Abstract
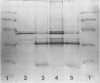
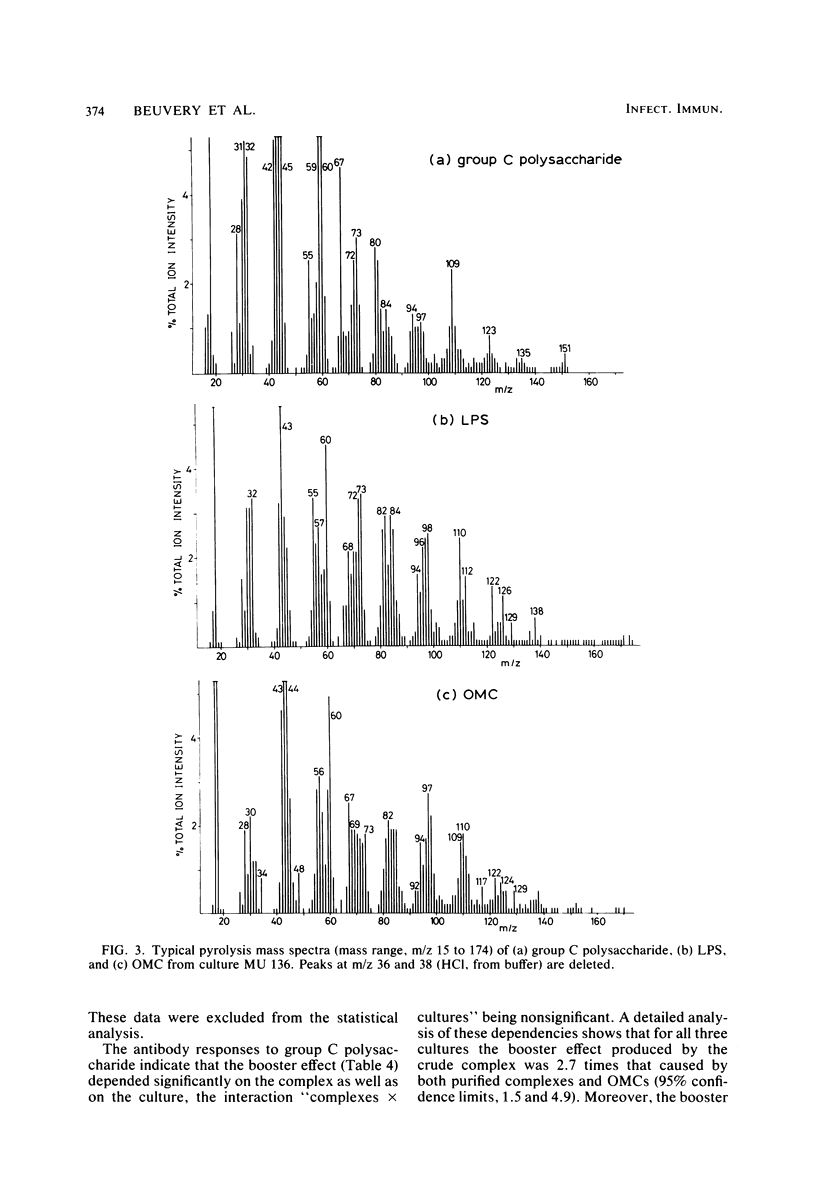
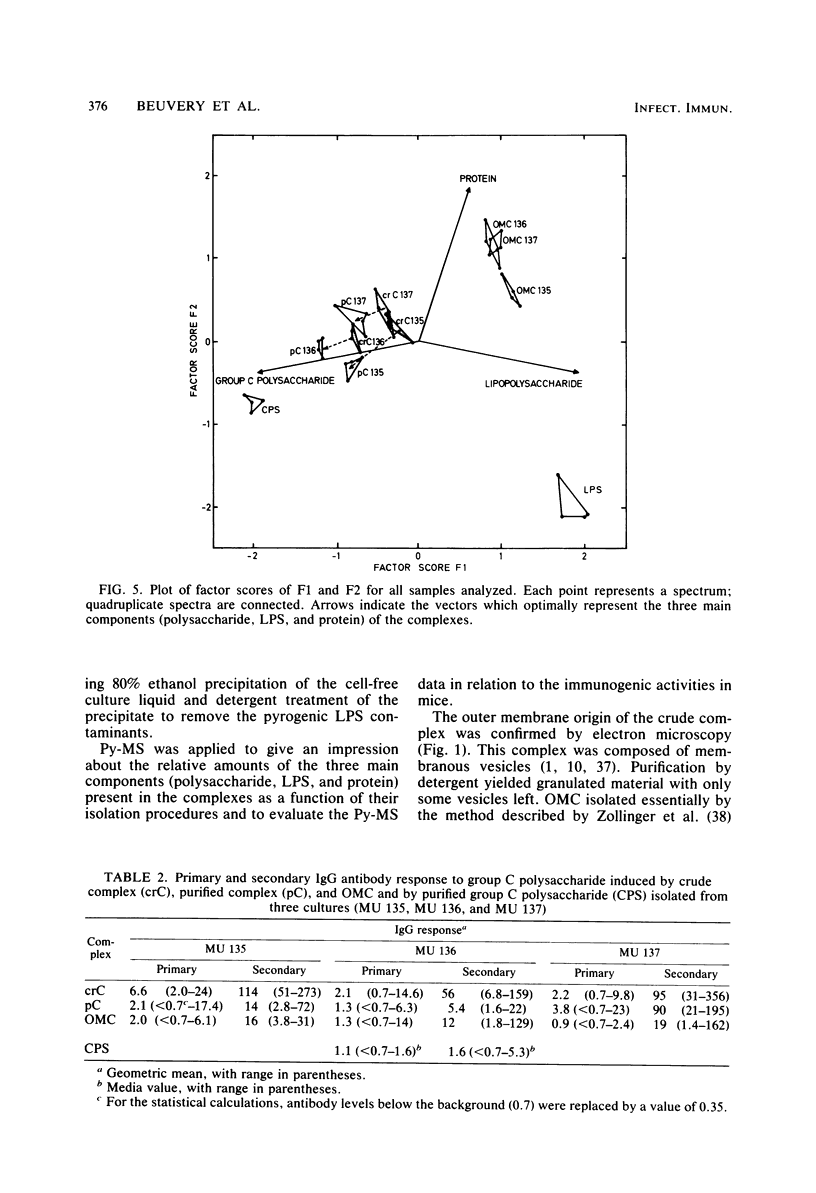
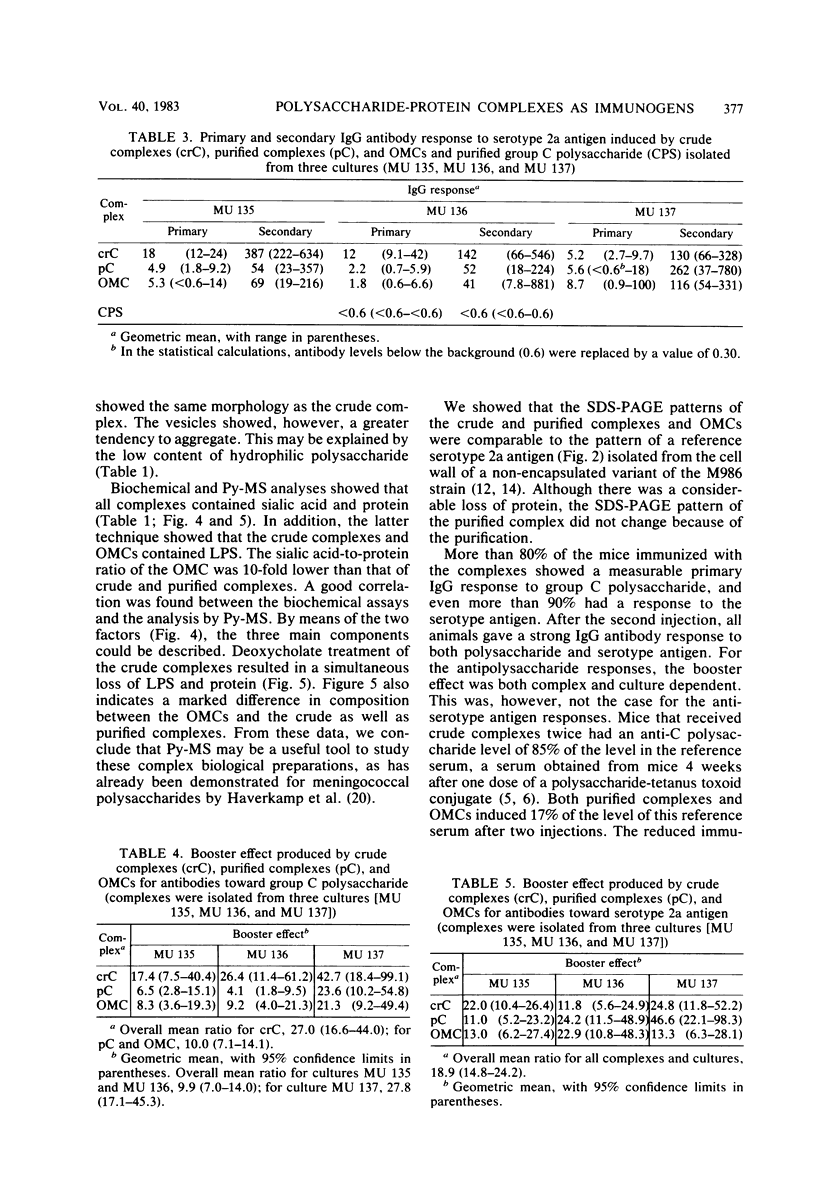
A crude complex containing group C polysaccharide, outer membrane proteins, and lipopolysaccharide (LPS) was isolated from the cell-free culture liquid of Neisseria meningitidis serogroup C, serotype 2a. Group C polysaccharide and LPS were removed from this complex, resulting in an outer membrane complex and a purified complex, respectively. Analysis by electron microscopy showed the outer membrane origin of the crude complex and the outer membrane complex, whereas such a structure was absent in the purified complex. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis patterns of the three complexes were identical. Pyrolysis-mass spectrometry data correlated well with those obtained by the biochemical assays and suggested a low LPS content in the purified complex and a low polysaccharide content in the outer membrane complex. The purified complex was shown to be nonpyrogenic and could be prepared with the same yield as that of purified polysaccharide. The immunogenic activities of the complexes were studied in mice. The antibodies were measured by the enzyme-linked immunosorbent assay; and the bactericidal antibody assay. All complexes induced immunoglobulin G antibodies to group C polysaccharide as well as to the serotype antigen, although the removal of polysaccharide and LPS resulted in a reduction of the immunogenic activities of outer membrane complex and purified complex, respectively. A second dose of all complexes produced a clear booster effect of both antibody responses. The antibodies were bactericidal.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B. M., Skjørten F., Solberg O. Loss of endotoxin liberation in Neisseria meningitidis. Acta Pathol Microbiol Scand B. 1981 Aug;89(4):271–278. doi: 10.1111/j.1699-0463.1981.tb00188_89b.x. [DOI] [PubMed] [Google Scholar]
- Baker P. H., Stashak P. W. Quantitative and qualitative studies on the primary antibody response to pneumococcal polysaccharides at ehe cellular level. J Immunol. 1969 Dec;103(6):1342–1348. [PubMed] [Google Scholar]
- Beuvery E. C. Immunisation against bacterial meningitis. J Infect. 1981 Mar;3(1 Suppl):71–79. doi: 10.1016/s0163-4453(81)80011-4. [DOI] [PubMed] [Google Scholar]
- Beuvery E. C., Leussink A. B., Van Delft R. W., Tiesjema R. H., Nagel J. Immunoglobulin M and G antibody responses and persistence of these antibodies in adults after vaccination with a combined meningococcal group A and group C polysaccharide vaccine. Infect Immun. 1982 Aug;37(2):579–585. doi: 10.1128/iai.37.2.579-585.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuvery E. C., van Rossum F., Nagel J. Comparison of the induction of immunoglobulin M and G antibodies in mice with purified pneumococcal type 3 and meningococcal group C polysaccharides and their protein conjugates. Infect Immun. 1982 Jul;37(1):15–22. doi: 10.1128/iai.37.1.15-22.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven D. E., Frasch C. E. Protection against group B meningococcal disease: evaluation of serotype 2 protein vaccines in a mouse bacteremia model. Infect Immun. 1979 Oct;26(1):110–117. doi: 10.1128/iai.26.1.110-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoe I. W., Gilchrist J. E. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J Exp Med. 1973 Nov 1;138(5):1156–1167. doi: 10.1084/jem.138.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz I. D. Growth Requirements of the Meningococcus. J Bacteriol. 1942 Jun;43(6):757–761. doi: 10.1128/jb.43.6.757-761.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Gotschlich E. C. An outer membrane protein of Neisseria meningitidis group B responsible for serotype specificity. J Exp Med. 1974 Jul 1;140(1):87–104. doi: 10.1084/jem.140.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Robbins J. D. Protection against group B meningococcal disease. III. Immunogenicity of serotype 2 vaccines and specificity of protection in a guinea pig model. J Exp Med. 1978 Mar 1;147(3):629–644. doi: 10.1084/jem.147.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E. Role of protein serotype antigens in protection against disease due to Neisseria meningitidis. J Infect Dis. 1977 Aug;136 (Suppl):S84–S90. doi: 10.1093/infdis/136.supplement.s84. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969 Jun 1;129(6):1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Austrian R., Cvjetanović B., Robbins J. B. Prospects for the prevention of bacterial meningitis with polysaccharide vaccines. Bull World Health Organ. 1978;56(4):509–518. [PMC free article] [PubMed] [Google Scholar]
- Haverkamp J., Meuzelaar H. L., Beuvery E. C., Boonekamp P. M., Tiesjema R. H. Characterization of Neisseria meningitidis capsular polysaccharides containing sialic acid by pyrolysis mass spectrometry. Anal Biochem. 1980 May 15;104(2):407–418. doi: 10.1016/0003-2697(80)90092-5. [DOI] [PubMed] [Google Scholar]
- Kreeftenberg J. G., Loggen H. G., van Ramshorst J. D., Beuvery E. C. The limulus amebocyte lysate test micromethod and application in the control of sera and vaccines. Dev Biol Stand. 1977;34:15–20. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Sivonen A., Mäkelä H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics. 1977 Nov;60(5):730–737. [PubMed] [Google Scholar]
- Peltola H., Mäkelä H., Käyhty H., Jousimies H., Herva E., Hällström K., Sivonen A., Renkonen O. V., Pettay O., Karanko V. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med. 1977 Sep 29;297(13):686–691. doi: 10.1056/NEJM197709292971302. [DOI] [PubMed] [Google Scholar]
- Poolman J. T., Hopman C. T., Zanen H. C. Immunochemical characterization of Neisseria meningitidis serotype antigens by immunodiffusion and SDS-polyacrylamide gel electrophoresis immunoperoxidase techniques and the distribution of serotypes among cases and carriers. J Gen Microbiol. 1980 Feb;116(2):465–473. doi: 10.1099/00221287-116-2-465. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E., Mocca L. F. Five structural classes of major outer membrane proteins in Neisseria meningitidis. J Bacteriol. 1981 Apr;146(1):69–78. doi: 10.1128/jb.146.1.69-78.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zollinger W. D., Kasper D. L., Veltri B. J., Artenstein M. S. Isolation and characterization of a native cell wall complex from Neisseria meningitidis. Infect Immun. 1972 Nov;6(5):835–851. doi: 10.1128/iai.6.5.835-851.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Altieri P., Berman S., Lowenthal J., Artenstein M. S. Safety and immunogenicity of a Neisseria meningitidis type 2 protein vaccine in animals and humans. J Infect Dis. 1978 Jun;137(6):728–739. doi: 10.1093/infdis/137.6.728. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Griffiss J. M., Altieri P., Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979 May;63(5):836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Pennington C. L., Artenstein M. S. Human antibody response to three meningococcal outer membrane antigens: comparison by specific hemagglutination assays. Infect Immun. 1974 Nov;10(5):975–984. doi: 10.1128/iai.10.5.975-984.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]