Abstract
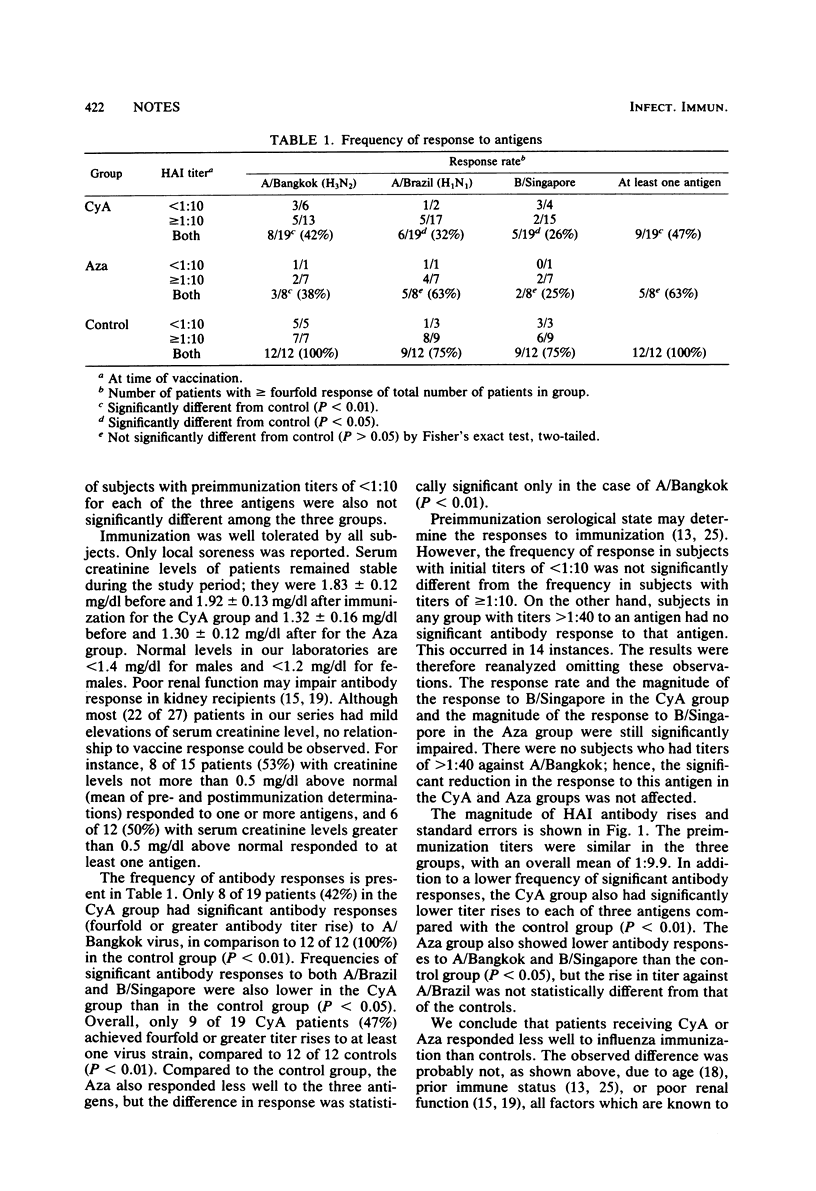
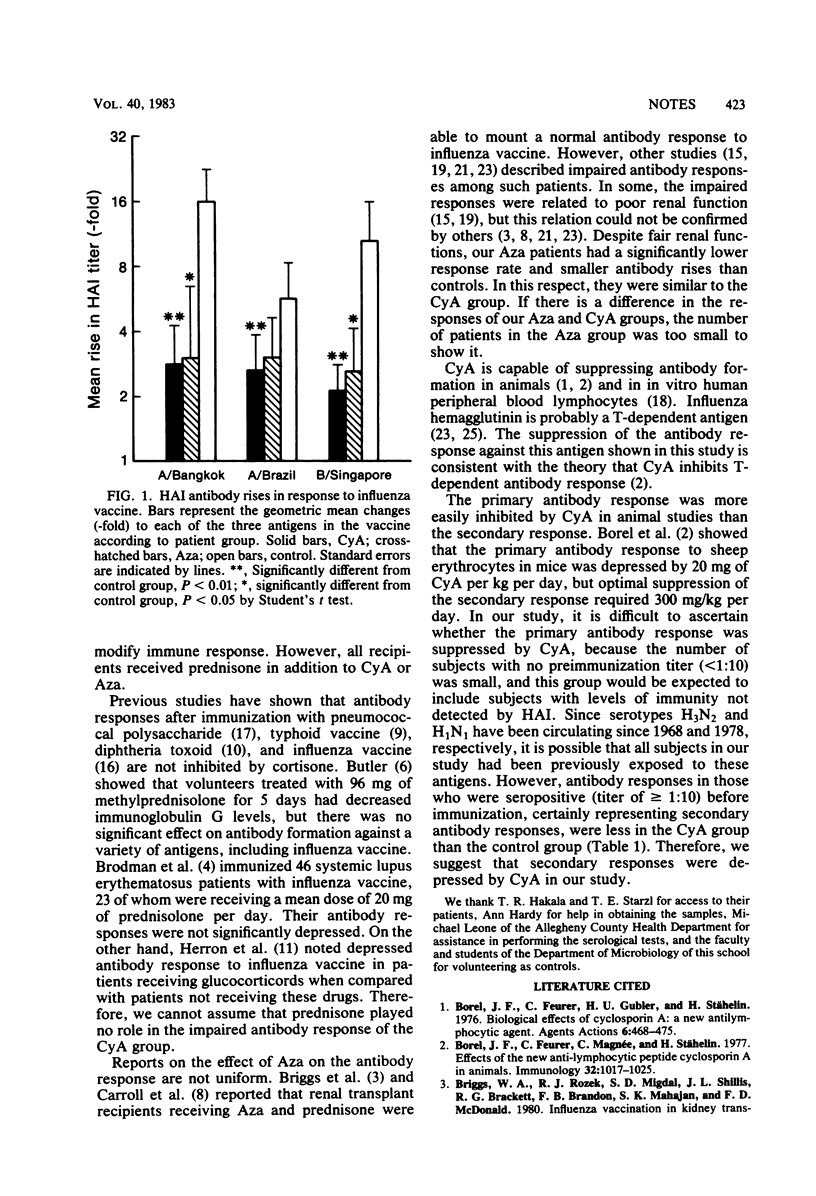
Nineteen renal transplant recipients receiving cyclosporin A and prednisone, eight kidney recipients receiving azathioprine and prednisone, and 12 healthy volunteers were immunized with 0.5 ml of trivalent influenza vaccine containing A/Bangkok/1/79 (H3N2), A/Brazil/11/78 (H1N1), and B/Singapore/222/79. Nine patients (47%) in the cyclosporin A group and five (63%) in the azathioprine group showed fourfold rises in titer to at least one virus strain compared with 12 (100%) in the control group.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borel J. F., Feurer C., Gubler H. U., Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976 Jul;6(4):468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Brodman R., Gilfillan R., Glass D., Schur P. H. Influenzal vaccine response in systemic lupus erythematosus. Ann Intern Med. 1978 Jun;88(6):735–740. doi: 10.7326/0003-4819-88-6-735. [DOI] [PubMed] [Google Scholar]
- Bunjes D., Hardt C., Röllinghoff M., Wagner H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol. 1981 Aug;11(8):657–661. doi: 10.1002/eji.1830110812. [DOI] [PubMed] [Google Scholar]
- Butler W. T. Corticosteroids and immunoglobulin synthesis. Transplant Proc. 1975 Mar;7(1):49–53. [PubMed] [Google Scholar]
- Calne R. Y., Rolles K., White D. J., Thiru S., Evans D. B., McMaster P., Dunn D. C., Craddock G. N., Henderson R. G., Aziz S. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979 Nov 17;2(8151):1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- Carroll R. N., Marsh S. D., O'Donoghue E. P., Breeze D. C., Shackman R. Response to influenza vaccine by renal transplant patients. Br Med J. 1974 Jun 29;2(5921):701–703. doi: 10.1136/bmj.2.5921.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN H. T. The influence of cortisone and hydrocortisone on the production of circulating antibody in human beings. J Allergy. 1953 Jul;24(4):342–347. doi: 10.1016/0021-8707(53)90179-8. [DOI] [PubMed] [Google Scholar]
- HAVENS W. P., Jr, SHAFFER J. M., HOPKE C. J., Jr The effect of ACTH and cortisone on the concentration of circulating diphtheria antitoxin. J Immunol. 1952 Apr;68(4):389–400. [PubMed] [Google Scholar]
- Herron A., Dettleff G., Hixon B., Brandwin L., Ortbals D., Hornick R., Hahn B. Influenza vaccination in patients with rheumatic diseases. Safety and efficacy. JAMA. 1979 Jul 6;242(1):53–56. [PubMed] [Google Scholar]
- Hess A. D., Tutschka P. J. Effect of cyclosporin A on human lymphocyte responses in vitro. I. CsA allows for the expression of alloantigen-activated suppressor cells while preferentially inhibiting the induction of cytolytic effector lymphocytes in MLR. J Immunol. 1980 Jun;124(6):2601–2608. [PubMed] [Google Scholar]
- Hobson D., Baker F. A., Curry R. L. Effect of influenza vaccines in stimulating antibody in volunteers with prior immunity. Lancet. 1973 Jul 21;2(7821):155–156. doi: 10.1016/s0140-6736(73)93106-1. [DOI] [PubMed] [Google Scholar]
- KUNIN C. M., SCHWARTZ R., YAFFE S., KNAPP J., FELLERS F. X., JANEWAY C. A., FINLAND M. Antibody response to influenza-virus vaccine in children with nephrosis: effect of cortisone. Pediatrics. 1959 Jan;23(1 Pt 1):54–62. [PubMed] [Google Scholar]
- Kumar S. S., Ventura A. K., VanderWerf B. Influenza vaccination in renal transplant recipients. JAMA. 1978 Feb 27;239(9):840–842. [PubMed] [Google Scholar]
- MIRICK G. S. The effects of ACTH and cortisone on antibodies in human beings. Bull Johns Hopkins Hosp. 1951 Apr;88(4):332–351. [PubMed] [Google Scholar]
- Paavonen T., Häyry P. Effect of cyclosporin A on T-dependent and T-independent immunoglobulin synthesis in vitro. Nature. 1980 Oct 9;287(5782):542–544. doi: 10.1038/287542a0. [DOI] [PubMed] [Google Scholar]
- Pabico R. C., Douglas R. G., Betts R. F., McKenna B. A., Freeman R. B. Antibody response to influenza vaccination in renal transplant patients: correlation with allograft function. Ann Intern Med. 1976 Oct;85(4):431–436. doi: 10.7326/0003-4819-85-4-431. [DOI] [PubMed] [Google Scholar]
- Parkman P. D., Hopps H. E., Rastogi S. C., Meyer H. M., Jr Summary of clinical trials of influenza virus vaccines in adults. J Infect Dis. 1977 Dec;136 (Suppl):S722–S730. doi: 10.1093/infdis/136.supplement_3.s722. [DOI] [PubMed] [Google Scholar]
- Rytel M. W., Niebojewski R. A., Rosenkranz M. A., Sedmak G. Humoral and cell mediated immune response to bivalent influenza A/NJ/76 and A/Vict/75 vaccine in renal allograft recipients. Dev Biol Stand. 1977 Jun 1;39:225–230. [PubMed] [Google Scholar]
- Starzl T. E., Klintmalm G. B., Porter K. A., Iwatsuki S., Schröter G. P. Liver transplantation with use of cyclosporin a and prednisone. N Engl J Med. 1981 Jul 30;305(5):266–269. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiver H. G., Graves P., Meiklejohn G., Schröter G., Eickhoff T. C. Impaired serum antibody response to inactivated influenza A and B vaccine in renal transplant recipients. Infect Immun. 1977 Jun;16(3):738–741. doi: 10.1128/iai.16.3.738-741.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauraso N. M., Gleckman R., Pedreira F. A., Sabbaj J., Yahwak R., Madoff M. A. Effect of dosage and route of inoculation upon antigenicity of inactivated influenza virus vaccine (Hong Kong strain) in man. Bull World Health Organ. 1969;41(3):507–516. [PMC free article] [PubMed] [Google Scholar]
- Virelizier J. L., Postlethwaite R., Schild G. C., Allison A. C. Antibody responses to antigenic determinants of influenza virus hemagglutinin. I. Thymus dependence of antibody formation and thymus independence of immunological memory. J Exp Med. 1974 Dec 1;140(6):1559–1570. doi: 10.1084/jem.140.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]


