Abstract
Lipopolysaccharide mutants of Salmonella typhimurium provoked diminished amounts of fluid in rabbit ileal loops as compared with the response to the wild type. The responses elicited by these mutants ranged from 0 to 60% of that caused by the parent strain. Two completely rough mutants and one leaky rough mutant were chosen for further study. Purified lipopolysaccharide from the parent and the mutant strains failed to stimulate fluid exsorption in ileal loop experiments. Histological studies revealed that the three lipopolysaccharide mutants were less invasive than wild type and were less able to generate an inflammatory reaction in the rabbit ileum. A Salmonella enterotoxin was present in culture filtrates from one rough mutant and the wild type; however, the rough mutant appeared to produce less toxin. Enterotoxic activity was absent in culture filtrates from the two other rough mutants. These results suggest that reductions in both invasiveness and the ability to produce Salmonella enterotoxin decreased the ability of these mutants to provoke fluid exsorption. Also, the results indicate that lipopolysaccharide mutations can have a profound effect on the enteropathogenic properties of S. typhimurium.
Full text
PDF


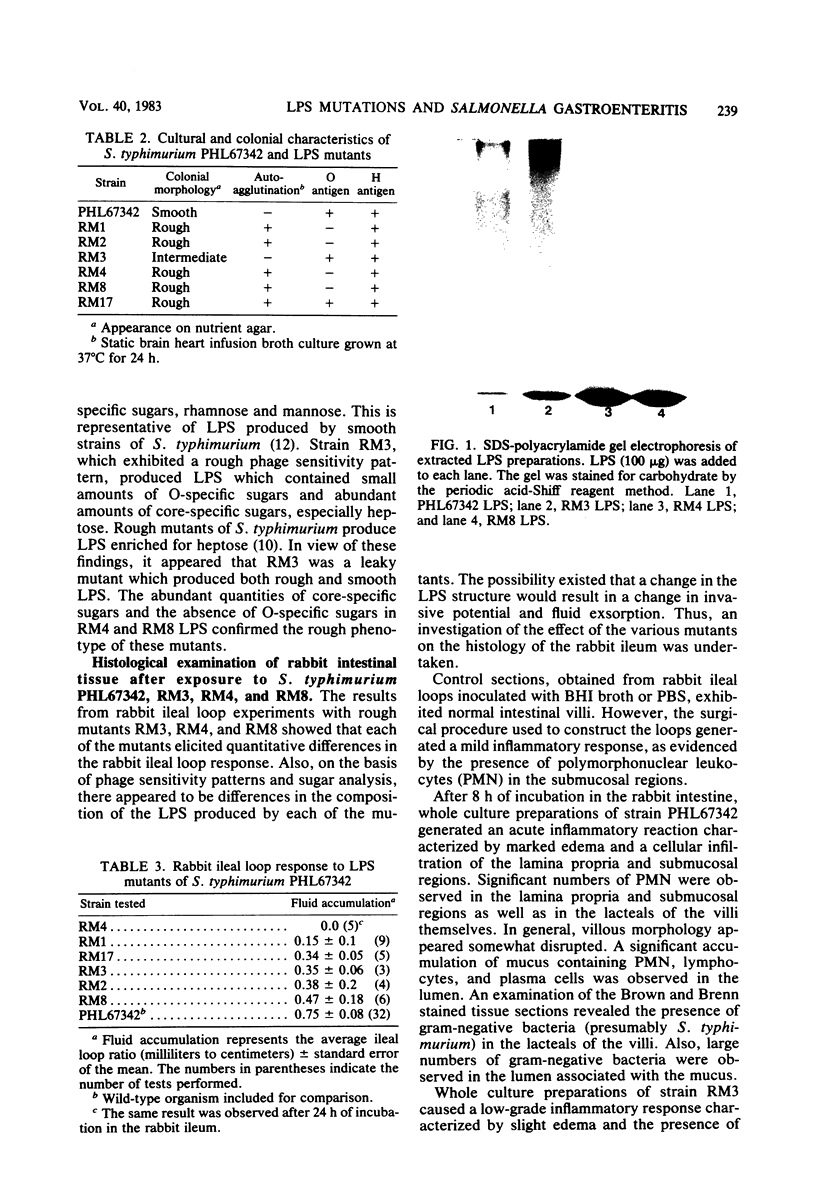





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edebo L., Normann B. Virulence and immunogenicity of mutant strains of Salmonella typhimurium. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(1):75–84. doi: 10.1111/j.1699-0463.1970.tb04271.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Giannella R. A., Formal S. B., Dammin G. J., Collins H. Pathogenesis of salmonellosis. Studies of fluid secretion, mucosal invasion, and morphologic reaction in the rabbit ileum. J Clin Invest. 1973 Feb;52(2):441–453. doi: 10.1172/JCI107201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Gots R. E., Charney A. N., Greenough W. B., 3rd, Formal S. B. Pathogenesis of Salmonella-mediated intestinal fluid secretion. Activation of adenylate cyclase and inhibition by indomethacin. Gastroenterology. 1975 Dec;69(6):1238–1245. [PubMed] [Google Scholar]
- Giannella R. A. Importance of the intestinal inflammatory reaction in salmonella-mediated intestinal secretion. Infect Immun. 1979 Jan;23(1):140–145. doi: 10.1128/iai.23.1.140-145.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Rout W. R., Formal S. B., Collins H. Role of plasma filtration in the intestinal fluid secretion mediated by infection with Salmonella typhimurium. Infect Immun. 1976 Feb;13(2):470–474. doi: 10.1128/iai.13.2.470-474.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeiner J., Schlecht S. Molecular organization of the outer membrane of Salmonella typhimurium. Eur J Biochem. 1979 Feb 1;93(3):609–620. doi: 10.1111/j.1432-1033.1979.tb12861.x. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Houston C. W., Koo F. C., Peterson J. W. Characterization of Salmonella toxin released by mitomycin C-treated cells. Infect Immun. 1981 May;32(2):916–926. doi: 10.1128/iai.32.2.916-926.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann B., Reske K., Jann K. Heterogeneity of lipopolysaccharides. Analysis of polysaccharide chain lengths by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Eur J Biochem. 1975 Dec 1;60(1):239–246. doi: 10.1111/j.1432-1033.1975.tb20996.x. [DOI] [PubMed] [Google Scholar]
- Koupal L. R., Deibel R. H. Assay, characterization, and localization of an enterotoxin produced by Salmonella. Infect Immun. 1975 Jan;11(1):14–22. doi: 10.1128/iai.11.1.14-22.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel S. L., Robertson D. C. Factors affecting release of heat-labile enterotoxin by enterotoxigenic Escherichia coli. Infect Immun. 1979 Mar;23(3):652–659. doi: 10.1128/iai.23.3.652-659.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A., Hellerqvist C. G. Bacteriophage attachment sites, serological specificity, and chemical composition of the lipopolysaccharides of semirough and rough mutants of Salmonella typhimurium. J Bacteriol. 1971 Jan;105(1):57–64. doi: 10.1128/jb.105.1.57-64.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A. Studies of a receptor for felix O-1 phage in Salmonella minnesota. J Gen Microbiol. 1967 Aug;48(2):225–233. doi: 10.1099/00221287-48-2-225. [DOI] [PubMed] [Google Scholar]
- Lyman M. B., Steward J. P., Roantree R. J. Characterization of the virulence and antigenic structure of Salmonella typhimurium strains with lipopolysaccharide core defects. Infect Immun. 1976 Jun;13(6):1539–1542. doi: 10.1128/iai.13.6.1539-1542.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Valtonen V. V., Valtonen M. Role of O-antigen (lipopolysaccharide) factors in the virulence of Salmonella. J Infect Dis. 1973 Jul;128(Suppl):81–85. doi: 10.1093/infdis/128.supplement_1.s81. [DOI] [PubMed] [Google Scholar]
- Nakano M., Saito K. Chemical components in the cell wall of Salmonella typhimurium affecting its virulence and immunogenicity in mice. Nature. 1969 Jun 14;222(5198):1085–1086. doi: 10.1038/2221085a0. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Sandefur P. D., Peterson J. W. Isolation of skin permeability factors from culture filtrates of Salmonella typhimurium. Infect Immun. 1976 Sep;14(3):671–679. doi: 10.1128/iai.14.3.671-679.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandefur P. D., Peterson J. W. Neutralization of Salmonella toxin-induced elongation of Chinese hamster ovary cells by cholera antitoxin. Infect Immun. 1977 Mar;15(3):988–992. doi: 10.1128/iai.15.3.988-992.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., MacAlister T., Costerton J. W., Cheng K. J. Permeability of lipopolysaccharide-deficient (rough) mutants of Salmonella typhimurium to antibiotics, lysozyme, and other agents. Can J Microbiol. 1974 Aug;20(8):1135–1145. doi: 10.1139/m74-176. [DOI] [PubMed] [Google Scholar]
- Sedlock D. M., Deibel R. H. Detection of Salmonella enterotoxin using rabbit ileal loops. Can J Microbiol. 1978 Mar;24(3):268–273. doi: 10.1139/m78-046. [DOI] [PubMed] [Google Scholar]
- Sedlock D. M., Koupal L. R., Deibel R. H. Production and partial purification of Salmonella enterotoxin. Infect Immun. 1978 May;20(2):375–380. doi: 10.1128/iai.20.2.375-380.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O., Edebo L. Phagocytosis of mutants of Salmonella typhimurium by rabbit polymorphonuclear cells. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(4):481–488. doi: 10.1111/j.1699-0463.1972.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Tannock G. W., Blumershine R. V., Savage D. C. Association of Salmonella typhimurium with, and its invasion of, the ileal mucosa in mice. Infect Immun. 1975 Feb;11(2):365–370. doi: 10.1128/iai.11.2.365-370.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weisberg P. B., Carlson G. M., Cohen S. Effect of Salmonella typhimurium on myoelectrical activity in the rabbit ileum. Gastroenterology. 1978 Jan;74(1):47–51. [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]



