Abstract
Whether mucosal immunization is required for optimal protective CD8 T cell memory at mucosal surfaces is controversial. In this study, using an adoptive transfer system, we compare the efficacy of two routes of acute lymphocytic choriomeningitis viral infection on the generation, maintenance, and localization of Ag-specific CD8 T cells in tissues, including the vaginal mucosa. Surprisingly, at day 8, i.p. infection results in higher numbers of Ag-specific CD8 T cells in the vaginal mucosa and iliac lymph node, as well as 2–3× more Ag-specific CD8 T cells that coexpress both IFN-γ and TNF-α in comparison to the intranasal route of infection. Expression of the integrin/activation marker CD103 (αEβ7) is low on vaginal mucosal Ag-specific CD8 T cells in comparison to gut mucosal intraepithelial lymphocytes. At memory, no differences are evident in the number, cytokine production, or protective function of Ag-specific CD8 T cells in the vaginal mucosa comparing the two routes of infection. However, differences persist in the cytokine profile of genital tract vs peripheral Ag-specific CD8 T cells. So although the initial route of infection, as well as tissue microenvironment, appear to influence both the magnitude and quality of the effector CD8 T cell response, both systemic and mucosal infection are equally effective in the differentiation of protective memory CD8 T cell responses against vaginal pathogenic challenge.
Many infectious pathogens enter the body via mucosal surfaces. Consequently, generating and sustaining protective immune responses at mucosal sites is an important goal in efforts to produce a successful vaccine against viral infections such as HIV-1, which is primarily transmitted worldwide through the female genital mucosa (1). Understanding factors regulating CD8 T cell immunity at sites such as the genital mucosa will be critical in these efforts.
Discrepancies in the research studies examining cellular immunity in the genital mucosa have led to some confusion regarding requirements for the generation and maintenance of CD8 T cell memory in mucosal sites. Several studies established that mucosal immunization was required for protective mucosal T cell responses in mice and nonhuman primates (2–5). However, several other studies have convincingly demonstrated that systemic immunization with live viral vectors effectively provides protection against mucosal challenge in nonhuman primates (6, 7). So although recent reviews continue to assert that mucosal immunization is required for effective mucosal T cell memory, other studies have demonstrated long-term T cell memory in the gut mucosa of mice following mucosal and systemic infection (8–10). Consequently, the importance of mucosal immunization in long-term cellular immunity in the genital tract should be re-examined.
The male and female genital tracts display several unique features not shared with other mucosal sites. These include the lack of distinctive lympho-epithelial structures of the intestinal Peyer’s patches, and the reduced ability to respond to foreign Ags (11). In this study, we sought to characterize protective CD8 T cell immune responses in the vaginal tract of mice following i.p. or intranasal (i.n.)3 infection with lymphocytic choriomeningitis virus (LCMV) to track and compare the magnitude and quality of Ag-specific CD8 T cell responses. LCMV is a natural pathogen of mice often spread via inhalation of aerosolized virus from infected excretions such as fecal matter, urine, and nasal secretions, in addition to infections by bites (12). Whether inhaled (i.n.) or given systemically (i.p.), LCMV initiates robust immune responses in mice (13, 14).
Because studying immune responses in the genital mucosa is technically challenging because of low lymphocyte recovery, we exploited a model system whereby we adoptively transferred Thy1.1 transgenic T cells specific for the DbGP33–41 peptide of the LCMV glycoprotein (15). Our results demonstrate that despite early differences in the magnitude and quality of the Ag-specific CD8 T cell response, both routes of infection are able to generate durable, protective memory CD8 T cells in the vaginal mucosa. These results suggest that early programming differences in effector mucosal CD8 T cell responses do not preclude development of a mucosal memory CD8 T cell population that can mediate protection to mucosal viral challenge.
Materials and Methods
Mice
C57BL/6 female mice were purchased from The Jackson Laboratory. Transgenic P14 Thy1.1 female mice were provided by Dr. R. Ahmed (Emory Vaccine Center, GA) and bred in house. All mice were maintained under specific pathogen-free conditions at the University of Tennessee in accordance with university Institutional Animal Care and Use Committee guidelines and used at 5–8 wk of age.
Adoptive transfer and infections
A total of 106 Thy1.1 P14 transgenic spleen cells were adoptively transferred into intact C57BL/6 mice followed by infection with 2 × 105 PFU of LCMV-Armstrong i.p. or i.n. 1 day later. For rechallenge studies, LCMV-Armstrong immune mice (4 mo postinfection) were progesterone synchronized with depo-provera 2 mg/mouse s.c. At day 5 after progesterone synchronization, mice were rechallenged intravaginally with 5 × 107 PFU of Vaccinia virus-expressing GP33–41 (16).
Tissue harvest and flow cytometry
At specified time points (day 8, > 40 postinfection, day 5 postchallenge), animals were sacrificed and lungs perfused with 5 ml cold PBS before removal and processing as previously described (9). Serum and vaginal washes were frozen. The organized nasal-associated lymphoid tissue (o-NALT) and vaginal tract were removed and pooled from three to four mice. The vaginal tracts were minced using a tissue chopper and incubated with HBSS plus 1.3 mM EDTA solution for 30 min at 37°C and then resuspended in 225 U/ml type I collagenase for 60 min at 37°C. Cell pellets were subjected to a Percoll density gradient and lymphocytes were collected (yield 1–5 × 105 cells). Single cell suspensions were stained with DbGP33–41 tetramer prepared as previously described (17) and mAbs purchased from BD Pharmingen (Thy1.1, CD8, CD44, IFN-γ, and TNF-α). Intracellular cytokine staining was assayed on cells from spleen and iliac lymph node (ILN). All samples were run on a FACSCalibur (BD Biosciences). All data were analyzed with FlowJo software (Tree Star). Unpaired Student t tests were performed to determine statistical significance with ** denoting p < 0.01, and *** denoting p < 0.001.
Confocal microscopy and Vaccinia plaque assay
Frozen sections of the vaginal tract (5–6 μm) were fixed and stained with mAbs Thy1.1, CD8 (BD Pharmingen) as described previously (18). Confocal microscopy was performed with a laser scanning confocal microscope (Leica SP2) and blinded samples were counted to determine Thy1.1+CD8+ cells. Plaque assays were performed on homogenized ovaries using M2-10B4 cells (19).
Results
Ag-specific CD8 T cell responses differ in the genital tract on day 8 following systemic or mucosal route of LCMV infection
Previous studies have determined that following viral infection, Ag-specific CD8 T cell responses are robust in many tissues (9, 20). To determine whether differences exist in the magnitude of the Ag-specific CD8 T cell response in different tissue compartments depending on route of infection, we directly compared mice that were adoptively transferred with transgenic splenocytes specific for the DbGP33–41 peptide of LCMV glycoprotein and were subsequently infected with 2 × 105 PFU of LCMV Armstrong either via a systemic route (i.p.) or a mucosal route (i.n.).
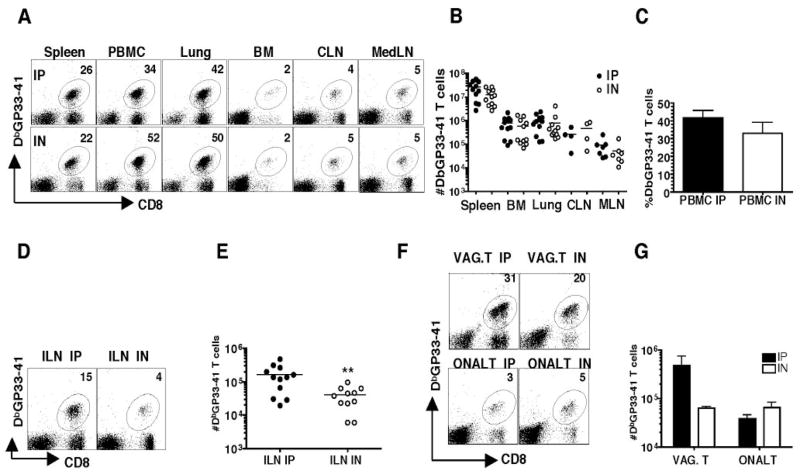
Both routes of infection generate robust CD8 T cell responses in several tissue compartments of individual mice including spleen, PBMC, lung, bone marrow, cervical lymph node, and mediastinal lymph node on day 8 (Fig. 1A). Overall, no significant differences were observed in the total Ag-specific responses in these tissues comparing i.p. infected vs i.n. infected mice (Fig. 1, B and C). However, significantly higher numbers of Ag-specific CD8 T cells are present in the ILN, the draining node of the genital tract, following i.p. infection compared with i.n. infection (Fig. 1, D and E). Moreover, whereas the Ag-specific CD8 T cell responses in the o-NALT at day 8 are similar, we show significantly higher numbers of Ag-specific CD8 T cells in the vaginal tract of mice comparing i.p. vs i.n. infection (Fig. 1, F and G). No differences were noted on the surface expression of several T cell activation markers between the two treatment groups (data not shown).
Figure 1.
Magnitude of the Ag-specific CD8 T cell response differs in the genital tract on day 8. Lymphocytes from spleen, peripheral blood, lung, bone marrow, CLN, MedLN, ILN, vaginal tract, and o-NALT were isolated and surface stained with DbGP33–41, CD8. A, Representative FACS staining of tissues from a day 8 postinfection LCMV Armstrong i.p. or i.n. infected mouse. The numbers represent the percentage of Ag-specific CD8 T cells in the indicated tissue compartment. B, Total number of Ag-specific CD8 T cells in tissues. C, Percentage of Ag-specific CD8 T cells in PBMC. D, Representative staining and E, Total numbers of Ag-specific T cells in the ILN. F, Representative staining and G, Total number of Ag-specific CD8 T cells in the vaginal tract and o-NALT. Black/filled symbols are i.p. and white/open symbols are i.n. The data represent three experiments; for o-NALT and vaginal tract tissues are pooled from four animals, each experiment. Not shown are uninfected control mice that showed no detectable Ag-specific responses.**, p < 0.01.
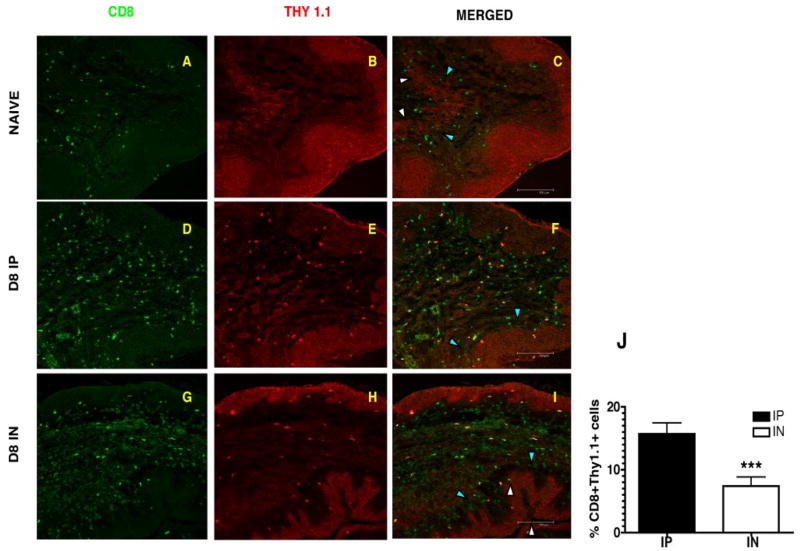
To independently confirm the surprising observations of differences in the magnitude of the Ag-specific CD8 T cell response in the vaginal tract on day 8, we examined micro sections of the vaginal tract using immunofluorescence staining and confocal microscopy because FACS analysis of the vaginal tract requires us to pool tissues from three to four mice per treatment group to obtain adequate numbers for analysis. Confocal analysis allows us to visualize and determine numbers of Ag-specific CD8 T cells in individual treated mice using costaining of CD8 and Thy1.1 Abs. The vaginal sections of naive animals show few CD8 T cells and this number increases significantly by day 8 postinfection (Fig. 2, A–C). However, similar to what we see by FACS analysis, we show higher numbers of Thy1.1+ CD8+ Ag-specific cells in the vaginal tract of mice infected with LCMV via the i.p. route on day 8 compared with mice infected via the intranasal route (Fig. 2, D–J).
Figure 2.
Confocal microscopy confirms differences in the magnitude of the Ag-specific CD8 T cell response in the vagina on day 8. Frozen sections of the vaginal tract were fixed and stained with CD8 and Thy 1.1 Abs. Thy1.1 staining shows Ag-specific CD8 T cell staining in the stroma of the vaginal mucosa on day 8 postinfection with LCMV. A, D, and G, CD8 T cells in green and B, E, H, Thy1.1 staining in red. C, F, I, Merged image shows Thy1.1 staining only on CD8 T cells of infected mice. Naive stained only for CD8 T cells. Images were captured using a 20× objective lens. The epithelial layer is indicated by the white arrowheads (luminal edge) and blue arrowheads (basement membrane). J, Percentage of Ag-specific CD8 T cells in vaginal tract of infected mice at day 8 postinfection. Thy1.1+ CD8+ cells were counted on blinded samples.***, p < 0.001.
Cytokine production is influenced by route of infection and tissue localization
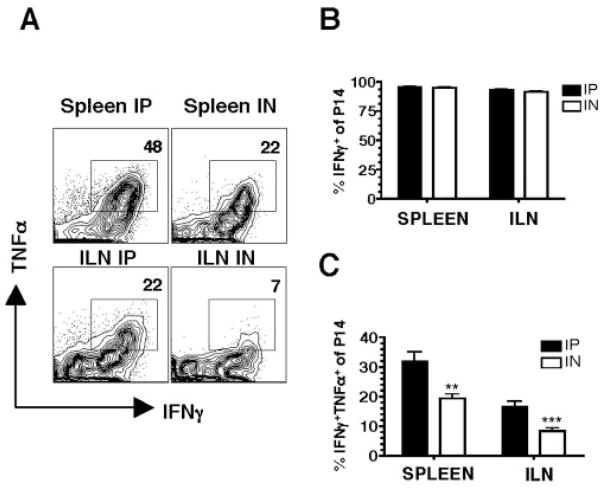
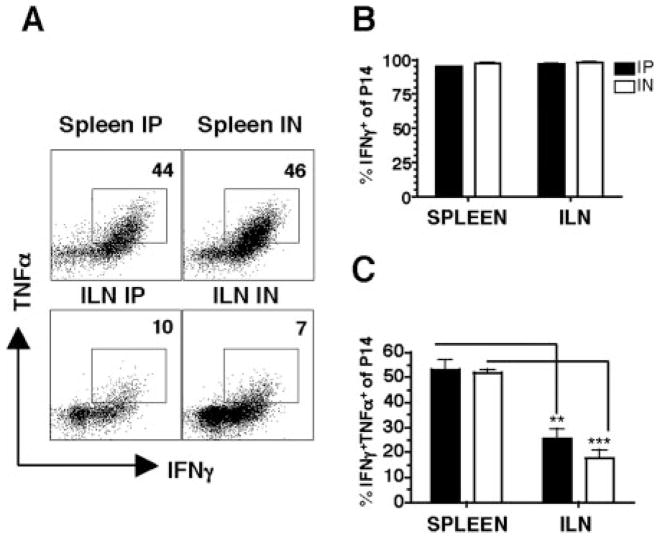
Because the magnitude of the Ag-specific CD8 T cell response differs in the vaginal mucosa on day 8, we also examined the quality of these cells by determining cytokine production. Intracellular cytokine analysis following ex vivo peptide stimulation with GP33–41 shows that T cells from both i.p. and i.n. infected mice show similar production of IFN-γ in spleen and ILN (Fig. 3, A and B). Notably however, more of the cells coproduce IFN-γ and TNF-α following the i.p. compared with i.n. route of infection (Fig. 3, A and C). Moreover, when we compare peripheral vs genital tract Ag-specific responses in the spleen and ILN respectively, we observe higher proportions of IFN-γ+TNF-α+coproducing cells in the spleen (Fig. 3, A and C). These results suggest that Ag-specific CD8 T cell responses in the genital tract are different in the production of inflammatory cytokines compared with peripheral cells. Higher proportions of these IFN-γ+TNF-α+Ag-specific responses are produced in response to systemic infection.
Figure 3.
Cytokine production is influenced by the route of immunization and tissue localization on day 8. Lymphocytes from spleen and ILN were isolated on day 8 of i.p. or i.n. LCMV infected mice and after 5 h ± peptide stimulation, stained with Thy1.1, CD8, IFN-γ, and TNF-α. A, Representative staining gating on Thy1.1+ CD8+ Ag-specific cells incubated with GP33–41 peptide. No staining was seen with no peptide (data not shown). B, Percentage of IFN-γ+ by Ag-specific T cells and C, Percentage of IFN-γ+TNF-α+by Ag-specific cells. Data are from three separate experiments.**, p < 0.01;***, p < 0.001.
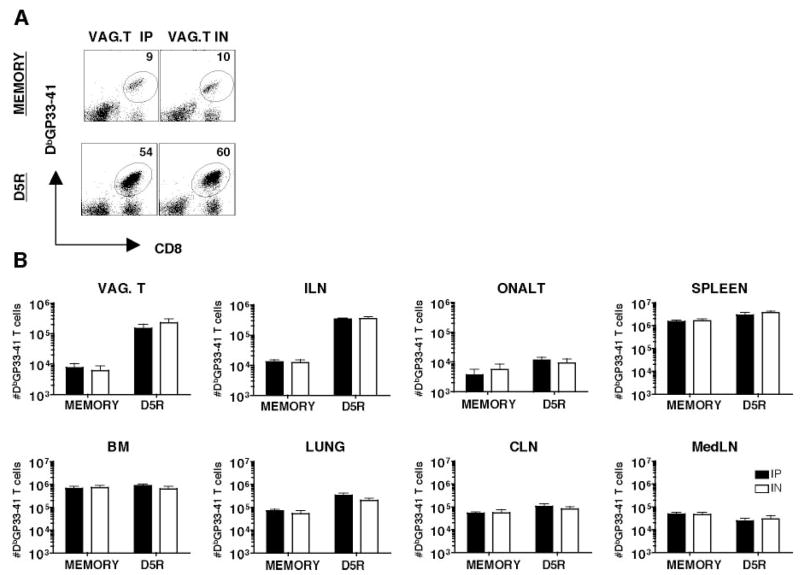
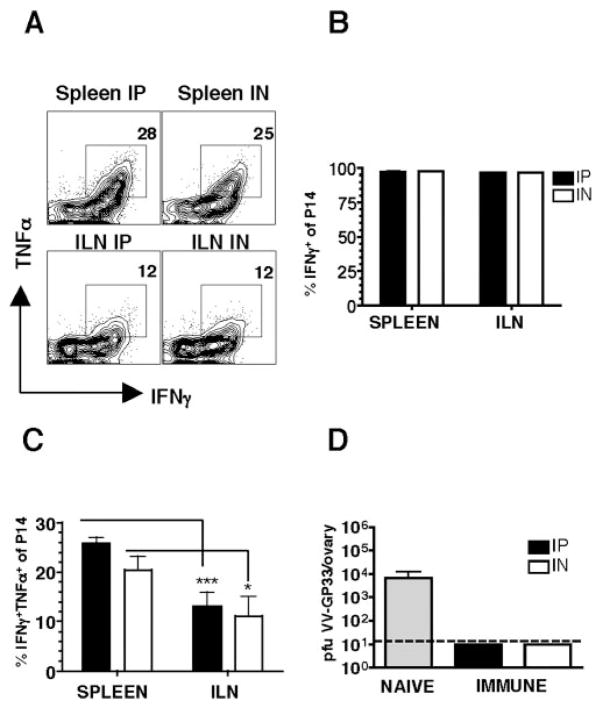
Given these differences in both the magnitude and quality of Ag-specific responses in the vaginal tract following these different infection routes at day 8, we asked whether these differences persist at memory. Examination of the vaginal tract by immunofluorescence staining and confocal microscopy show no differences in the magnitude of the response at memory time points (data not shown). Examination of spleen, PBMC, lung, bone marrow, cervical lymph node, mediastinal lymph node, ILN, o-NALT, and vaginal tract reveal no significant differences in the percentages or total numbers of Ag-specific CD8 T cells following i.p or i.n. infection at memory (Fig. 6 and data not shown). Furthermore, we observe no differences in the proportion of Ag-specific memory CD8 T cells coproducing IFN-γ and TNF-α comparing i.n. vs i.p. infection (Fig. 4). Importantly however, significant differences persist in cytokine production between the genital tract and peripheral Ag-specific T cells, with higher proportions of IFN-γ+ TNF-α+ in the spleen vs ILN irrespective of route of infection (Fig. 4, A and C). Thus, localization of Ag-specific CD8 T cells in the genital tract appears to influence cytokine production.
Figure 6.
Intravaginal rechallenge generate protective secondary responses in the genital tract of systemic and mucosal immune mice. Lymphocytes were isolated from indicated tissues of immune mice and day 5 intravaginal rechallenge with vaccinia expressing the GP33–41 peptide of the LCMV glycoprotein. A, Representative FACS staining of vaginal tract lymphocytes from i.p. and i.n. day 45 memory and day 5 intravaginal rechallenge mice. B, Total number of Ag-specific CD8 T cells in indicated tissue compartments of day 80 i.p. or i.n. immune mice and day 5 intravaginal rechallenge mice. Black bars are i.p. and white bars are i.n. For o-NALT and vaginal tracts, tissues pooled from four animals in each experiment.
Figure 4.
Cytokine production of memory T cells is influenced by tissue localization. Lymphocytes from spleen and ILN were isolated at day 80 and after 5 h ± peptide stimulation stained with Thy 1.1, CD8, IFN-γ, and TNF-α. Gating on Thy 1.1+ CD8+ cells, stimulation with GP33–41 specific peptide results in IFN-γ and TNF-α. No staining of IFN-γ or TNF-α was seen with no peptide controls (data not shown). A, Representative staining showing reduced TNF-α coexpressing cells in the ILN compared with spleen. B, Percentage of IFN-γ+ and C, Percentage of IFN-γ+ TNF-α+ Ag-specific cells.**, p < 0.01;***, p < 0.001.
Ag-specific CD8 T cells in the genital tract show low levels of expression of the integrin and activation marker CD103 (αEβ7)
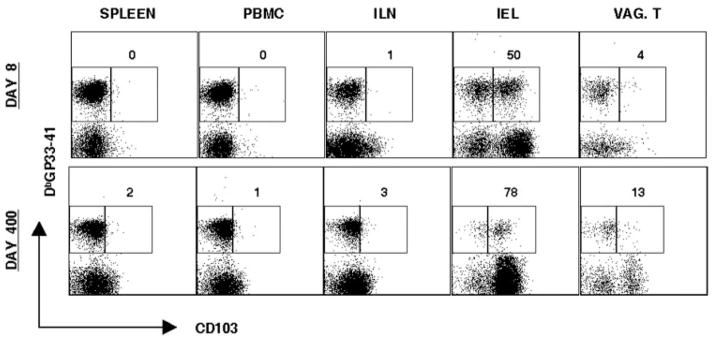
Lymphocytes in mucosal environments have been shown to express several specific adhesion molecules (21). CD103 is an integrin and activation marker that has been shown to bind E-cadherin, an integrin receptor shown to have high expression on intestinal endothelial cells and tonsil (21, 22). CD103 expression has been shown to be high on Ag-specific memory intestinal intraepithelial lymphocytes (IELs) and EBV-specific tonsil resident effector-memory CTLs (21, 23). We determined CD103 expression on Ag-specific CD8 T cells in the spleen, PBMC, ILN, IEL, and vaginal tract on day 8 and day 400 of memory (Fig. 5). CD103 expression is high on Ag-specific gut IELs as has been shown previously, but remains low on Ag-specific CD8 T cells in the vaginal tract and ILN at day 8 and memory (Fig. 5) (23). CD103 expression did not differ on vaginal tract T cells from i.n. vs i.p. infected mice (data not shown). This result demonstrates that characteristics of Ag-specific T cells of different mucosal compartments can be unique.
Figure 5.
Expression of CD103 on Ag-specific CD8 T cells in genital mucosa. Lymphocytes were isolated from the spleen, PBMC, ILN, IEL, and vaginal tract from day 8 and day 400 LCMV Armstrong i.p. Cells were stained with CD8, CD103, and DbGP33–41. Representative staining is shown, gating on CD8 T cells. Numbers represent percentage of CD103high Ag-specific CD8 T cells in indicated tissue compartments. For vaginal tract, tissues are pooled from four to six mice.
Ag-specific CD8 responses in the vaginal tract of systemic and mucosal immune mice are protective to mucosal challenge
Although we observe no obvious differences in the magnitude or quality of memory Ag-specific T cell populations in the i.p. vs i.n. immune mice, it is possible that subtle differences persist that we have not identified. To directly compare the protective function of the memory Ag-specific CD8 T cell population in the absence of potential differences in the humoral response (data not shown), we challenged mice with an intravaginal infection of vaccinia expressing the GP33–41 peptide of the LCMV glycoprotein (16). Both groups of immune mice demonstrate robust amnestic responses at day 5R, with large increases seen in the vaginal tract and draining ILN (Fig. 6). Intracellular cytokine production at day 5R of Ag-specific CD8 T cells show no differences in the proportion of IFN-γ+ TNF-α+ cells in i.p. vs i.n. immune mice, although again we note that Ag-specific T cells in the genital tract show a reduced proportion of these cells compared with cells in the spleen (Fig. 7, A–C). Importantly, both groups of immune mice clear virus while naive control mice have detectable virus in the ovaries at day 5 (Fig. 7D). Similar results were seen at day 3 of rechallenge (data not shown).
Figure 7.
Cytokine production of secondary effectors following intra-vaginal rechallenge. A, Representative staining showing IFN-γ+ TNF-α+ coexpressing cells in the ILN vs spleen. B and C, Production of IFN-γ and TNF-α by Ag-specific CD8 T cells. D, Viral titers in the ovaries on day 5 mice challenged intravaginally with 5 × 105 PFU vaccinia expressing the GP33–41 peptide of the LCMV glycoprotein. Gray bar represents naive mice; black bars are i.p. immune; and white bars are i.n. immune; n = 10–12 mice in each group;*, p < 0.5;***, p < 0.001.
Discussion
In summary, our study demonstrates, using a TCR transgenic transfer model and LCMV systemic or mucosal route of infection in mice, that functional, protective CD8 T cell memory is achieved regardless of the route of infection, despite early differences in the magnitude and quality of the Ag-specific CD8 effector population in the genital mucosa. Future studies will address whether these early differences in genital tract CD8 T cell quality and quantity are absent later due to a redistribution of these cells as they differentiate to memory.
Our study extends recent work demonstrating that activated CD8 T cells migrate pervasively to nonlymphoid tissues, including the gut mucosa, regardless of the site of activation using several viral models (10). Together, these studies demonstrate that irrespective of the site of initial Ag encounter, Ag-specific CD8 T cells widely disseminate to many tissues, including the gut and vaginal mucosa, and differentiate to functional protective memory T cells in these sites. Such widespread dissemination likely ensures elimination of the pathogen and provides early sentinels in the event of secondary infection. Although we cannot solely attribute protection against reinfection to resident memory T cells in different tissue compartments, localization of memory T cells at these sites likely play an important role in early confrontation of pathogens, significantly compromising the ability of the pathogen to spread.
Earlier studies have suggested that following systemic immunization, there is a failure to develop protective immune responses at mucosal sites (2, 3, 5, 24). One significant difference between those studies suggesting mucosal immunization is required for mucosal protection vs those suggesting systemic immunization can provide protection is the type of Ag used. In those studies that demonstrate compartmentalization of the immune response based on the route of immunization, Ag replication was absent or abortive (2, 3, 5, 24). Our study, in addition to the studies done in nonhuman primates, used viral systems that initiate via a systemic or mucosal route, and subsequently disseminate systemically, as is characteristic of HIV infection (6, 7). In many of the live viral vector studies, functional, protective CD8 T cell memory is achieved in mucosal compartments, regardless of route of immunization (10, 23). Thus, we suggest that both Ag replication and Ag spread are likely important determinants in the ability to form functional, protective memory CD8 responses in mucosal compartments.
Finally, our study also demonstrates that Ag-specific CD8 T cell responses in the genital mucosa are characterized by a lower proportion of IFN-γ+ TNF-α+ cells compared with responses in the spleen. CD103 expression on Ag-specific CD8 T cells differs between the gut mucosa and vaginal mucosa, with most Ag-specific CD8 T cells in the vaginal mucosa having low CD103 expression at day 8 and memory. Thus, despite the same TCR expression, it appears that localization within the genital tract influences cytokine expression/activation marker profiles of Ag-specific T cells, suggesting that localization within the genital mucosa leads to a unique differentiation program of memory T cells. This is supportive of recent work demonstrating differences in the quality of Ag-specific responses in the intestinal mucosa and lung airways (9, 25). Future studies will further delineate characteristics of genital mucosal memory T cells and the possible role that mucosal dendritic cells may play in modulating unique memory differentiation programs (26–28).
Acknowledgments
We thank Elizabeth Bonney, Mark Sangster, Tim Sparer, and Scott Mueller for helpful comments, Barry Rouse for critical review of the manuscript, and Susmit Suvas for help with the confocal studies.
Footnotes
This work was supported by National Institutes of Health Grant AI05771901 and University of Tennessee start-up funds to T.M.O.
Abbreviations used in this paper: i.n., intranasal; ILN, iliac lymph node; LCMV, lymphocytic choriomeningitis virus; IEL, intraepithelial lymphocytes; o-NALT, organized nasal-associated lymphoid tissue.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 2.Gallichan WS, Rosenthal KL. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J Exp Med. 1996;184:1879–1890. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belyakov IM, Derby MA, Ahlers JD, Kelsall BL, Earl P, Moss B, Strober W, Berzofsky JA. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc Natl Acad Sci USA. 1998;95:1709–1714. doi: 10.1073/pnas.95.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belyakov IM, Hel Z, Kelsall B, Kuznetsov VA, Ahlers JD, Nacsa J, Watkins DI, Allen TM, Sette A, Altman J, et al. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat Med. 2001;7:1320–1326. doi: 10.1038/nm1201-1320. [DOI] [PubMed] [Google Scholar]
- 5.Qimron U, Paul L, Bar-Haim E, Bloushtain N, Eisenbach L, Staats HF, Porgador A. Non-replicating mucosal and systemic vaccines: quantitative and qualitative differences in the Ag-specific CD8+ T cell population in different tissues. Vaccine. 2004;22:1390–1394. doi: 10.1016/j.vaccine.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 6.Pal R, Venzon D, Santra S, Kalyanaraman VS, Montefiori DC, Hocker L, Hudacik L, Rose N, Nacsa J, Edghill-Smith Y, et al. Systemic immunization with an ALVAC-HIV-1/protein boost vaccine strategy protects rhesus macaques from CD4+ T-cell loss and reduces both systemic and mucosal simian-human immunodeficiency virus SHIVKU2 RNA levels. J Virol. 2006;80:3732–3742. doi: 10.1128/JVI.80.8.3732-3742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevceva L, Alvarez X, Lackner AA, Tryniszewska E, Kelsall B, Nacsa J, Tartaglia J, Strober W, Franchini G. Both mucosal and systemic routes of immunization with the live, attenuated NYVAC/simian immunodeficiency virus SIV(gpe) recombinant vaccine result in gag-specific CD8+ T-cell responses in mucosal tissues of macaques. J Virol. 2002;76:11659–11676. doi: 10.1128/JVI.76.22.11659-11676.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 9.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 10.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrancois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 11.Mestecky J, Moldoveanu Z, Russell MW. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am J Reprod Immunol. 2005;53:208–214. doi: 10.1111/j.1600-0897.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 12.Buchmeier MJ, Bowen MD, Peters CJ. Arenaviridae: the viruses and their replication. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, et al., editors. Fields Virology. 4. Lippincott Williams and Wilkins; Philadelphia: 2001. pp. 1635–1668. [Google Scholar]
- 13.Khanolkar A, Fuller MJ, Zajac AJ. CD4 T cell-dependent CD8 T cell maturation. J Immunol. 2004;172:2834–2844. doi: 10.4049/jimmunol.172.5.2834. [DOI] [PubMed] [Google Scholar]
- 14.Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol. 2001;2:1067–1076. doi: 10.1038/ni727. [DOI] [PubMed] [Google Scholar]
- 15.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 16.Harrington LE, van der Most R, Whitton JL, Ahmed R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J Virol. 2002;76:3329–3337. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 18.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 19.Lutarewych MA, Quirk MR, Kringstad BA, Li W, Verfaillie CM, Jordan MC. Propagation and titration of murine cytomegalovirus in a continuous bone marrow-derived stromal cell line (M2–10B4) J Virol Methods. 1997;68:193–198. doi: 10.1016/s0166-0934(97)00126-2. [DOI] [PubMed] [Google Scholar]
- 20.Onami TM, Harrington LE, Williams MA, Galvan M, Larsen CP, Pearson TC, Manjunath N, Baum LG, Pearce BD, Ahmed R. Dynamic regulation of T cell immunity by CD43. J Immunol. 2002;168:6022–6031. doi: 10.4049/jimmunol.168.12.6022. [DOI] [PubMed] [Google Scholar]
- 21.Woodberry T, Suscovich TJ, Henry LM, August M, Waring MT, Kaur A, Hess C, Kutok JL, Aster JC, Wang F, et al. α E β7 (CD103) expression identifies a highly active, tonsil-resident effector-memory CTL population. J Immunol. 2005;175:4355–4362. doi: 10.4049/jimmunol.175.7.4355. [DOI] [PubMed] [Google Scholar]
- 22.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the α E β 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 23.Kim SK, Schluns KS, Lefrancois L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J Immunol. 1999;163:4125–4132. [PubMed] [Google Scholar]
- 24.Jogler C, Hoffmann D, Theegarten D, Grunwald T, Uberla K, Wildner O. Replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species. J Virol. 2006;80:3549–3558. doi: 10.1128/JVI.80.7.3549-3558.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohlmeier JE, Miller SC, Woodland DL. Cutting edge: antigen is not required for the activation and maintenance of virus-specific memory CD8+ T cells in the lung airways. J Immunol. 2007;178:4721–4725. doi: 10.4049/jimmunol.178.8.4721. [DOI] [PubMed] [Google Scholar]
- 26.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]