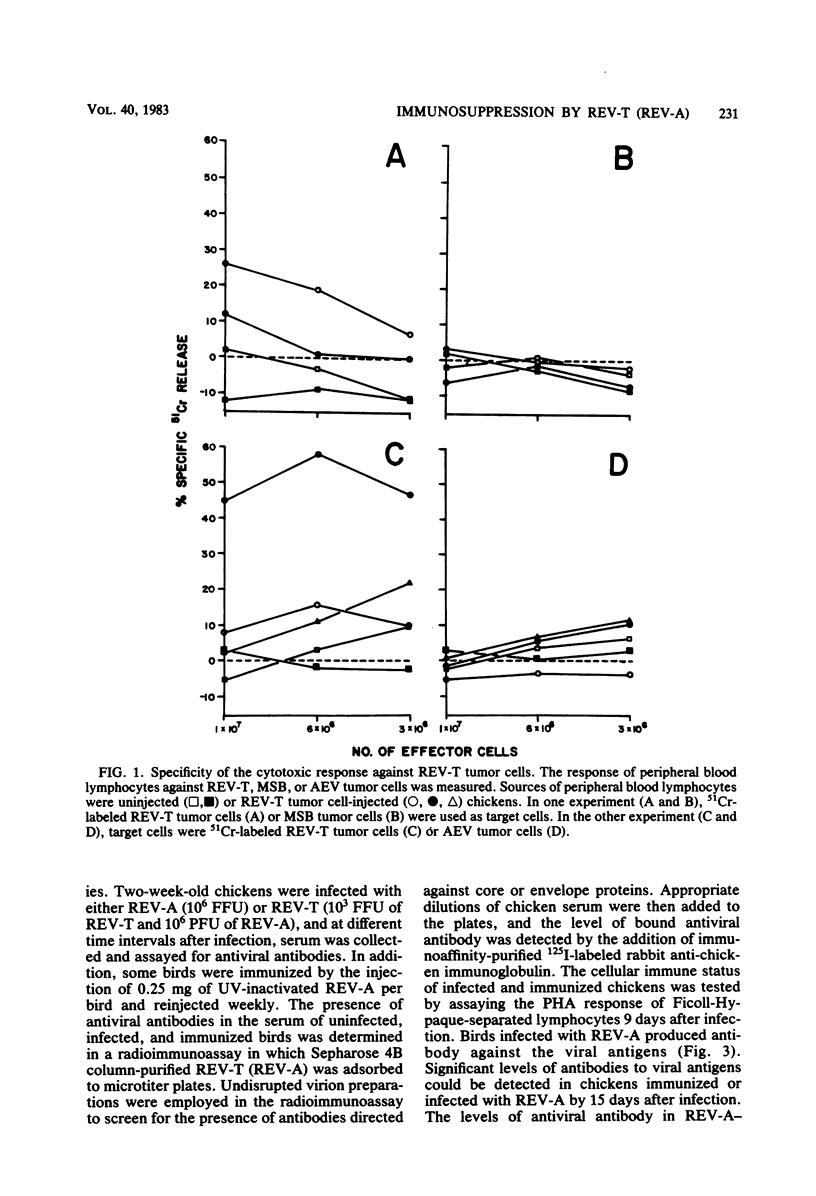
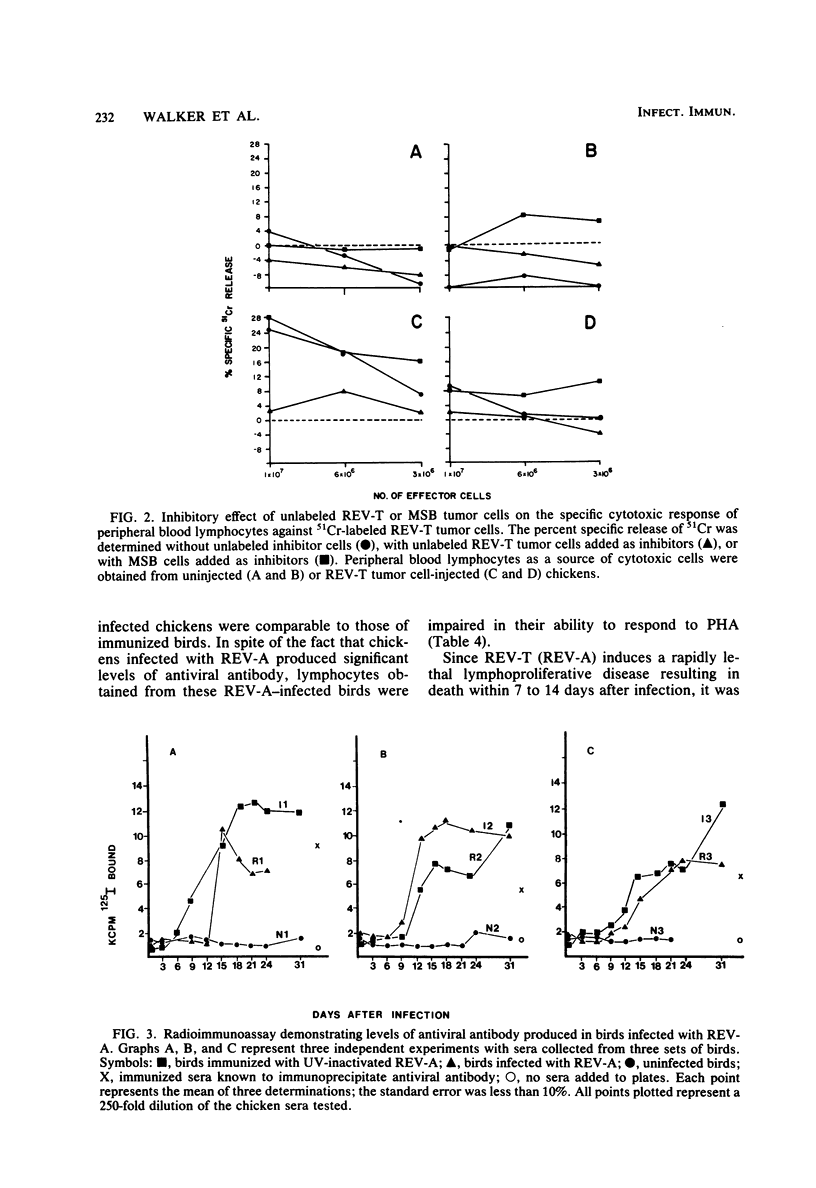
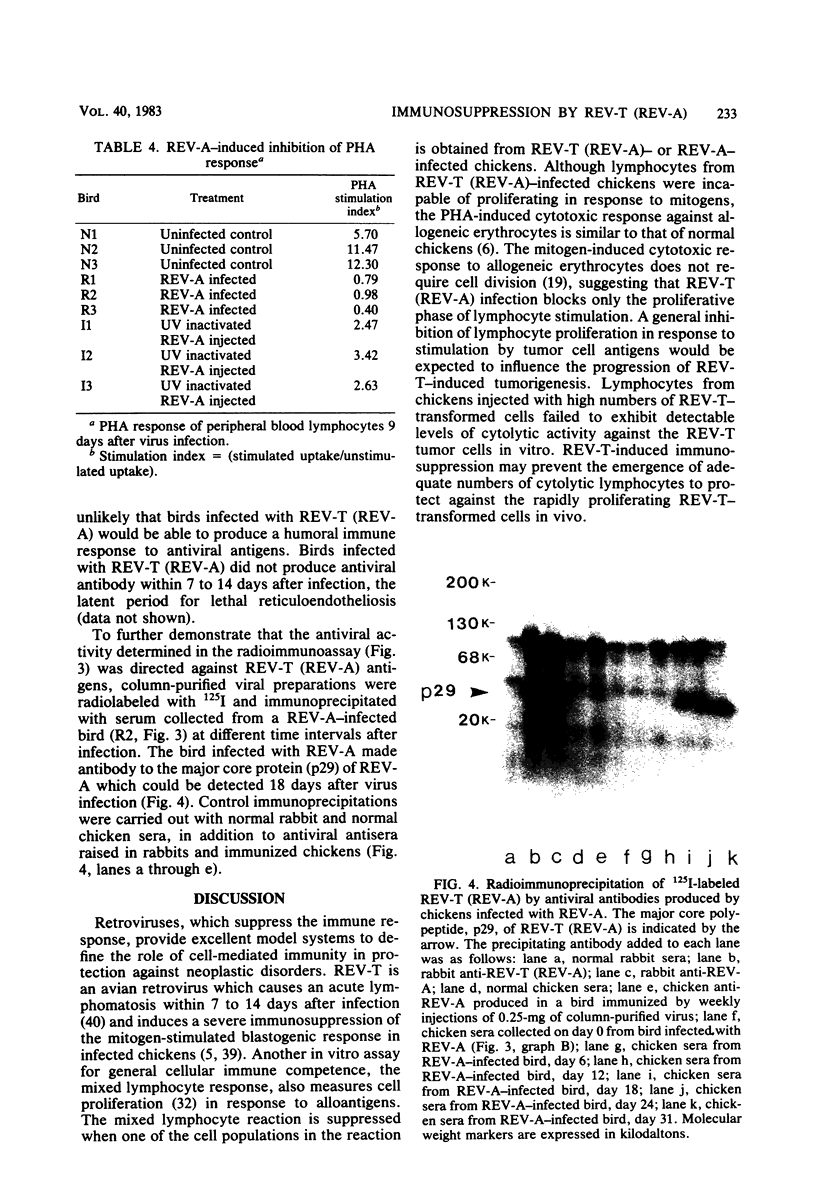
Several parameters of the cellular and humoral immune responses of chickens infected with reticuloendotheliosis virus (REV-T), an avian defective acute leukemia virus, or with its helper virus, reticuloendotheliosis-associated virus (REV-A), were evaluated. Spleen cells from chickens infected with REV-T (REV-A) or REV-A exhibited depressed mixed lymphocyte and mitogen responses in vitro. Allograft rejection was delayed by 6 to 14 days in birds infected with REV-A. The specific antitumor cell immune response was also studied by a 51Cr-release cytotoxicity assay. Lymphocytes from chickens infected with low numbers of the REV-T-transformed cells exhibited significant levels of cytolytic reactivity against the 51Cr-labeled REV-T tumor cells in vitro. The mitogen response of lymphocytes from these injected birds was similar to that of uninjected chickens. In contrast, lymphocytes from chickens injected with higher numbers of REV-T-transformed cells exhibited suppressed mitogen reactivity and failed to develop detectable levels of cytotoxic activity directed against the REV-T tumor cells. These results suggest that the general depression of cellular immune competence which occurs during REV-T (REV-A) infection could contribute to the development of this acute leukemia by inhibiting the proliferation of cytotoxic cells directed against the tumor cell antigens. The cytotoxic effect observed after the injection of chickens with non-immunosuppressive levels of REV-T-transformed cells appears to be specific for the REV-T tumor cell antigens since cells transformed by Marek's disease virus or avian erythroblastosis virus were not lysed. In marked contrast, birds whose cellular immune responses were suppressed by infection with REV-A were capable of producing a humoral immune response to viral antigens. Detectable levels of viral antibody, however, did not appear until 12 to 15 days after REV-A infection. Since REV-T (REV-A) induced an acute leukemia resulting in death within 7 to 14 days, it appears unlikely that the ability of chickens to make antiviral antibody influences the development of lethal reticuloendotheliosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Kato S. Two cell lines from lymphomas of Marek's disease. Biken J. 1974 Sep;17(3):105–116. [PubMed] [Google Scholar]
- Bernstein I. D., Wright P. W., Cohen E. Generation of cytotoxic lymphocytes in vitro: response of immune rat spleen cells to a syngeneic gross virus-induced lymphoma in mixed lymphocyte-tumor culture. J Immunol. 1976 May;116(5):1367–1372. [PubMed] [Google Scholar]
- COCK A. G., CLOUGH M. Successful skin homografts in inbred chickens. Nature. 1956 Jul 21;178(4525):136–137. doi: 10.1038/178136a0. [DOI] [PubMed] [Google Scholar]
- CRAIG J. V., McDERMID E. M. Prolonged skin homograft survival and erythrocyte (B-locus) antigens in young chicks. Transplantation. 1963 Apr;1:191–200. doi: 10.1097/00007890-196301020-00007. [DOI] [PubMed] [Google Scholar]
- Carpenter C. R., Bose H. R., Rubin A. S. Contact-mediated suppression of mitogen-induced responsiveness by spleen cells in reticuloendotheliosis virus-induced tumorigenesis. Cell Immunol. 1977 Oct;33(2):392–401. doi: 10.1016/0008-8749(77)90167-8. [DOI] [PubMed] [Google Scholar]
- Carpenter C. R., Kempf K. E., Bose H. R., Rubin A. S. Characterization of the interaction of reticuloendotheliosis virus with the avian lymphoid system. Cell Immunol. 1978 Sep;39(2):307–315. doi: 10.1016/0008-8749(78)90106-5. [DOI] [PubMed] [Google Scholar]
- Carpenter C. R., Rubin A. S., Bose H. R., Jr Suppression of the mitogen-stimulated blastogenic response during reticuloendotheliosis virus-induced tumorigenesis: investigations into the mechanism of action of the suppressor. J Immunol. 1978 Apr;120(4):1313–1320. [PubMed] [Google Scholar]
- Collavo D., Zanovello P., Biasi G., Chieco-Bianchi L. T lymphocyte tolerance and early appearance of virus-induced cell surface antigens in Moloney-murine leukemia virus neonatally injected mice. J Immunol. 1981 Jan;126(1):187–193. [PubMed] [Google Scholar]
- Essex M., Cotter S. M., Hardy W. D., Jr, Hess P., Jarrett W., Jarrett O., Mackey L., Laird H., Perryman L., Olsen R. G. Feline oncornavirus-associated cell membrane antigen. IV. Antibody titers in cats with naturally occurring leukemia, lymphoma, and other diseases. J Natl Cancer Inst. 1975 Aug;55(2):463–467. [PubMed] [Google Scholar]
- Essex M., Sliski A., Hardy W. D., Jr, Cotter S. M. Immune response to leukemia virus and tumor-associated antigens in cats. Cancer Res. 1976 Feb;36(2 Pt 2):640–645. [PubMed] [Google Scholar]
- Franklin R. B., Maldonado R. L., Bose H. R. Isolation and characterization of reticuloendotheliosis virus transformed bone marrow cells. Intervirology. 1974;3(5-6):342–352. doi: 10.1159/000149771. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser M., Kirchner H., Holden H. T., Herberman R. B. Inhibition of cell-mediated cytotoxicity against tumor-associated antigens by suppressor cells from tumor-bearing mice. J Natl Cancer Inst. 1976 Apr;56(4):865–867. doi: 10.1093/jnci/56.4.865. [DOI] [PubMed] [Google Scholar]
- Heininger D., Touton M., Chakravarty A. K., Clark W. R. Activation of cytotoxic function in T lymphocytes. J Immunol. 1976 Dec;117(6):2175–2180. [PubMed] [Google Scholar]
- Herberman R. B. Cell-mediated immunity to tumor cells. Adv Cancer Res. 1974;19(0):207–263. doi: 10.1016/s0065-230x(08)60055-x. [DOI] [PubMed] [Google Scholar]
- Hoelzer J. D., Franklin R. B., Bose H. R., Jr Transformation by reticuloendotheliosis virus: development of a focus assay and isolation of a nontransforming virus. Virology. 1979 Feb;93(1):20–30. doi: 10.1016/0042-6822(79)90272-1. [DOI] [PubMed] [Google Scholar]
- Hoelzer J. D., Lewis R. B., Wasmuth C. R., Bose H. R., Jr Hematopoietic cell transformation by reticuloendotheliosis virus: characterization of the genetic defect. Virology. 1980 Jan 30;100(2):462–474. doi: 10.1016/0042-6822(80)90536-x. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L. H., Rufner R., Sevoian M. Isolation and development of a reticuloendotheliosis virus-transformed lymphoblastoid cell line from chicken spleen cells. Infect Immun. 1979 Aug;25(2):694–701. doi: 10.1128/iai.25.2.694-701.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Chused T. M., Herberman R. B., Holden H. T., Lavrin D. H. Evidence of suppressor cell activity in spleens of mice bearing primary tumors induced by Moloney sarcoma virus. J Exp Med. 1974 Jun 1;139(6):1473–1487. doi: 10.1084/jem.139.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leclerc J. C., Gomard E., Plata F., Levy J. P. Cell-mediated immune reaction against tumors induced by oncornaviruses. II. Nature of the effector cells in tumor-cell cytolysis. Int J Cancer. 1973 Mar 15;11(2):426–432. doi: 10.1002/ijc.2910110220. [DOI] [PubMed] [Google Scholar]
- Leslie G. A., Clem L. W. Phylogen of immunoglobulin structure and function. 3. Immunoglobulins of the chicken. J Exp Med. 1969 Dec 1;130(6):1337–1352. doi: 10.1084/jem.130.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. P., Leclerc J. C. The murine sarcoma virus-induced tumor: exception or general model in tumor immunology? Adv Cancer Res. 1977;24:1–66. doi: 10.1016/s0065-230x(08)61012-x. [DOI] [PubMed] [Google Scholar]
- Lewis R. B., McClure J., Rup B., Niesel D. W., Garry R. F., Hoelzer J. D., Nazerian K., Bose H. R., Jr Avian reticuloendotheliosis virus: identification of the hematopoietic target cell for transformation. Cell. 1981 Aug;25(2):421–431. doi: 10.1016/0092-8674(81)90060-x. [DOI] [PubMed] [Google Scholar]
- Maldonado R. L., Bose H. R., Jr Group-specific antigen shared by the members of the reticuloendotheliosis virus complex. J Virol. 1976 Mar;17(3):983–990. doi: 10.1128/jvi.17.3.983-990.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miggiano V. C., Birgen I., Pink J. R. The mixed leukocyte reaction in chickens. Evidence for control by the major histocompatibility complex. Eur J Immunol. 1974 Jun;4(6):397–401. doi: 10.1002/eji.1830040602. [DOI] [PubMed] [Google Scholar]
- Naor D. Suppressor cells: permitters and promoters of malignancy? Adv Cancer Res. 1979;29:45–125. doi: 10.1016/s0065-230x(08)60846-5. [DOI] [PubMed] [Google Scholar]
- Perryman L. E., Hoover E. A., Yohn D. S. Immunologic reactivity of the cat: immunosuppression in experimental feline leukemia. J Natl Cancer Inst. 1972 Nov;49(5):1357–1365. [PubMed] [Google Scholar]
- Rup B. J., Hoelzer J. D., Bose H. R., Jr Helper viruses associated with avian acute leukemia viruses inhibit the cellular immune response. Virology. 1982 Jan 15;116(1):61–71. doi: 10.1016/0042-6822(82)90403-2. [DOI] [PubMed] [Google Scholar]
- Rup B. J., Spence J. L., Hoelzer J. D., Lewis R. B., Carpenter C. R., Rubin A. S., Bose H. R., Jr Immunosuppression induced by avian reticuloendotheliosis virus: mechanism of induction of the suppressor cell. J Immunol. 1979 Sep;123(3):1362–1370. [PubMed] [Google Scholar]
- Scofield V. L., Bose H. R., Jr Depression of mitogen response in spleen cells from reticuloendotheliosis virus-infected chickens and their suppressive effect on normal lymphocyte response. J Immunol. 1978 Apr;120(4):1321–1325. [PubMed] [Google Scholar]
- Sharma J. M., Coulson B. D. Cell-mediated cytotoxic response to cells bearing Marek's disease tumor-associated surface antigen in chickens infected with Marek's disease virus. J Natl Cancer Inst. 1977 Jun;58(6):1647–1651. doi: 10.1093/jnci/58.6.1647. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Van Eldik L. J. Characterization of the immunosuppression accompanying virus-induced avian osteopetrosis. Infect Immun. 1978 Nov;22(2):452–461. doi: 10.1128/iai.22.2.452-461.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutman O. Delayed tumour appearance and absence of regression in nude mice infected with murine sarcoma virus. Nature. 1975 Jan 10;253(5487):142–144. doi: 10.1038/253142a0. [DOI] [PubMed] [Google Scholar]
- Waite M. R., Allen P. T. RNA-directed DNA polymerase activity of reticuloendotheliosis virus: characterization of the endogenous and exogenous reactions. J Virol. 1975 Oct;16(4):872–879. doi: 10.1128/jvi.16.4.872-879.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]