Abstract
Biomechanical regulation of tumor phenotypes have been noted for several decades, yet the function of mechanics in the co-evolution of the tumor epithelium and altered cancer extracellular matrix has not been appreciated until fairly recently. In this review, we examine the dynamic interaction between the developing epithelia and the extracellular matrix, and discuss how similar interactions are exploited by the genetically modified epithelium during tumor progression. We emphasize the process of mechanoreciprocity, which is a phenomenon observed during epithelial transformation, in which tension generated within the extracellular microenvironment induce and cooperate with opposing reactive forces within transformed epithelium to drive tumor progression and metastasis. We highlight the importance of matrix remodeling, and present a new, emerging paradigm that underscores the importance of tissue morphology as a key regulator of epithelial cell invasion and metastasis.
Keywords: tension, integrins, migration, extracellular matrix, epithelial cell, force
Introduction
The majority of adult human cancers originate from the epithelial cells that line the surfaces of our bodies. Recent work has highlighted the mechanical changes associated with epithelial carcinomas, including elevated extracellular matrix (ECM) stiffness and increased interstitial pressure. Despite the association between mechanical force and tumors, however, cancer research has historically focused primarily on defining the role of genetic and biochemical changes in tumor progression. Nevertheless, a novel paradigm has emerged over the past few decades that brings a three-dimensional (3D) tissue perspective to epithelial cancers and that views cancer as a dynamic organ that exploit similar biochemical and biomechanical stimuli utilized during development to drive tumor evolution (Lelievre et al., 1996; Wiseman and Werb, 2002; Nelson and Bissell, 2006).
Among the greater than 200 cell types in our bodies, epithelial cells have unique interactions with their microenvironment such that they maintain three distinct types of interfaces along their cell surfaces. The apical surface of a simple epithelium is free of adhesion contact, whereas the lateral surfaces of the cells interact with neighboring cells through adhesions such as gap and adherens junctions. The basal surface of the epithelium, on the other hand, interacts with a specialized ECM that is rich in extracellular matrix laminin protein and is called the basement membrane (BM). The entire epithelium together with the BM thereafter is embedded within a collagen rich interstitial matrix. Through these different adhesive interactions, biochemical and biomechanical cues regulate epithelial cell fate to direct the development of the tissue and contribute to disease (Helmlinger et al., 1997; Farge, 2003; Keller et al., 2003; Brancaccio et al., 2006). At the cellular level there exist a number of molecular mechanisms through which cells sense and transduce biochemical and mechanical cues that are localized within the membrane, the cytoskeleton and at specific cell-matrix complexes (Hamill and Martinac, 2001; Tamada et al., 2004; Chiquet et al., 2007). Although we know much about the effect of biochemical cues on epithelial behavior, we know relatively less about how force could influence cell and tissue fate. Nevertheless, branched epithelial structures, such as the mammary gland ductal tree, present multiple opportunities for force sensing and transmission that undoubtedly modify its structure, integrity and function. For instance, epithelial ducts are often embedded within an architecturally complex extracellular microenvironment that broadly encompasses cellular (fibroblasts, adipocytes, endothelial cells and immune cells) and non-cellular (structural extracellular and soluble factors such as cytokines and growth factors) components. In the context of tissues such as the breast, lung and heart, mechanical loading can physically alter the conformation of extracellular receptor complexes present in stromal cells such as fibroblasts and in the epithelial cells. In response to force, domains within these adhesion complexes can be stretched or compressed, either directly or indirectly, and these biomechanical changes thereafter elicit alterations in the structure and function of the ECM receptor complexes to actively influence signaling. Force can also modify the activity and function of other membrane complexes such as growth factor receptors, cytokine receptors, ion channels and cell–cell junctional complexes (Silver and Siperko, 2003). During tumor progression, importantly, the relationship between the epithelium and the ECM becomes increasingly perturbed. As tissues transform and metastasize, a dynamic interaction is established wherein changes in the ECM enable cells to undergo uncontrolled cell proliferation, resist apoptosis and acquire an invasive phenotype.
Epithelial tumor cell invasion and metastasis is the leading cause of mortality amongst cancer patients. Before the tumor epithelium can move away from its site of origin and become metastatic, tumor cells must first detach from neighboring cells, remodel the ECM and attain a migratory phenotype. In this review, we examine how directed ECM remodeling conspires with genetically transformed cells to promote cancer progression and metastasis. Although changes in the makeup of the tumor microenvironment ultimately affect all stages of tumor progression, this review specifically focuses on describing how forces generated between cells and the ECM influence cell orientation at the tissue, cell and molecular level to regulate tissue homeostasis. We begin the review by examining how epithelial cell/ECM interactions evolve during development to produce the different patterns seen in tissues that undergo morphogenetic programs such as branching morphogenesis. We describe the unique function that the BM has in the establishment of cell and tissue polarity. We then outline epithelial cell transformation and detail the reciprocal changes that occur between the epithelial cells and the ECM during tumor evolution, and discuss how these affect the morphology, orientation and metastatic behavior of transformed tissues. Finally, we present various technologies that have been developed to help us understand how force could modulate cancer progression.
Dynamic reciprocity in development
The development of distinct tissues and specialized organs require precise spatial and temporal coordination of cell growth and differentiation. The heterogeneity of cell types with distinct positioning within epithelial tissues is the result of molecular pathways that establish tissue polarity to appropriately orient epithelial cells so that the cell’s apical surface faces the luminal space and the basal surface is positioned towards the basal lamina. The development of this cellular orientation within a tissue depends upon a combination of internal and external biochemical and biophysical cues. In this regard, studies examining the phenomena of branching morphogenesis have provided important insight into how microenvironmental signals direct the spatial orientation of cells within epithelial tissues such as the lungs, kidneys and the mammary gland. Thus to generate epithelial structures that bud or branch, a cell or group of cells within the epithelium must correctly interpret microenvironmental cues to proliferate or migrate with the proper orientation to the established plane of tissue growth while maintaining the growth of the neighboring cells along the established polarity plane. Clearly biochemical signals such as growth factors or hormones have a key function in dictating tissue patterning (Chrenek et al., 2001; Sternlicht et al., 2006; Robinson, 2007). Nevertheless, biophysical cues also induce local changes in developmental processes such as branching morphogenesis, affecting a variety of cellular processes such as the rate of proliferation, the establishment of cell and tissue polarity, determination of cell shape and even specification of cell fate (Wang et al., 2001; Wong et al., 2003; Paszek et al., 2005).
The development of epithelial tissues is tightly coupled to the production of the BM and interstitial matrix at all stages ranging from the newly formed embryonic endoderm and ectoderm (Leivo, 1983) to the remodeled pregnant mammary gland in the adult organism (Watson, 2006). As epithelial cells proliferate and differentiate, they remodel the BM and interstitial matrix to facilitate proper development and orientation in a process termed dynamic reciprocity (Bissell et al., 1982). The ECM affects the behavior of cells through a variety of biochemical and biophysical mechanisms. For example, the composition of the BM and the interstitial matrix and the topology of the ECM cooperate to determine cell phenotype by triggering biochemical responses within a cell that alter gene expression, as well as protein synthesis and function (Kleinman et al., 2003; Larsen et al., 2006). ECM components also modulate cell phenotype by generating tensional forces within the matrix, as well as through matrix topology cues, that is, the spatial orientation of matrix fibrils. Cells interpret and respond to physical cues in their external matrix by generating tensional forces through cytoskeletal remodeling and actomyosin contractility by a process termed mechanoreciprocity (Paszek and Weaver, 2004; Polte et al., 2004; Ghosh et al., 2007). In this fashion, mechanoreciprocity critically modulates branching morphogenesis of epithelial tissues by regulating cell shape, polarity, motility and proliferation.
The interplay between biochemical and biophysical cues from the ECM and their influence on developmental processes such as epithelial branching morphogenesis has been elegantly described during lung alveolar expansion and branching morphogenesis (Cardoso and Lu, 2006). During lung development, the topology of the matrix governs the formation of epithelial buds that direct the fractal arrangement of ducts found in the mature lung. Force regulates ductal development as revealed by experiments using mouse pulmonary rudiments, which require cellular tension to undergo epithelial bud formation. Studies have demonstrated that local thinning of the BM, possibly induced through mechanical force, predicts the localization of epithelial cell budding revealed by the presence of a thicker BM in the quiescent tissue regions surrounding the areas undergoing localized budding. That tensional forces generated by the epithelial cells themselves could drive branching morphogenesis was illustrated through the use of inhibitory pharmacological agents that modify the activity of Rho-associated kinase (ROCK), myosin light chain kinase, myosin ATPase and via microfilament toxins which showed that following treatment with these agents, actomyosin tension was greatly diminished and epithelial budding was tempered with minimal effects on BM integrity. In contrast, when Rho GTPase was activated using CFN-1, epithelial budding was enhanced and branching morphogenesis was stimulated, with evident localized thinning of BM that correlated with budding and cleft formation (Moore et al., 2005). Biochemical assays further revealed that branching morphogenesis is not solely due to reduced Rho activity but was more closely associated with cell contractility. Interestingly, these studies showed that the rate of epithelial or mesenchymal cell proliferation was not widely affected by these treatments, despite extensive alterations in tissue patterning.
The mammary gland is a unique and dynamic organ that undergoes a variety of different gross morphological changes during development differentiation, and pregnancy. Mammary gland development is governed by biochemical and biophysical cues that influence all stages of branching morphogenesis, differentiation and involution. As with the lung, the mammary epithelium is subject to a dynamic interplay between epithelial cells and the ECM stroma. Thus, either accelerating or inhibiting ECM turnover by modulating the activity or levels of matrix metalloproteases (MMPs) has a profound effect on the branching phenotype of the mammary gland. For example, inhibition of MMP-dependent ECM turnover by pharmaceutical inhibition or through genetic ablation or mutation reduces the degree of epithelial branching (Reviewed in Unger and Weaver, 2003; Page-McCaw et al., 2007; Butcher et al., 2008), whereas the introduction of an ectopically expressed MMP enhances ECM turnover and induces precocious branching morphogenesis (Simian et al., 2001). Such experimental observations imply that ECM integrity is necessary for epithelial tissue homeostasis and that localized remodeling of the BM is required for tissue patterning. Similar to the lung epithelium, the mammary epithelium generates tension to modulate MEC behavior through actin cytoskeleton remodeling and through activation of actomyosin elements (Paszek et al., 2005) (reviewed in Paszek and Weaver, 2004). For instance, primary cultures of murine MECs differentiate and assemble polarized growth-arrested acini that differentiate in response to lactogenic hormones when embedded in floating type I collagen gels. In contrast, these same cells assemble non-polarized continuously growing colonies when embedded in mechanically restrained collagen I gels (Barcellos-Hoff et al., 1989). Biochemical cues from the BM are also important for normal tissue behavior as emphasized by the observation that a mixed MEC cell population, isolated from pre-lactating mouse mammary glands, neither polarize nor form functionally differentiated acini, unless they retain the ability to produce and assemble their own BM (Emerman and Pitelka, 1977; Barcellos-Hoff et al., 1989).
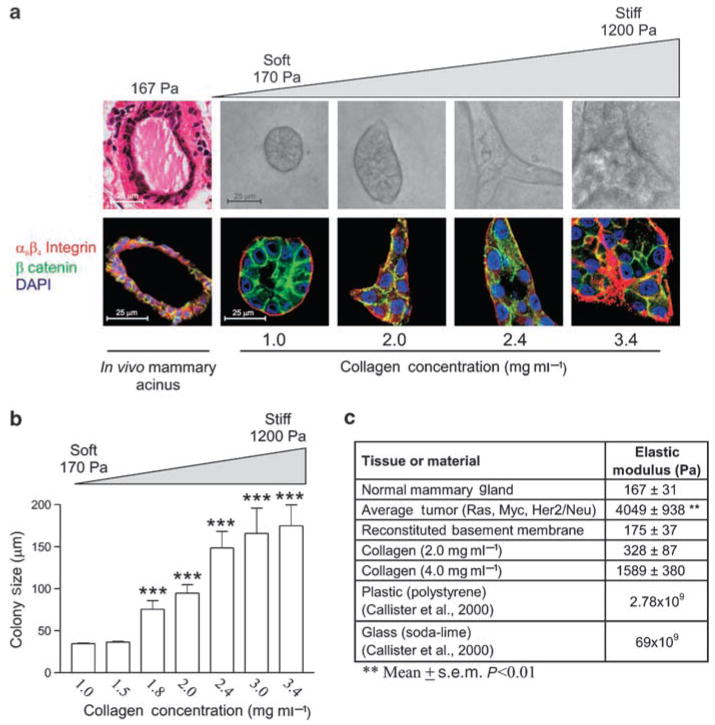
Experiments performed in our laboratory have highlighted the interplay between physical force from the ECM and MECs during epithelial morphogenesis. Human MECs form growth-arrested, polarized, acini with cleared lumens when grown within compliant collagen I + recombinant BM (Figure 1a, first two columns). Yet we could show that as the matrix is progressively stiffened, MECs assemble colonies in which cell–cell junction and tissue polarity are compromised, luminal clearance fails and growth control is perturbed (Figure 1a. latter 3 columns, 1b). Importantly, we observed that the MECs within the structures interacting with the most compliant matrices form immature nascent focal contacts that mature into focal adhesions only when the ECM is significantly stiffened or the cells are exposed to exogenously applied force. We could additionally show that this process depends upon actomyosin contractility and substantial actin remodeling through experiments illustrating that introducing active V17Rho promotes focal adhesion maturation in MECs interacting with highly compliant matrices (Paszek et al., 2005).
Figure 1.
MEC growth and morphogenesis reflect changes in matrix stiffness. (a) Confocal images of MEC grown in 3D cultures. As MECs grow in progressively stiffened matrices (170–1200 Pa), MEC morphology becomes progressively disrupted. Irregular MEC changes are characterized by disrupted cell–cell adherens junctions and tissue polarity, illustrated by a loss of β-catenin (green) and loss of β4 integrin (red) organization (nuclei =blue). (b) Normal MEC acini reaches a proliferative growth-arrested phase when cultured in soft gels that is lost as they are cultured in stiffer matrices. (c) Measured elastic modulus for a variety of substrates. Values represent the mean ± s.e.m. of four measurements from multiple mice and gels. **P≤0.01, ***P≤0.001. (Reproduced with modification and proper permission obtained from Elsevier as published in Paszek et al., 2005.)
Although the idea of mechanoreciprocity is relatively new, evidence that ECM and actomyosin tensional force could influence cell shape and behavior has been observed for decades (Rodriguez-Boulan et al., 1983; Ingber et al., 1986). To this end Madin–Darby Canine Kidney (MDCK) cells are a valuable resource to study the molecular mechanisms directing the establishment of tissue polarity. Using the MDCK cell system, studies examining the phenomenon of polarity reversal have illustrated how the BM provides critical cues necessary for establishing apical–basal polarity. Thus, MDCK cells grown in 3D collagen gels form polarized cysts with appropriate apical and basal orientation. However, in the absence of an exogenous ECM, MDCK cells form cysts with reversed polarity such that the apical surface of the cell faces the periphery of the cyst whereas the basal surface is oriented towards the lumen face which contains deposited BM proteins (Chambard et al., 1984; Wang et al., 1990a). Yet, when these reverse polarity cysts were challenged with a second ECM cue by re-embedding the cysts within 3D collagen gels, appropriate tissue polarity could be induced, suggesting BM is a critical regulator of tissue polarity. Importantly, in these experiments epithelial re-polarization was contingent upon loss of the inappropriate apical BM cue, because inhibition of luminal BM degradation compromised acini morphogenesis and led to the generation of aberrant multiple de novo lumens formation (Wang et al., 1990a, b). The importance of BM in polarity was directly demonstrated by studies showing how MDCK fail to polarize when BM synthesis and assembly are inhibited (O’Brien et al., 2001).
Transformation
Epithelial tissue homeostasis is defined as the maintenance of a polarized cellular monolayer in which cell growth and survival are tightly regulated and differentiation is promoted. Consistently, loss of tissue integrity is a hallmark of cancer, and compromised cell and tissue polarity indicates epithelial cell dedifferentiation that often precedes malignant transformation. Given that normal tissue homeostasis depends upon appropriate stromal–epithelial interactions, it is not surprising that tumor progression is frequently associated with changes in the extracellular stroma and BM. Indeed, tumors are characterized by profound ECM remodeling that alters their composition, topology and mechanical properties.
The progression of epithelial cancers from normal to malignant disease is characterized by genetic changes in the epithelium as well as modifications within the stroma termed tissue desmoplasia. Indeed, the induction of tissue desmoplasia can drive cancer progression, and inhibiting the reactive stroma can restrict and in some instances even prevent, tumor development (Bissell et al., 1999; Unger and Weaver, 2003). Thus, cancer is a disease, the behavior of which is regulated by biochemical and biophysical cues not only at the cellular level, but also at the tissue, organ and system level. For instance, uncontrolled epithelial cell proliferation that increases tumor cell mass also elevates compressive forces on the BM and the surrounding ECM that can induce growth factor and MMP secretion, enhance growth factor and cytokine signaling and reduce BM integrity to enhance cancer cell invasion (Paszek et al., 2005; reviewed in Paszek and Weaver 2004). Factors released by tumor cells can also activate the fibroblasts within the stroma and stimulate inflammatory cells to induce tumor cell migration. Activated fibroblasts deposit and remodel ECM proteins including Collagens I, III and IV, fibronectin, elastin and tenascin (Bissell et al., 2002; Coussens and Werb, 2002; Wiseman and Werb, 2002). Increased deposition of matrix components and tumor mass expansion coupled with global and local changes in the quality and topology of the ECM, collectively generate a microenvironment that can be up to an order of magnitude stiffer than that observed in normal tissues, and that has been correlated with high histological tumor grade (Paszek et al., 2005; Rutkowski and Swartz, 2007; Samani et al., 2007). Thus, fibrotic premalignant lesions are consistently 3- to 6-fold stiffer than normal tissue and high grade ductal carcinomas are up to 13-fold stiffer (Samani et al., 2007). Such changes in the material properties of the tissue are likely the result of chronic ECM remodeling, increased cell mass and altered tumor cell rheology that profoundly alter tumor phenotype and pathophysiology.
Loss of polarity, epithelial-to-mesenchymal transition (EMT), resistance to apoptosis and cell proliferation are all force-dependent phenotypes. Although the overall importance of mechanical force to tissue behavior is generally acknowledged, much remains to be discovered about cell and tissue mechanotransduction and little is known about how such mechanosensory signals might guide cellular behavior. Investigators are just beginning to elucidate how mechanical stimulation induces structural, compositional and functional changes at the cellular level and how these physical cues could alter the structural integrity and function of differentiated tissues. What is known is that compressive forces are generated by the expanding fibrotic tumor mass and reciprocal resistance to the cellular expansion by the extracellular tissue adjacent to the transformed tissue (Volokh, 2006). Mechanical loading in the form of compression force alters gene expression and modifies cell signaling and can potentially induce MMP-dependent ECM remodeling. For instance, IL-8 and NF-κB ligand production are increased by compression (Ichimiya et al., 2007; Muroi et al., 2007) as is FGF-mediated ERK activation (Vincent et al., 2007). Indeed, the significance of compression force as a key regulator of tumor cell behavior has been illustrated by studies on TGFβ function. These TGFβ studies showed how dynamic compression and contraction can lead to the activation of latent ECM bound TGFβ, which thereafter stimulates a fibrotic response by the tumor-associated fibroblasts that then feeds back to induce epithelial-to-mesenchymal transition of the tumor (Leivonen and Kahari, 2007; Willis and Borok, 2007; Wipff et al., 2007). TGFβ activation also stimulates the production of matrix remodeling enzymes such as MMPs and lysyl-oxidase (LOX) to alter matrix topology and induce matrix stiffening that, in turn, can also alter tumor cell behavior (Heinemeier et al., 2007). In this regard, studies that have examined hypertropic scars could show that MMP-9 and MMP-28 secretion and activity is enhanced when the tissue is mechanically loaded, and emphasize how compression force can induce ECM remodeling (Reno et al., 2002, 2005).
The ECM stroma adjacent to an expanding tumor mass responds to the tumor-generated compression force by exerting a reciprocal resistance force on the expanding tumor mass. This tumor-initiated resistance force increases tensional forces within the tumor cells to alter their behavior, in part by regulating the activity of various biochemical-signaling cascades, and also by actomyosin-induced cytoskeletal reorganization. For instance, tensional forces are transmitted from tumor cell to tumor cell though adhesion plaques, and within the tumor cells through the cytoskeleton to cell–ECM adhesions (Katsumi et al., 2004). In addition, the expanding tumor mass and actively migrating tumor cells can each independently deliver direct forces to cell–ECM and cell–cell adhesion plaques, thereby impacting tumor cell behavior by physically distorting the ECM (Figure 2).
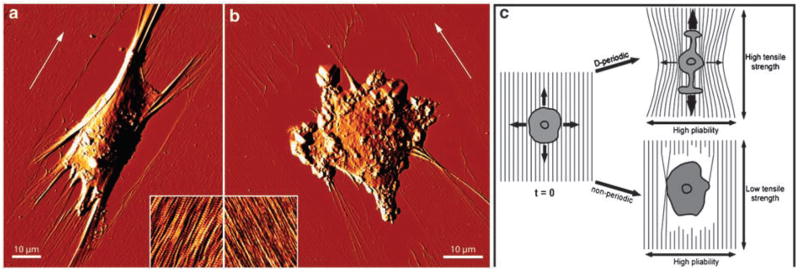
Figure 2.
SAOS-A2 cells were seeded on (a) D-periodic, or (b) non-periodic collagen matrices and allowed to spread for 45 min. Subsequently, cells were glutaraldehyde/paraformaldehyde-fixed and AFM deflection images representing the error signal were recorded while scanning the sample in contact mode. White arrows indicate the orientation of the collagen fibrils within the matrices. (a) On D-periodic collagen, cells polarize strongly and deform the D-periodic matrix perpendicular to the fibril direction, as indicated by the exposed surface. Collagen fibrils are bundled at the front and back of the cell without rupturing. The inset (3 μm × 3 μm) shows an AFM contact mode topograph of ≈ 3 nm thick collagen matrices assembled on freshly-cleaved mica in the presence of potassium ions. (b) Cell adhesion causes frequent rupture of non-periodic collagen fibrils, as demonstrated by the frayed appearance of the fibril ends and the widespread exposure of the mica surface in the cell periphery. The inset (3 μm × 3 μm) shows an AFM contact mode topograph of ≈ 3 nm thick collagen matrices assembled in the absence of potassium ions. (c) Model illustrating how differences in matrix rigidity between D-periodic and non-periodic collagen matrices affect cell polarization. Upon seeding, cells explore the mechanical properties of the surrounding D-periodic or non-periodic matrix by forming protrusions in all directions. Subsequently, cells form adhesion complexes and begin to exert pulling forces on the matrix. The high tensile strength of D-periodic collagen fibrils permits the establishment of strong cellular traction along the fibril direction. In contrast, the high pliability of the fibrils prevents traction when cells pull perpendicular to the fibril orientation. As a result of the directional traction the cells elongate. The low tensile strength of non-periodic collagen fibrils avoids traction build-up in the fibril direction, preventing cells from polarizing (Reproduced with modifications and proper permission obtained from Elsevier as published in Friedrichs et al., 2007)
External tensile forces can effect changes in cellular phenotype either by altering biochemical signaling within cells to alter gene expression and protein function or by inducing cytoskeletal remodeling to change cell shape and signaling, modify tissue organization and alter cell growth, survival and motility. Thus, conformational changes in membrane cytoskeletal proteins such as vinculin, that are induced by tensional force, influence signaling within the cell by altering the activity of ion channels or by promoting integrin clustering and activation to alter cytokine and growth factor receptor signaling (Paszek et al., 2005; Gupta and Grande-Allen, 2006). Examples of these effects include experiments showing how αVβ3 integrins cluster in response to the application of an extracellular tensile force that potentiate JNK signaling (Katsumi et al., 2005). These data demonstrate how an elevated tensile force can induce VEGF expression to drive vascular growth (Quinn et al., 2002). Indeed, cyclic strain can induce p38 SAPK2, ErbB2 and AT1 activity while simultaneously stimulating PDGF production in a PI3K-dependent manner (Nguyen et al., 2000; Adam et al., 2003). Wnt, β-catenin, IGF-1, CREB, c-myc and Stat1/3 are examples of other intracellular signaling molecules whose activity can be modulated by external tensional force (Avvisato et al., 2007; Reichelt, 2007; Triplett et al., 2007). Given space limitations, we have chosen not to delve into the details of how force could alter cell signaling and elicit biochemical changes in proteins and nucleic acids and instead refer the reader to several excellent, recent reviews (Pedersen and Swartz, 2005; Wang et al., 2006; Schwartz and Desimone, 2008).
Integral membrane proteins such as integrins or dystroglycan couple extracellular tensional forces with intracellular cytoskeletal tension (Muschler et al., 2002; Katsumi et al., 2004). β-integrins for instance respond to extracellular force stimuli by forming intracellular interactions with cytoskeletal adaptor proteins such as talin or α-actinin and thereafter recruit a plethora of adhesion plaque proteins and cytoskeletal interactions to reciprocally transduce extracellular and intracellular forces. Force-dependent ECM-mediated integrin ligation activate and oligomerize integrins, stimulate Rho GTPases and drive cytoskeletal rearrangements that promote the maturation of focal adhesions and influence adhesion and growth factor signaling (Figure 3). Mature focal adhesions generate intrinsic cellular traction forces through actomyosin-induced contractility and through cytoskeletal remodeling (Beningo and Wang, 2002; Mogilner and Oster, 2003). The small GTPases Ras, Rho and Rac respond to tensile stimuli and can mediate focal adhesion formation by promoting contractility thereby inducing cell proliferation, survival and motility (Clark et al., 1998; Cox et al., 2001; del Pozo et al., 2004).
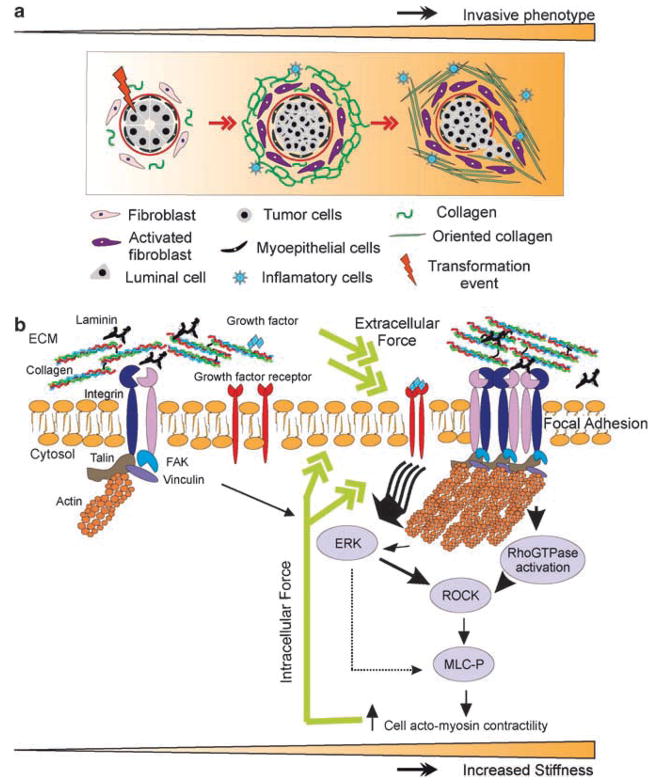
Figure 3.
Malignant transformation of mammary epithelial cells is regulated by matrix stiffness. Breast transformation ensues through progressive acquisition of genetic alterations in the luminal epithelial cells residing within the mammary ducts. The tissue stroma responds to these epithelial alterations by initiating a desmoplastic response that is characterized by activation and transdifferentiation of fibroblasts, infiltration of immune cells, increased secretion of growth factors and cytokines, and elevated matrix synthesis and remodeling that manifests as matrix stiffening. (a) Cartoon depicting the stages of breast tumorigenesis (from left to right; normal ducts, ductal carcinoma in situ and invasive phenotype), highlighting key desmoplastic changes within the tissue stroma. (b) Force-dependent focal adhesion maturation mediated by elevated tumor matrix stiffness. Integrins are bidirectional mechanosensors that integrate biochemical and biophysical cues from the matrix and the actin cytoskeleton and transduce cell-generated force to the surrounding microenvironment. Activated integrins bind to ECM proteins via cooperative interactions between their alpha and beta extracellular domains and form nascent highly dynamic adhesion signaling complexes. In response to external mechanical force or elevated cell-generated contractility integrin clustering is enhanced and the recruitment of multiple integrin adhesion plaque proteins including talin and vinculin is favored. These, in turn, associate with the actin cytoskeleton and multiple signaling proteins including focal adhesion kinase (FAK), Src family kinases, and integrin-linked kinase, to promote cell growth, survival, migration and differentiation. Matrix stiffening, which reflects elevated matrix deposition, linearization and cross-linking, can co-operate with oncogenic signaling to enhance cell-generated contractility to foster integrin associations and focal adhesion maturation. Maturation of focal adhesions promotes cell generated forces by enhancing Rho GTPase and ERK-mediated acto-myosin contractility–which feed forward to further promote integrin clustering and focal adhesion assembly and transmit acto-myosin-generated cellular forces to the ECM. (Reprinted with appropriate permission obtained from Elsevier as published in Kass et al., 2007.)
Traction force microscopy is a recently developed tool that permits the visualization and quantification of actomyosin and cytoskeletal generated forces. Thus, mechanoreciprocity can be clearly observed and quantified using traction force microscopy, which demonstrates how cells generate actomyosin contractility tensional forces of increasing magnitude in response to matrices of incremental stiffness. This reciprocal relationship between the cell and its mechanical substrate can influence the behavior and phenotype of the cell by altering the degree of cell spreading, the rate of cell growth, the amount of cell survival and even the speed and direction of cell motility (Wang et al., 2001; Wong et al., 2003; Paszek et al., 2005).
Migration and metastasis
Metastasis is the major cause of cancer fatality, underscoring the urgency of understanding the molecular mechanisms regulating this process. A key step in tumor metastasis is destabilization of tissue structure and thereafter the directed migration of tumor cells towards the vasculature or lymphatics. Both of these steps require dynamic modulation of cell and tissue polarity and are influenced by force. What we and others now appreciate is that the mechanical and topological features of the ECM influence tumor metastasis by promoting directed tumor cell invasion into the parenchyma and by fostering rapid and efficient tumor cell extravasation and colonization at distant tissue sites. Evidence to support these conclusions is given by work showing how matrix stiffness and topology can enhance cell migration speed and facilitate directed cell motility. For example, in one study fibroblasts seeded on a substrate made up of materials of two distinct stiffnesses, with similar ligand densities, durotaxed towards the stiffer substrate, regardless of matrix composition or density. The fibroblasts seeded on the compliant substrate could ‘sense’ the stiffer substrate, project membrane structures towards the rigid matrix, and showed persistent migration toward the stiffer substrate. In the vicinity of the stiff matrix the associated cells exhibited greater cell motility yet remained locally adherent (Lo et al., 2000). Intriguingly, micro-aspiration experiments in which the substrate was deformed using a micro pipettor demonstrated how the directionality of this fibroblast movement was enhanced by tensional forces induced within the matrix (that is, a micropipettor was used to gently pull the substrate away from the cell to generate a directed tensile force (Lo et al., 2000)). Similarly, vascular endothelial cells and smooth muscle cells exhibit directed and rapid cell migration termed ‘durotaxis’ in response to a gradient of matrix stiffness, emphasizing how this mechanically regulated process is likely highly conserved (Wong et al., 2003).
Cells exhibit widely divergent responses to an exogenous force and evidence to date emphasize how mechanoresponsiveness is cell and tissue specific (Yeung et al., 2005; Wells and Discher, 2008). For example, neutrophils exert a very low force and are themselves highly sensitive to an exogenous force such that they respond to even small changes in sheer stress, typically in the range of 1 Pa (Fukuda and Schmid-Schonbein, 2003). On the other hand, mechanically resistant cells such as osteoblasts require much larger exogenous force stimuli, typically in the 20 MPa range, before they will modify their behavior (Grodzinsky et al., 2000). Indeed, tissue pathology is often accompanied by an altered mechanoresponsiveness of the cells within the tissue. This phenomenon has been observed most prominently during transformation in which the rheology and mechanosensitivity of the cancer cells are known to differ substantially from that of their non-transformed counterparts (compare Figures 1a–c). In this regard, we showed that transformed human MECs spread appreciably, migrate rapidly and exert significantly higher actomyosin-dependent cellular force as compared with nonmalignant MECs interacting with a similar compliant matrix (Paszek et al., 2005; Kass et al., 2007). These data imply that small changes in ECM composition or remodeling such as localized fibrillogenesis could theoretically induce profound changes in tumor cell behavior including altered cell polarity and directed cell migration.
Matrix topology-directed migration has been observed in MECs in vivo and likely facilitates tumor cell metastasis. Using two-photon intravital imaging coupled with second harmonic generation the directed, rapid epidermal growth factor-stimulated migration of MECs along prominent collagen bundles adjacent to blood vessels has been observed (Condeelis and Segall, 2003; Ingman et al., 2006; Wyckoff et al., 2006). Although the molecular mechanisms regulating such directed cellular migration in vivo have yet to be delineated, bundled, linearized collagens are characteristically stiff, while we showed that matrix stiffness enhances EGF-induced signaling (Paszek et al., 2005; unpublished observations) and increases the speed of cell migration ((Wong et al., 2003); unpublished observations). These and other data imply that the altered matrix material properties and changes in ECM topology associated with tumor progression could foster directed tumor cell migration towards the vasculature to facilitate tumor cell intravasation and metastasis. Although it is well known that the stroma surrounding developing breast tumors is stiffer and the collagen fibrils are highly oriented and bundled (Demou et al., 2005; Paszek et al., 2005; Samani et al., 2007), to date no direct evidence exists to substantiate such claims. In this regard, using atomic force microscopy to probe the mechanical properties of developing transgenic tumors, we could show that matrix stiffness increases in association with tumor progression and that the stroma at the front of the invading tumor, where we and others have observed prominent linear collagen bundling, is substantially stiffer than the noninvasive edge. We also determined that the stroma adjacent to peripheral bloody vessels, in regions where rapidly migrating breast tumor cells have been observed, is also quite rigid (unpublished observations; (Condeelis and Segall, 2003; Ingman et al., 2006; Wyckoff et al., 2006)).
Matrix orientation and mechanical integrity influence cell migration by modifying the direction and composition of integrin adhesions. Thus, fibroblasts orient themselves on rigid collagen I fibers so that they are able to generate maximal traction forces that stabilize integrin adhesions to promote focal adhesion maturation in the direction of the collagen fiber alignment (Figures 3a and c). This phenotype is not favored if the cells are perpendicularly oriented to the fibrils or if they interact with collagen gels of low tensile strength where cellular force is neither reinforced nor greatly resisted (Figures 3a–c). Consistently, atomic force microscopy has shown that traction forces directed along parallel collagen I fibers develop in cells plated on these oriented substrates (Friedrichs et al., 2007). The directed maturation of focal adhesions permit cells to adopt a shape and orientation that optimizes their migration in the direction of collagen fiber alignment.
Although tumor cells respond to matrix material properties and topology, they are not merely passive participants and themselves respond to the mechanical and topological properties of the ECM. Tumor cells actively remodel their local extracellular microenvironment either by directly releasing ECM remodeling enzymes such as MMPs, serine and cysteine proteases or hyaluronidases (Lopez-Otin and Matrisian, 2007; Lokeshwar et al., 2008; Stern, 2008), or indirectly by stimulating stromal cells to deposit, process and reorganize their ECMs. Thus, the ECM-associated remodeling observed with tumor desmoplasia is the net result of stromally-induced matrix remodeling as well as matrix deposition, degradation and cross-linking mediated by the tumor cells (Egeblad and Werb, 2002; Strongin, 2006; Payne et al., 2007). Indeed, tumor progression is associated with altered expression of a number of ECM proteins including cellular fibronectin, collagens I, III and IV, tenascin and various proteoglycans produced by the cellular stroma and the tumor cells that collectively promote tumor migration, proliferation and survival (Bissell et al., 2002; Coussens and Werb, 2002; Wiseman and Werb, 2002). Increased expression and activity of MMPs expressed by fibroblasts, infiltrating immune cells and the transformed cells together release growth factors trapped in the stromal matrix to stimulate invasion, and contribute significantly to ECM remodeling to facilitate cell invasion and metastasis through the vasculature. For instance, MMP2 and -14 secreted by tumors cells can cleave pro laminin-5 to expose a cryptic site within laminin-5 that promotes cell migration (Giannelli et al., 1997). Both fibroblasts and tumors secrete matrix cross-linkers that alter the topology of the ECM and enhance its material properties or stiffness. In particular, TBGβ and HIF-1α induce the expression of lysyl oxidases (LOX), which in turn cross-link type I and III collagens to increase their stiffness (Erler and Giaccia, 2006). The importance of matrix cross-linking in metastatic disease is underscored by the observation that increased LOX expression is positively associated with the most advanced stage of renal cell carcinoma and highly expressed in invasive and metastatic breast cancer cell lines (Kirschmann et al., 2002). In fact, ductal breast carcinomas and fibrotic tissue show elevated levels of LOX (Decitre et al., 1998) and inhibiting LOX activity reduces tumor cell invasion in vitro (Kirschmann et al., 2002), and reduces breast cancer cell metastasis in vivo (Erler and Giaccia, 2006). Indeed, enzymes and proteins such as transglutaminase and the proteoglycans lumican and decorin also modify tumor cell behavior and might do so by modifying the mechanical properties and topology of the ECM (Decitre et al., 1998; Wiseman and Werb, 2002; Akiri et al., 2003; Alowami et al., 2003; Eshchenko et al., 2007). Thus, a dynamic physical and biochemical dialog between the tumor cells and their microenvironment contribute to tumor progression and metastasis.
Investigating epithelial mechanotransduction
Future research in the area of cell mechanobiology will require novel experimental and theoretical methodologies to determine the type and magnitude of the forces experienced at the cellular and sub-cellular levels, and to identify the force sensors/receptors that initiate the cascade of cellular and molecular events. To investigate epithelial morphogenesis and malignant transformation, 3D culture systems have been developed to model the in vivo environment of epithelial cells. Original 3D culture models were designed to completely embed epithelial cells within a polymerized ECM to closely recapitulate the structure and composition of the polarized structures in vitro. These models have now been modified to study how mechanical properties affect morphogenesis and biochemical processes (Hebner et al., 2007). We now present an overview of these 3D models and the modifications used to study mechanotransduction. In addition, we discuss engineering approaches to modulate the physical forces applied to cells.
3D culture systems
As discussed in this review, ECM composition and architecture are frequently altered in breast transformation and can influence epithelial cell growth, differentiation and migration. Methods to study these biological processes traditionally involve culturing isolated cells on a 2D surface. However, cells in vivo exist in a complex 3D microenvironment. To more accurately study epithelial cell morphogenesis in vitro, models have been developed to recapitulate the in vivo 3D environment. The simplest 3D models involve embedding a single type of cell in a biocompatible scaffold. These biocompatible scaffolds provide cells with a prefabricated ECM, which is often modifiable by the embedded cells. Scaffolding materials commonly used for complete embedment of epithelial cells include rBMs produced and isolated from Engelbreth–Holm–Swarm mouse tumor matrices, collagen I and fibrin. Laminin 1, collagen IV, entactin and heparin sulfate proteoglycans are the major components of the EHS rBM. rBM has been utilized extensively to study morphogenesis and transformation in normal, non-transformed epithelial cells, such as MDCK and MCF-10A cells (Petersen et al., 1992; Weaver et al., 1995, 1997; Debnath et al., 2003). Indeed, when various non-transformed epithelial cells (primary and cell lines) are mixed with rBM they form polarized structures, with hollow lumens, and assemble their own endogenous BM that is surrounded by the polymerized gel (Gudjonsson et al., 2002; Kenny et al., 2007). A modified version of this method utilizes a rBM undercoat on the tissue culture surface, with cells plated on top of the rBM, and then adds another layer of rBM on top of the plated cells, resulting in a pseudo-3D system (Debnath et al., 2003; Hebner et al., 2007). Although the cells are not mixed directly with the rBM, they are nevertheless surrounded by rBM and are able to form acinar structures, presumably by remodeling the rBM. Drawbacks of using rBM matrices include the fact that the matrix is not fully characterized and that it has considerable lot-to-lot variability. Another commonly used embedment scaffold, collagen I, is better defined than rBM derived from EHS. However, although certain epithelial cells polarize in collagen I, such as MDCK cells, many others either fail to undergo acinar morphogenesis, or assemble colonies with reversed polarity (Aunins, 1990). One advantage collagen I has over rBM is that the mechanical properties can be modified by tittering the collagen concentration and extent of cross-linking. Yet, collagen is also biologically derived, and therefore is subject to variability between preparations, species and processing techniques (for instance, intact- versus telopeptide-free collagen).
Synthetic materials have been developed and are being applied to manipulate substrate stiffness and matrix composition and material properties for cell preparation. For instance, bis poly acrylamide gels, traditionally used to separate biomolecules, have been manipulated in the 3D culture system and illustrate the effect of substrate stiffness on cell and tissue phenotype (Pelham and Wang, 1997). By varying the concentration of poly acrylamide to bis acrylamide cross-linker, a range of quantifiable matrix stiffnesses can be achieved onto which ligand of choice can be conjugated. The limitation of this system is that non-polymerized poly acrylamide is cytotoxic and therefore the matrix does not lend itself to a bona fide pseudo-3D embedment protocol. Thus the system is limited to 2D manipulation or pseudo 3D assays (Schmedlen et al., 2002). Nevertheless, using this approach, gels have been prepared with a precisely calibrated modulus range (200–10 000 Pa) and 2D and pseudo 3D systems have been used successfully to study matrix stiffness and composition on the behavior of cells (Paszek et al., 2005).
Engineered bioreactors
Knowledge of the types, magnitude and duration of forces within the cell and tissue are essential to understand the molecular mechanisms regulating mechano-transduction. The cellular response to mechanical stimulation depends upon the type of force applied, with tensile and compressive forces being applied perpendicular to the surface of the cell or 3D construct and shear forces being applied parallel to the cell or 3D construct surface. The cellular response also depends upon on the magnitude, frequency and duration of the applied stimuli. To delineate the role of physical force in cell behavior and tissue homeostasis, researchers apply physiologically relevant mechanical stimuli at the cell and tissue level using specially engineered devices which have been designed to control the temporal, spatial and intensity of the force parameter.
There are two general approaches to study cellular mechanotransduction. The first approach uses multiple cells which are collectively mechanically stimulated and which mimic the forces that the cell would typically experience within their physiological microenvironment (tissue). This first set of techniques includes the simple application of hydrostatic pressure, compression, tension and shear stress to cell monolayers, or tissue fragments (ex vivo explant culture or cells embedded in tissue-engineered scaffolds). For instance, flow chambers have been developed that apply shear stresses to cell monolayers, either through pressure-driven systems that apply a parabolic laminar flow profile or cone-and-plate flow chambers which apply a uniform shear stress with a linear flow profile (Davies, 1995). The role of hydrostatic pressure in cell and tissue growth and differentiation has been investigated in a 2D format by applying a transmembrane pressure to cells plated on a porous, stiff substrate. Similarly, the effect of hydrostatic pressure in 2D or 3D has been assessed by directing compressed air or a column of fluid over a culture of cells (reviewed in Paszek and Weaver, 2004). Alternatively, techniques investigating the mechanoresponse of cells to tensile stress involve the application of static or cyclic, axial or biaxial strains to monolayers of cells plated on a deformable membrane, or within a deformable 3D scaffold (Vanderploeg et al., 2004; Wall et al., 2007). In addition, mechanical devices have been used since the 1970s to deconstruct the role of static and dynamic compression in cell growth and metabolism (Panjabi et al., 1979).
A recent approach to studying cellular mechanotransduction has been used to investigate the response of individual cells to a directed mechanostimuli. This approach uses sophisticated devices that apply pico- or nano-Newton forces to individual cell membranes, receptors or cytoskeletal elements. These methods include the use of particle attachment to apply precise forces to the surface of cells, using small microbeads coated with adhesive ligands or antibodies that bind to a specific cell surface receptor through which the force can be directly transmitted (Huang et al., 2004; Gan, 2007). Different techniques for applying these types of forces include optical trapping, micropipette aspiration and the application of both linear and torsional forces by magnetic manipulation (Pommerenke et al., 1996; Choquet et al., 1997; Hochmuth, 2000). Similar, methods have been developed that can assess and measure cellular and material forces at the nano-scale level. This includes atomic force microscopy and traction force microscopy that can be used to determine the material properties, as well as the forces generated by single cells (Munevar et al., 2001; Garcia et al., 2007; Sabass et al., 2008). The development of such innovative approaches and tools now permits endless approaches to obtain insight into the fundamental processes regulating mechanotransduction and for analyzing specific physical interactions between cells and their surrounding micro-environment.
Conclusions
Stromal–epithelial interactions drive developmental processes such as polarity, and maintain tissue homeostasis through a network of physical and biochemical processes that operate within the 3D epithelial tissue. Although this review focuses on the effect of micro-environmental force on cell and tissue polarity, force also influences many other aspects of normal tissue behavior and tumor biology including cancer initiation, transformation, metastasis and treatment efficacy and our understanding of these effects are just now beginning to be appreciated. For instance, force appears to exert a profound effect on apoptosis resistance and likely alters the efficacy of drug delivery to tumors (Chen et al., 1997; Numaguchi et al., 2003; Padera et al., 2004). Furthermore, cellular and ECM interactions evolve over time, dynamically guiding and responding to development. Integral to this process is the complex interplay between soluble factors, cell–cell and cell–ECM interactions and the mechanical microenvironment, which cooperatively drive epithelial morphogenesis and differentiation, and regulate tissue homeostasis.
Mechanical force elicits a myriad of biochemical responses in a cell, altering how a cell responds to an exogenous signal, and dramatically influencing how differentiation decisions are made during development. Tumor progression is associated with pronounced changes in cell and tissue force, including increased compression, altered ECM composition, stiffness and topology that elevate extracellular tension and the elastic modulus of the tissue, modify the cytoskeleton, enhance cell rheology and tension and induce interstitial pressure to alter cellular mechanotransduction. Compromised mechanotransduction perturbs mechanohomeostasis and contributes to tumor progression. Yet, although the overall importance of mechanical force to tumor etiology is slowly becoming acknowledged, much still remains to be discovered about how mechanotransduction is regulated at the cell and tissue level. Furthermore, we know little about how mechanosensory mechanisms might guide critical processes such as cell and tissue polarity. Thus the quest to elucidate how mechanical stimulation induces structural, compositional and functional changes at the cellular and tissue levels has just begun.
Acknowledgments
We apologize to the authors whose work is not cited because of space limitations. This work was supported by NIH Grant 7R01CA078731-07, DOD Breast Cancer Research Era of Hope Grant W81XWH-05-1-330 (BC044791), CIRM Grant RS1-00449 and DOE Grant A107165 to VMW, NIH NCI Training Grant 5T32CA108462-04 to JL and DOD Breast Cancer Research Era of Hope Grant BC06262 to JM.
References
- Adam RM, Roth JA, Cheng HL, Rice DC, Khoury J, Bauer SB, et al. Signaling through PI3K/Akt mediates stretch and PDGF-BB-dependent DNA synthesis in bladder smooth muscle cells. J Urol. 2003;169:2388–2393. doi: 10.1097/01.ju.0000063980.99368.35. [DOI] [PubMed] [Google Scholar]
- Akiri G, Sabo E, Dafni H, Vadasz Z, Kartvelishvily Y, Gan N, et al. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 2003;63:1657–1666. [PubMed] [Google Scholar]
- Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson PH. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 2003;5:R129–R135. doi: 10.1186/bcr622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aunins JGWDIC. Experimental collision efficiencies of polymer-flocculated animal cells. Biotechnol Prog. 1990;6:54–61. doi: 10.1021/bp00001a009. [DOI] [PubMed] [Google Scholar]
- Avvisato CL, Yang X, Shah S, Hoxter B, Li W, Gaynor R, et al. Mechanical force modulates global gene expression and beta-catenin signaling in colon cancer cells. J Cell Sci. 2007;120:2672–2682. doi: 10.1242/jcs.03476. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, Wang YL. Flexible substrata for the detection of cellular traction forces. Trends Cell Biol. 2002;12:79–84. doi: 10.1016/s0962-8924(01)02205-x. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Weaver VM, Lelievre SA, Wang F, Petersen OW, Schmeichel KL. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59:1757s–1763s. discussion 1763s–1764s. [PubMed] [Google Scholar]
- Brancaccio M, Hirsch E, Notte A, Selvetella G, Lembo G, Tarone G. Integrin signalling: the tug-of-war in heart hypertrophy. Cardiovasc Res. 2006;70:422–433. doi: 10.1016/j.cardiores.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumor progression. Nat Rev Cancer. 2008 doi: 10.1038/nrc2544. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- Chambard M, Verrier B, Gabrion J, Mauchamp J. Polarity reversal of inside-out thyroid follicles cultured within collagen gel: reexpression of specific functions. Biol Cell. 1984;51:315–325. doi: 10.1111/j.1768-322x.1984.tb00310.x. [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Tunc-Civelek V, Sarasa-Renedo A. Gene regulation by mechanotransduction in fibroblasts. Appl Physiol Nutr Metab. 2007;32:967–973. doi: 10.1139/H07-053. [DOI] [PubMed] [Google Scholar]
- Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin- cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- Chrenek MA, Wong P, Weaver VM. Tumour-stromal interactions. Integrins and cell adhesions as modulators of mammary cell survival and transformation. Breast Cancer Res. 2001;3:224–229. doi: 10.1186/bcr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer. 2003;3:921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox EA, Sastry SK, Huttenlocher A. Integrin-mediated adhesion regulates cell polarity and membrane protrusion through the Rho family of GTPases. Mol Biol Cell. 2001;12:265–277. doi: 10.1091/mbc.12.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Decitre M, Gleyzal C, Raccurt M, Peyrol S, Aubert-Foucher E, Csiszar K, et al. Lysyl oxidase-like protein localizes to sites of de novo fibrinogenesis in fibrosis and in the early stromal reaction of ductal breast carcinomas. Lab Invest. 1998;78:143–151. [PubMed] [Google Scholar]
- del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, Schwartz MA. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- Demou ZN, Awad M, McKee T, Perentes JY, Wang X, Munn LL, et al. Lack of telopeptides in fibrillar collagen I promotes the invasion of a metastatic breast tumor cell line. Cancer Res. 2005;65:5674–5682. doi: 10.1158/0008-5472.CAN-04-1682. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Erler JT, Giaccia AJ. Lysyl oxidase mediates hypoxic control of metastasis. Cancer Res. 2006;66:10238–10241. doi: 10.1158/0008-5472.CAN-06-3197. [DOI] [PubMed] [Google Scholar]
- Eshchenko TY, Rykova VI, Chernakov AE, Sidorov SV, Grigorieva EV. Expression of different proteoglycans in human breast tumors. Biochemistry (Mosc) 2007;72:1016–1020. doi: 10.1134/s0006297907090143. [DOI] [PubMed] [Google Scholar]
- Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- Friedrichs J, Taubenberger A, Franz CM, Muller DJ. Cellular remodelling of individual collagen fibrils visualized by time-lapse AFM. J Mol Biol. 2007;372:594–607. doi: 10.1016/j.jmb.2007.06.078. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Schmid-Schonbein GW. Regulation of CD18 expression on neutrophils in response to fluid shear stress. Proc Natl Acad Sci USA. 2003;100:13152–13157. doi: 10.1073/pnas.2336130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y. Invited review article: a review of techniques for attaching micro- and nanoparticles to a probe’s tip for surface force and near-field optical measurements. Rev Sci Instrum. 2007;78:081101. doi: 10.1063/1.2754076. [DOI] [PubMed] [Google Scholar]
- Garcia R, Magerle R, Perez R. Nanoscale compositional mapping with gentle forces. Nat Mater. 2007;6:405–411. doi: 10.1038/nmat1925. [DOI] [PubMed] [Google Scholar]
- Ghosh K, Pan Z, Guan E, Ge S, Liu Y, Nakamura T, et al. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials. 2007;28:671–679. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Grande-Allen KJ. Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc Res. 2006;72:375–383. doi: 10.1016/j.cardiores.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- Hebner C, Weaver VM, Debnath J. Modeling morphogenesis and oncogenesis in three-dimensional breast epithelial cultures. Annu Rev Pathol. 2007;3:313–339. doi: 10.1146/annurev.pathmechdis.3.121806.151526. [DOI] [PubMed] [Google Scholar]
- Heinemeier KM, Olesen JL, Haddad F, Langberg H, Kjaer M, Baldwin KM, et al. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol. 2007;582:1303–1316. doi: 10.1113/jphysiol.2007.127639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ, Jain RK. Solid stress inhibits the growth of multicellular tumor spheroids. Nat Biotechnol. 1997;15:778–783. doi: 10.1038/nbt0897-778. [DOI] [PubMed] [Google Scholar]
- Hochmuth RM. Micropipette aspiration of living cells. J Biomech. 2000;33:15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- Huang H, Kamm RD, Lee RT. Cell mechanics and mechanotransduction: pathways, probes, and physiology. Am J Physiol Cell Physiol. 2004;287:C1–11. doi: 10.1152/ajpcell.00559.2003. [DOI] [PubMed] [Google Scholar]
- Ichimiya H, Takahashi T, Ariyoshi W, Takano H, Matayoshi T, Nishihara T. Compressive mechanical stress promotes osteoclast formation through RANKL expression on synovial cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:334–341. doi: 10.1016/j.tripleo.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Madri JA, Folkman J. A possible mechanism for inhibition of angiogenesis by angiostatic steroids: induction of capillary basement membrane dissolution. Endocrinology. 1986;119:1768–1775. doi: 10.1210/endo-119-4-1768. [DOI] [PubMed] [Google Scholar]
- Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn. 2006;235:3222–3229. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- Kass L, Erler JT, Dembo M, Weaver VM. Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int J Biochem Cell Biol. 2007;39:1987–1994. doi: 10.1016/j.biocel.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem. 2005;280:16546–16549. doi: 10.1074/jbc.C400455200. [DOI] [PubMed] [Google Scholar]
- Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- Keller R, Davidson LA, Shook DR. How we are shaped: the biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschmann DA, Seftor EA, Fong SF, Nieva DR, Sullivan CM, Edwards EM, et al. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002;62:4478–4483. [PubMed] [Google Scholar]
- Kleinman HK, Philp D, Hoffman MP. Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 2003;14:526–532. doi: 10.1016/j.copbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Larsen M, Artym VV, Green JA, Yamada KM. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol. 2006;18:463–471. doi: 10.1016/j.ceb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Leivo I. Structure and composition of early basement membranes: studies with early embryos and teratocarcinoma cells. Med Biol. 1983;61:1–30. [PubMed] [Google Scholar]
- Leivonen SK, Kahari VM. Transforming growth factor-beta signaling in cancer invasion and metastasis. Int J Cancer. 2007;121:2119–2124. doi: 10.1002/ijc.23113. [DOI] [PubMed] [Google Scholar]
- Lelievre S, Weaver VM, Bissell MJ. Extracellular matrix signaling from the cellular membrane skeleton to the nuclear skeleton: a model of gene regulation. Recent Prog Horm Res. 1996;51:417–432. [PMC free article] [PubMed] [Google Scholar]
- Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokeshwar VB, Gomez P, Kramer M, Knapp J, McCornack MA, Lopez LE, et al. Epigenetic regulation of hyal-1 hyaluronidase expression: identification of hyal-1 promoter. J Biol Chem. 2008 doi: 10.1074/jbc.M801101200. (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–808. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- Mogilner A, Oster G. Force generation by actin polymerization II: the elastic ratchet and tethered filaments. Biophys J. 2003;84:1591–1605. doi: 10.1016/S0006-3495(03)74969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Polte T, Huang S, Shi B, Alsberg E, Sunday ME, et al. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev Dyn. 2005;232:268–281. doi: 10.1002/dvdy.20237. [DOI] [PubMed] [Google Scholar]
- Munevar S, Wang Y, Dembo M. Traction force microscopy of migrating normal and H-ras transformed 3T3 fibroblasts. Biophys J. 2001;80:1744–1757. doi: 10.1016/s0006-3495(01)76145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroi Y, Kakudo K, Nakata K. Effects of compressive loading on human synovium-derived cells. J Dent Res. 2007;86:786–791. doi: 10.1177/154405910708600819. [DOI] [PubMed] [Google Scholar]
- Muschler J, Levy D, Boudreau R, Henry M, Campbell K, Bissell MJ. A role for dystroglycan in epithelial polarization: loss of function in breast tumor cells. Cancer Res. 2002;62:7102–7109. [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HT, Adam RM, Bride SH, Park JM, Peters CA, Freeman MR. Cyclic stretch activates p38 SAPK2-, ErbB2-, and AT1-dependent signaling in bladder smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C1155–C1167. doi: 10.1152/ajpcell.2000.279.4.C1155. [DOI] [PubMed] [Google Scholar]
- Numaguchi Y, Huang S, Polte TR, Eichler GS, Wang N, Ingber DE. Caldesmon-dependent switching between capillary endothelial cell growth and apoptosis through modulation of cell shape and contractility. Angiogenesis. 2003;6:55–64. doi: 10.1023/a:1025821517679. [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, et al. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodeling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjabi MM, White AA, III, Wolf JW., Jr A biomechanical comparison of the effects of constant and cyclic compression on fracture healing in rabbit long bones. Acta Orthop Scand. 1979;50:653–661. doi: 10.3109/17453677908991288. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Payne SL, Hendrix MJ, Kirschmann DA. Paradoxical roles for lysyl oxidases in cancer—a prospect. J Cell Biochem. 2007;101:1338–1354. doi: 10.1002/jcb.21371. [DOI] [PubMed] [Google Scholar]
- Pedersen JA, Swartz MA. Mechanobiology in the third dimension. Ann Biomed Eng. 2005;33:1469–1490. doi: 10.1007/s10439-005-8159-4. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells [published erratum appears in Proc Natl Acad Sci USA 1993 Mar 15;90(6):2556] Proc Natl Acad Sci USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polte TR, Eichler GS, Wang N, Ingber DE. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am J Physiol Cell Physiol. 2004;286:C518–C528. doi: 10.1152/ajpcell.00280.2003. [DOI] [PubMed] [Google Scholar]
- Pommerenke H, Schreiber E, Durr F, Nebe B, Hahnel C, Moller W, et al. Stimulation of integrin receptors using a magnetic drag force device induces an intracellular free calcium response. Eur J Cell Biol. 1996;70:157–164. [PubMed] [Google Scholar]
- Quinn TP, Schlueter M, Soifer SJ, Gutierrez JA. Cyclic mechanical stretch induces VEGF and FGF-2 expression in pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L897–L903. doi: 10.1152/ajplung.00044.2001. [DOI] [PubMed] [Google Scholar]
- Reichelt J. Mechanotransduction of keratinocytes in culture and in the epidermis. Eur J Cell Biol. 2007;86:807–816. doi: 10.1016/j.ejcb.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Reno F, Grazianetti P, Stella M, Magliacani G, Pezzuto C, Cannas M. Release and activation of matrix metalloproteinase-9 during in vitro mechanical compression in hypertrophic scars. Arch Dermatol. 2002;138:475–478. doi: 10.1001/archderm.138.4.475. [DOI] [PubMed] [Google Scholar]
- Reno F, Sabbatini M, Stella M, Magliacani G, Cannas M. Effect of in vitro mechanical compression on Epilysin (matrix metalloproteinase-28) expression in hypertrophic scars. Wound Repair Regen. 2005;13:255–261. doi: 10.1111/j.1067-1927.2005.130307.x. [DOI] [PubMed] [Google Scholar]
- Robinson GW. Cooperation of signalling pathways in embryonic mammary gland development. Nat Rev Genet. 2007;8:963–972. doi: 10.1038/nrg2227. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Paskiet KT, Sabatini DD. Assembly of enveloped viruses in Madin–Darby canine kidney cells: polarized budding from single attached cells and from clusters of cells in suspension. J Cell Biol. 1983;96:866–874. doi: 10.1083/jcb.96.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski JM, Swartz MA. A driving force for change: interstitial flow as a morphoregulator. Trends Cell Biol. 2007;17:44–50. doi: 10.1016/j.tcb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Sabass B, Gardel ML, Waterman CM, Schwarz US. High resolution traction force microscopy based on experimental and computational advances. Biophys J. 2008;94:207–220. doi: 10.1529/biophysj.107.113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samani A, Zubovits J, Plewes D. Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Phys Med Biol. 2007;52:1565–1576. doi: 10.1088/0031-9155/52/6/002. [DOI] [PubMed] [Google Scholar]
- Schmedlen RH, Masters KS, West JL. Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials. 2002;23:4325–4332. doi: 10.1016/s0142-9612(02)00177-1. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Desimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008 doi: 10.1016/j.ceb.2008.05.005. (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver FH, Siperko LM. Mechanosensing and mechanochemical transduction: how is mechanical energy sensed and converted into chemical energy in an extracellular matrix? Crit Rev Biomed Eng. 2003;31:255–331. doi: 10.1615/critrevbiomedeng.v31.i4.10. [DOI] [PubMed] [Google Scholar]
- Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development. 2001;128:3117–3131. doi: 10.1242/dev.128.16.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R. Hyaluronidases in cancer biology. Semin Cancer Biol. 2008;18:275–280. doi: 10.1016/j.semcancer.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Kouros-Mehr H, Lu P, Werb Z. Hormonal and local control of mammary branching morphogenesis. Differentiation. 2006;74:365–381. doi: 10.1111/j.1432-0436.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strongin AY. Mislocalization and unconventional functions of cellular MMPs in cancer. Cancer Metastasis Rev. 2006;25:87–98. doi: 10.1007/s10555-006-7892-y. [DOI] [PubMed] [Google Scholar]
- Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell. 2004;7:709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Triplett JW, O’Riley R, Tekulve K, Norvell SM, Pavalko FM. Mechanical loading by fluid shear stress enhances IGF-1 receptor signaling in osteoblasts in a PKCzeta-dependent manner. Mol Cell Biomech. 2007;4:13–25. [PubMed] [Google Scholar]
- Unger M, Weaver VM. The tissue microenvironment as an epigenetic tumor modifier. Methods Mol Biol. 2003;223:315–347. doi: 10.1385/1-59259-329-1:315. [DOI] [PubMed] [Google Scholar]
- Vanderploeg EJ, Imler SM, Brodkin KR, Garcia AJ, Levenston ME. Oscillatory tension differentially modulates matrix metabolism and cytoskeletal organization in chondrocytes and fibrochondrocytes. J Biomech. 2004;37:1941–1952. doi: 10.1016/j.jbiomech.2004.02.048. [DOI] [PubMed] [Google Scholar]
- Vincent TL, McLean CJ, Full LE, Peston D, Saklatvala J. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthr Cartil. 2007;15:752–763. doi: 10.1016/j.joca.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Volokh KY. Stresses in growing soft tissues. Acta Biomater. 2006;2:493–504. doi: 10.1016/j.actbio.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Wall ME, Weinhold PS, Siu T, Brown TD, Banes AJ. Comparison of cellular strain with applied substrate strain in vitro. J Biomech. 2007;40:173–181. doi: 10.1016/j.jbiomech.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. I. Uncoupling the roles of cell–cell and cell-substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990a;95 (Part 1):137–151. doi: 10.1242/jcs.95.1.137. [DOI] [PubMed] [Google Scholar]
- Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. II. Disassembly and assembly of plasma membrane domains during reversal of epithelial cell polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990b;95 (Part 1):153–165. doi: 10.1242/jcs.95.1.153. [DOI] [PubMed] [Google Scholar]
- Wang HB, Dembo M, Hanks SK, Wang Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci USA. 2001;98:11295–11300. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Thampatty BP. An introductory review of cell mechanobiology. Biomech Model Mechanobiol. 2006;5:1–6. doi: 10.1007/s10237-005-0012-z. [DOI] [PubMed] [Google Scholar]
- Watson CJ. Involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res. 2006;8:203. doi: 10.1186/bcr1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Howlett AR, Langton-Webster B, Petersen OW, Bissell MJ. The development of a functionally relevant cell culture model of progressive human breast cancer. Semin Cancer Biol. 1995;6:175–184. doi: 10.1006/scbi.1995.0021. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, et al. Reversion of the malignant phenotype of human breast cells in three- dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells RG, Discher DE. Matrix elasticity, cytoskeletal tension, and TGF-beta: the insoluble and soluble meet. Sci Signal. 2008;1:pe13. doi: 10.1126/stke.110pe13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JY, Velasco A, Rajagopalan P, Pham Q. Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir. 2003;19:1908–1913. [Google Scholar]
- Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol. 2006;16:1515–1523. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]