Abstract
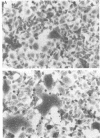
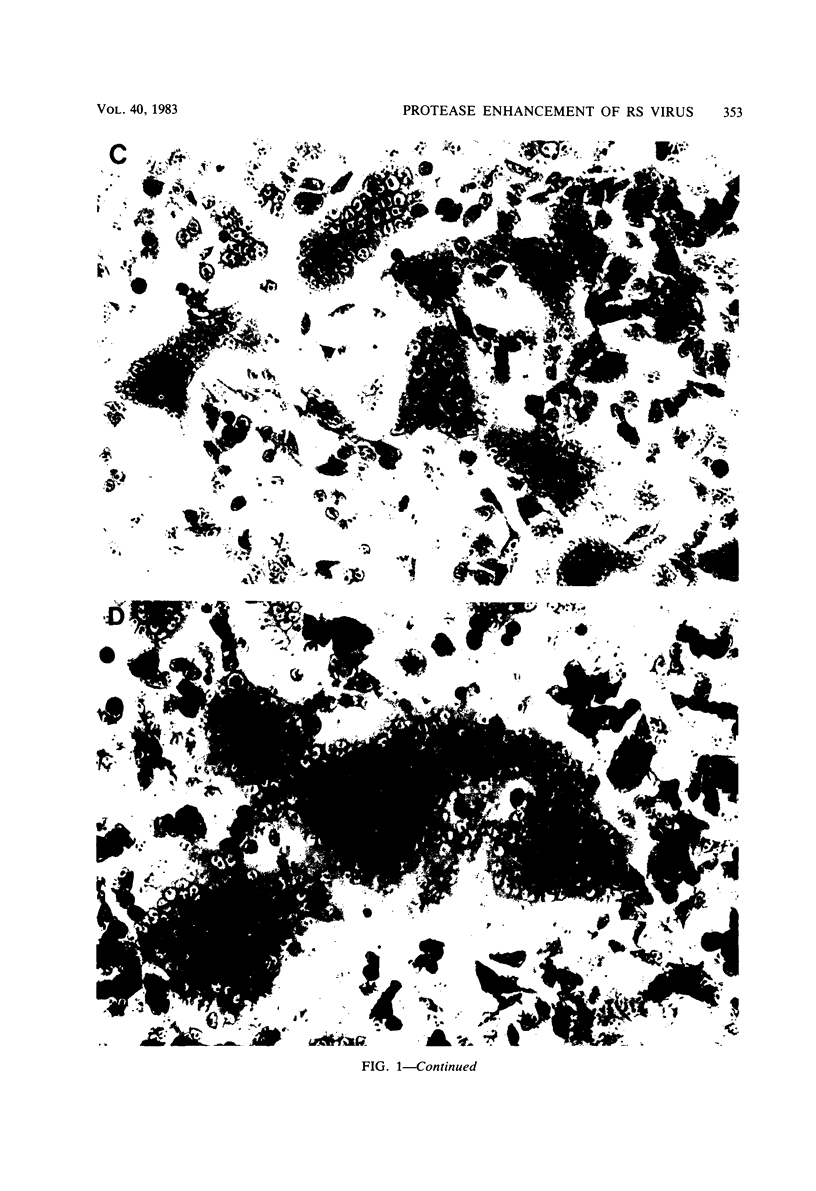
A series of proteases of diverse substrate specificity were tested for their effect on respiratory syncytial virus-induced cytopathology. Three of the enzymes, thrombin, plasmin, and trypsin, were able to augment significantly the fusion of virus-infected A549 cells. On a concentration basis, thrombin was the most active promoter, followed by plasmin and then trypsin. Hirudin, a specific thrombin inhibitor, blocked the fusion-enhancing property of thrombin, yet had no influence on the basal rate of fusion in the absence of the enzyme. By contrast, the amidine-type inhibitors of trypsin-like proteases, bis(5-amidino-2-benzimidazolyl)-methane (BABIM), blocked not only the thrombin effect, but also the fusion in the thrombin-free controls. The suppressive activity of BABIM was observed at concentrations so low as to exclude any direct inhibitory effect on thrombin itself. These results make it seem very likely that thrombin advances cell fusion by activating a BABIM-sensitive protease. Plasmin and trypsin can be expected to act in a similar manner.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruhn H. D. Growth regulation of vessel wall cells and of tumor cells by thrombin, factor XIII and fibronectin. Thromb Haemost. 1981 Dec 23;46(4):762–762. [PubMed] [Google Scholar]
- Carney D. H., Cunningham D. D. Role of specific cell surface receptors in thrombin-stimulated cell division. Cell. 1978 Dec;15(4):1341–1349. doi: 10.1016/0092-8674(78)90059-4. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):131–135. doi: 10.1073/pnas.72.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B., Teng N. N., Buchanan J. M. Mitogenicity of thrombin and surface alterations on mouse splenocytes. Exp Cell Res. 1976 Aug;101(1):41–46. doi: 10.1016/0014-4827(76)90409-2. [DOI] [PubMed] [Google Scholar]
- Czervionke R. L., Smith J. B., Hoak J. C., Fry G. L., Haycraft D. L. Use of a radioimmunoassay to study thrombin-induced release of PGI2 from cultured endothelium. Thromb Res. 1979;14(4-5):781–786. doi: 10.1016/0049-3848(79)90132-4. [DOI] [PubMed] [Google Scholar]
- Dubovi E. J., Geratz J. D., Shaver S. R., Tidwell R. R. Inhibition of respiratory syncytial virus-host cell interactions by mono- and diamidines. Antimicrob Agents Chemother. 1981 Apr;19(4):649–656. doi: 10.1128/aac.19.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J., Geratz J. D., Tidwell R. R. Inhibition of respiratory syncytial virus by bis(5-amidino-2-benzimidazolyl)methane. Virology. 1980 Jun;103(2):502–509. doi: 10.1016/0042-6822(80)90207-x. [DOI] [PubMed] [Google Scholar]
- Fernie B. F., Gerin J. L. Immunochemical identification of viral and nonviral proteins of the respiratory syncytial virus virion. Infect Immun. 1982 Jul;37(1):243–249. doi: 10.1128/iai.37.1.243-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten W., Bosch F. X., Linder D., Rott R., Klenk H. D. Proteolytic activation of the influenza virus hemagglutinin: The structure of the cleavage site and the enzymes involved in cleavage. Virology. 1981 Dec;115(2):361–374. doi: 10.1016/0042-6822(81)90117-3. [DOI] [PubMed] [Google Scholar]
- Geratz J. D., Shaver S. R., Tidwell R. R. Inhibitory effect of amidino-substituted heterocyclic compounds on the amidase activity of plasmin and of high and low molecular weight urokinase and on urokinase-induced plasminogen activation. Thromb Res. 1981 Oct 1;24(1-2):73–83. doi: 10.1016/0049-3848(81)90033-5. [DOI] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Korant B., Chow N., Lively M., Powers J. Virus-specified protease in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2992–2995. doi: 10.1073/pnas.76.6.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz S. G., Goldberg A. R., Choppin P. W. Proteolytic cleavage by plasmin of the HA polypeptide of influenza virus: host cell activation of serum plasminogen. Virology. 1973 Nov;56(1):172–180. doi: 10.1016/0042-6822(73)90296-1. [DOI] [PubMed] [Google Scholar]
- MacDonald N. E., Hall C. B., Suffin S. C., Alexson C., Harris P. J., Manning J. A. Respiratory syncytial viral infection in infants with congenital heart disease. N Engl J Med. 1982 Aug 12;307(7):397–400. doi: 10.1056/NEJM198208123070702. [DOI] [PubMed] [Google Scholar]
- Martin B. M., Wasiewski W. W., Fenton J. W., 2nd, Detwiler T. C. Equilibrium binding of thrombin to platelets. Biochemistry. 1976 Nov 2;15(22):4886–4893. doi: 10.1021/bi00667a021. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Importance of antibodies to the fusion glycoprotein of paramyxoviruses in the prevention of spread of infection. J Exp Med. 1980 Feb 1;151(2):275–288. doi: 10.1084/jem.151.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Scott A., Dittmar K. E., Owada M. Effect of p15-associated protease from an avian RNA tumor virus on avian virus-specific polyprotein precursors. J Virol. 1980 Feb;33(2):680–688. doi: 10.1128/jvi.33.2.680-688.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M., Homma M. Trypsin action on the growth of Sendai virus in tissue culture cells. V. An activating enzyme for Sendai virus in the chorioallantoic fluid of the embryonated chicken egg. Microbiol Immunol. 1980;24(2):113–122. doi: 10.1111/j.1348-0421.1980.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D., Rott R. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology. 1976 Jul 15;72(2):494–508. doi: 10.1016/0042-6822(76)90178-1. [DOI] [PubMed] [Google Scholar]
- Palmenberg A. C., Pallansch M. A., Rueckert R. R. Protease required for processing picornaviral coat protein resides in the viral replicase gene. J Virol. 1979 Dec;32(3):770–778. doi: 10.1128/jvi.32.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue J. F., Lubenskyi W., Kivity E., Sonder S. A., Fenton J. W., 2nd Protease mitogenic response of chick embryo fibroblasts and receptor binding/processing of human alpha-thrombin. J Biol Chem. 1981 Mar 25;256(6):2767–2776. [PubMed] [Google Scholar]
- Rifkin D. B. Plasminogen activator synthesis by cultured human embryonic lung cells: characterization of the suppressive effect of corticosteroids. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):421–428. doi: 10.1002/jcp.1040970317. [DOI] [PubMed] [Google Scholar]
- Roblin R., Young P. L. Dexamethasone regulation of plasminogen activator in embryonic and tumor-derived human cells. Cancer Res. 1980 Aug;40(8 Pt 1):2706–2713. [PubMed] [Google Scholar]
- SHERMER R. W., MASON R. G., WAGNER R. H., BRINKHOUS K. M. Studies on thrombin-induced platelet agglutination. J Exp Med. 1961 Dec 1;114:905–920. doi: 10.1084/jem.114.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Protease activation mutants of sendai virus. Activation of biological properties by specific proteases. Virology. 1976 Jan;69(1):265–277. doi: 10.1016/0042-6822(76)90213-0. [DOI] [PubMed] [Google Scholar]
- Shigeta S., Hinuma Y., Suto T., Ishida N. The cell to cell infection of respiratory syncytial virus in HEp-2 monolayer cultures. J Gen Virol. 1968 Jul;3(1):129–131. doi: 10.1099/0022-1317-3-1-129. [DOI] [PubMed] [Google Scholar]
- Tidwell R. R., Geratz J. D., Dann O., Volz G., Zeh D., Loewe H. Diarylamidine derivatives with one or both of the aryl moieties consisting of an indole or indole-like ring. Inhibitors of arginine-specific esteroproteases. J Med Chem. 1978 Jul;21(7):613–623. doi: 10.1021/jm00205a005. [DOI] [PubMed] [Google Scholar]
- Tidwell R. R., Geratz J. D., Dubovi E. J. Aromatic amidines: comparison of their ability to block respiratory syncytial virus induced cell fusion and to inhibit plasmin, urokinase, thrombin, and trypsin. J Med Chem. 1983 Feb;26(2):294–298. doi: 10.1021/jm00356a036. [DOI] [PubMed] [Google Scholar]
- Yoshikura H., Tejima S. Role of protease in mouse hepatitis virus-induced cell fusion. Studies with a cold-sensitive mutant isolated from a persistent infection. Virology. 1981 Sep;113(2):503–511. doi: 10.1016/0042-6822(81)90178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]