Abstract
Background
With the recent completion of numerous sequenced bacterial genomes, notable advances have been made in understanding the level of conservation between various species. However, relatively little is known about the genomic diversity among strains. We determined the complete genome sequence of the Florida strain of Anaplasma marginale, and near complete (>96%) sequences for an additional three strains, for comparative analysis with the previously fully sequenced St. Maries strain genome.
Results
These comparisons revealed that A. marginale has a closed-core genome with few highly plastic regions, which include the msp2 and msp3 genes, as well as the aaap locus. Comparison of the Florida and St. Maries genome sequences found that SNPs comprise 0.8% of the longer Florida genome, with 33.5% of the total SNPs between all five strains present in at least two strains and 3.0% of SNPs present in all strains except Florida. Comparison of genomes from three strains of Mycobacterium tuberculosis, Bacillus anthracis, and Nessieria meningiditis, as well as four Chlamydophila pneumoniae strains found that 98.8%–100% of SNPs are unique to each strain, suggesting A. marginale, with 76.0%, has an intermediate level of strain-specific SNPs. Comparison of genomes from other organisms revealed variation in diversity that did not segregate with the environmental niche the bacterium occupies, ranging from 0.00% to 8.00% of the larger pairwise-compared genome.
Conclusion
Analysis of multiple A. marginale strains suggests intracellular bacteria have more variable SNP retention rates than previously reported, and may have closed-core genomes in response to the host organism environment and/or reductive evolution.
Background
While the recent boom in genome sequencing projects has provided a wealth of information about bacterial metabolism and evolution, we know little about interstrain variation. A firm understanding of the rates and sites of variation is useful in determining genotypic differences associated with phenotypic traits and in formulating control strategies for a number of pathogens. Further, knowledge about the pan-genome of organisms will aid in determining the core genomic requirements, as well as shed more light on events that occur in the various environmental niches bacteria occupy.
Most studies of bacterial diversity to date have either utilized specific genomic loci [1,2] or have examined metagenomics of specific environmental niches [3,4]. While these types of studies help elucidate the extent of diversity, there is still a key component that has not yet been investigated – a measurement of diversity within bacterial species. Obtaining a true measure of species diversity is difficult, as the strains selected for whole-genome sequencing are generally chosen to examine a particular phenotypic trait, subjecting any resultant measures of diversity to selection bias.
The level of interstrain diversity can have a significant impact on the direction of research. Selection of pathogen strains for sequencing is typically based on differences in virulence [5], host preference [6], or tissue tropism [7]. Using these selection criteria may artificially skew the level of diversity in the studied genome sequences, resulting in a biased level of diversity which does not accurately reflect the true genetic diversity of the species. However, since the diversity among strains has only been examined in a small number of species, determining if there is a skew is difficult. For example, analysis of several genome sequences of Bacillus anthracis found a low number of single-nucleotide polymorphisms (SNPs) [8], which led to development of other techniques for examining the epidemiology of outbreak strains [9]. B. anthracis is an example of a "closed core" genome – that is, after sequencing several strains (four for B. anthracis), no strain-specific genes are added to the pan-genome [10], which may be a result of a clonal population split of B. anthracis from B. cereus. Thus, the closed-core genome may be the result of small evolutionary distance, and may be a rare finding for organisms with larger evolutionary distance. The alternative is an "open core" genome, where each new sequenced strain adds at least one unique gene to the pan-genome. This is exemplified by Streptococcus agalactiae, which has approximately 30 new strain-specific genes for each additional genome sequenced, regardless of the total number of strains compared.
What influences the pan-genome? Is the pan-genome content fixed, or does it drift with time? Do all non-clonal populations have open-core genomes, or is this influenced by the environment a bacterium occupies? While answering all of these questions will require sequencing many more genomes, Anaplasma marginale makes an excellent system for studying the last question for a number of reasons. A. marginale is a member of the order Rickettsiales and a well-established obligate intracellular bacterial model. A. marginale is the most globally prevalent vector-borne pathogen of cattle, causing cyclic anemia, decreased production, and possibly death [11]. A previous genome sequence for the St. Maries strain [11] establishes that this organism has a small genome size due to reductive evolution, and is related to several other intracellular pathogens, including those in the genera Anaplasma, Ehrlichia, and Rickettsia [12]. In addition, A. marginale has a number of characterized strains, with each strain defined by msp1α genotype [13,14]. While previous studies have utilized specific genes to examine differences between these strains [15-17], no studies have examined the species diversity of A. marginale. A number of studies have described strains that vary in geographic location and phenotypic traits [15,18,19], and these are available for determination of the true level of genetic diversity in this species, subsequent analysis of the status of core genes, and determination if these are correlated with the intracellular lifestyle, geographic location, tick-transmissible status, or other characteristics of these organisms.
To answer this question, we obtained genome sequences for four strains of A. marginale that have differing abilities to be transmitted by Dermacentor andersoni, with each phenotype represented by at least two geographically distinct isolations. We sequenced the Florida strain to completion using a BAC-based clone by clone approach, and obtained high coverage genome sequence data for three additional strains. The resulting DNA sequences were then analyzed and compared to both the previously sequenced St. Maries genome [11], as well as other bacterial species in the Order Rickettsiales. Further, the genomes of several other non-rickettsial bacteria were examined with similar genome comparison techniques to determine if diversity and pan-genome content are related to pathogenicity or an intracellular lifestyle.
Results
Microbial genome diversity
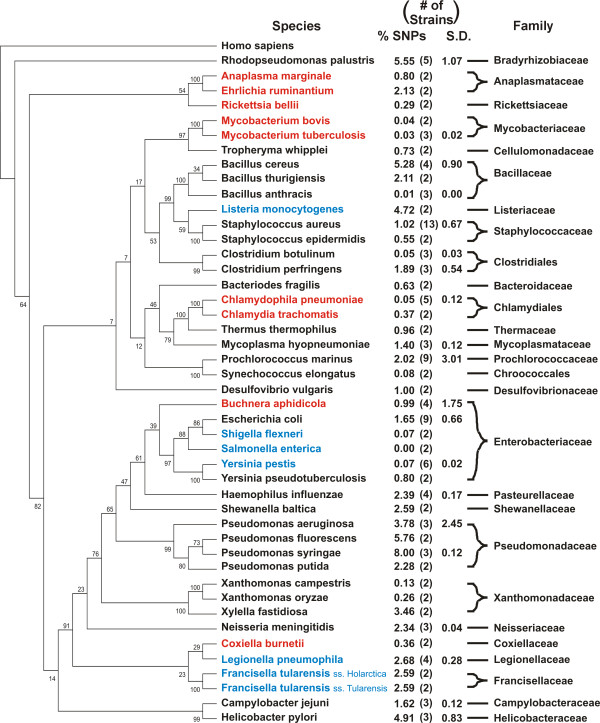
Previous studies [6,20] have shown high levels of variation between the genomes of different strains of obligate intracellular bacteria. To compare the levels found in A. marginale to other genomes, similar comparisons were made for organisms meeting the following criteria: organisms with 1) a single chromosome, 2) more than one sequenced strain, and 3) assembled and finished genome sequences deposited in Genbank, including free-living, facultative intracellular, and obligate intracellular bacteria (Figure 1). Single-factor analysis of variance (ANOVA) finds no significant differences in the level of variation between obligate intracellular, facultative, and free-living bacteria. The number of SNPs ranged from 0.00% to 8.00% of the larger genome, with significant intraspecies and intragenera variation.
Figure 1.
Comparison of the level of SNP diversity among sequenced genomes. Bacteria listed in blue are obligate intracellular, while those in red are facultatively intracellular. Each organism lists the average level of SNPs as a percentage of the largest genome.
General genome features and comparison of the St. Maries and Florida strains
The Anaplasma marginale Florida strain genome is composed of a single 1,202,435 bp circular chromosome predicted to contain 942 coding sequences (CDS) (Table 1). Similar to most other previously sequenced Anaplasmataceae, there are no plasmids and no identifiable insertion sequences. Compared to the previously sequenced St. Maries strain genome [11], there are seven fewer CDSs despite the larger genome size, due primarily to differences in split open reading frames (ORFs) and annotation differences. The high degree of synteny between these two strains is disrupted by two inversions; one approximately 30 kb long is flanked by repeat elements (msp3 pseudogenes), while the other is a single gene flanked by short duplicated hypothetical genes.
Table 1.
Comparison of the St. Maries and Florida genome features
| Anaplasma marginale | ||
| St. Maries | Florida | |
| Genome Size (bp) | 1,197,687 | 1,202,435 |
| CDS features | 949 | 942 |
| GC content (%) | 49.86% | 49.86% |
| Coding density (%) | 85.40% | 85.50% |
| Average gene length | 1078 | 1091 |
| rRNA genes | 3 | 3 |
| tRNA genes | 37 | 37 |
| Functional pseudogenes | 16 | 17 |
Split ORFs were first described in the Rickettsia conori genome [21], and are postulated to represent genes that are in the first stage of reductive evolution. The idea that these ORFs have split recently is consistent with the findings in Anaplasma, as different ORFs are split in the two completely sequenced strains. The four split ORFs annotated in the St. Maries genome (mutL, murC, aatA, and aspS) [11] are intact in the Florida genome, and two tandem genes annotated as hypothetical in the St. Maries genome (AM574 and AM576) are fused in the Florida genome (AMF_437). Only one split ORF, petA, is found in the Florida genome. Four small ORFs in the St. Maries genome (AM380, AM395, AM974, and AM976), ranging in size from 204 bp to 378 bp are not present in the Florida genome. These ORFs are flanked by repetitive DNA sequences, and appear to be missing due to recombination events.
Genes mediating genome plasticity
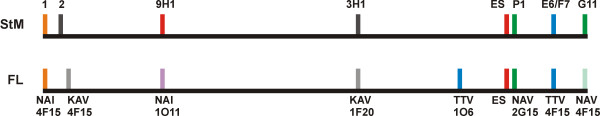
The msp2 superfamily is a group of related A. marginale genes encoding surface proteins [11]. Msp2 encodes a highly antigenic protein that varies over time during infection by gene conversion of functional pseudogenes into a single expression site, to create new antigenic variants capable of evading the existing immune response. Compared to the St. Maries genome, the Florida genome has one additional msp2 functional pseudogene. Of the eight Florida msp2 functional pseudogenes, four are identical to those in the St. Maries genome. The Florida genome has two sets of duplicated functional pseudogenes, TTV 4F15/TTV 1O6 and KAV 4F15/KAV 1F20 (Figure 2); while St. Maries was found to have duplicated functional pseudogenes, this was not noted in a functional pseudogene-targeted examination of other strains [22]. Florida has a set of duplicated functional pseudogenes in the same genome positions as St. Maries (2/3H1 in St. Maries, and KAV 4F15/KAV 1F20 in Florida). As obligate intracellular bacteria are not thought to undergo lateral gene transfer, identical functional pseudogenes indicates the sequence is either evolutionarily conserved or has been selected independently in both strains due to a fitness advantage. Interestingly, both copies in Florida have a change encoding 15 amino acids at the 5' end of the hypervariable region compared to their St. Maries counterparts; either both strains duplicated a functional pseudogene after this change occurred in an ancestral strain, or both copies in one of the strains acquired identical changes after the ancestral strain duplicated the original functional pseudogene. In contrast, only two of the seven MSP3 functional pseudogenes are identical between Florida and St. Maries (msp3 C/msp3-1, and msp3 4L1/msp3 6). The omp1-15 genes are present in both genomes, with a high degree of conservation between the predicted amino acid sequences (85.3–100% identity) as previously reported [17].
Figure 2.
Physical map of the MSP2 functional pseudogenes in the St. Maries (StM) and Florida (FL) strains. Vertical bars indicate relative position of functional pseudogenes in the genome (not to scale). Bars with the same color indicate identical functional pseudogenes, while similar colors indicate functional pseudogenes with segmental changes. ES represents the msp2 expression site.
Aaap gene family
The aaap gene was first recognized and characterized as an Anaplasma appendage associated protein [23]. Subsequently, additional related genes were identified that appear to be tandemly-duplicated copies that have diverged to have relatively low levels of sequence identity (Table 2). There is expansion of this locus in the Florida strain relative to the St. Maries strain, with a duplicated copy of the aaap gene. Because of the repetitive nature of this gene family, these sequences tend to be missing from pyrosequenced genome assemblies; therefore, we examined the status of this locus in several the strains via Southern analysis, revealing that this locus is highly plastic both within and between strains (Figure 3).
Table 2.
Identity between deduced AAAP amino acid sequences from the St. Maries and Florida strains
| St. Maries | ||||
| aaap | AM879 | AM880 | ||
| Florida | aaap | 45.0% | 49.4% | 49.6% |
| alp1 | 46.1% | 73.8% | 24.1% | |
| alp2 | 33.4% | 40.3% | 58.2% | |
| alp3 | 11.7% | 30.7% | 24.6% | |
Figure 3.
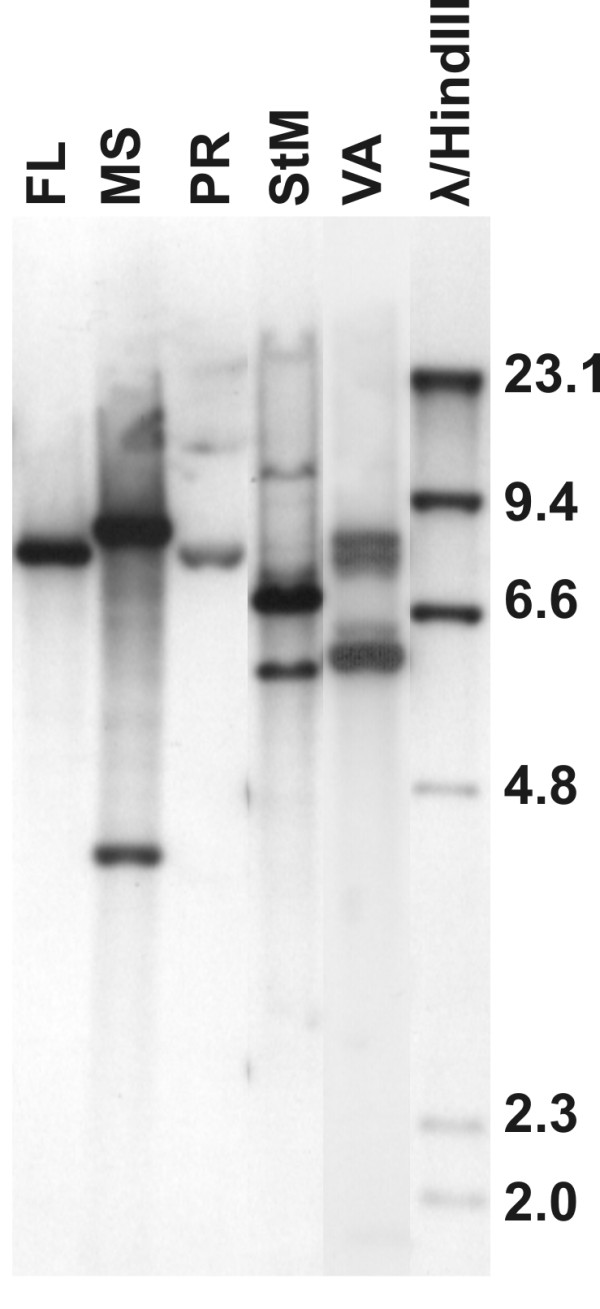
Southern blot of the aaap locus in A. marginale strains. FL – Florida strain, MS – Mississippi strain, PR – Puerto Rico strain, StM – St. Maries strain, VA – Virginia strain. DNA marker sizes are listed in kbp.
High density sequence coverage of additional strains
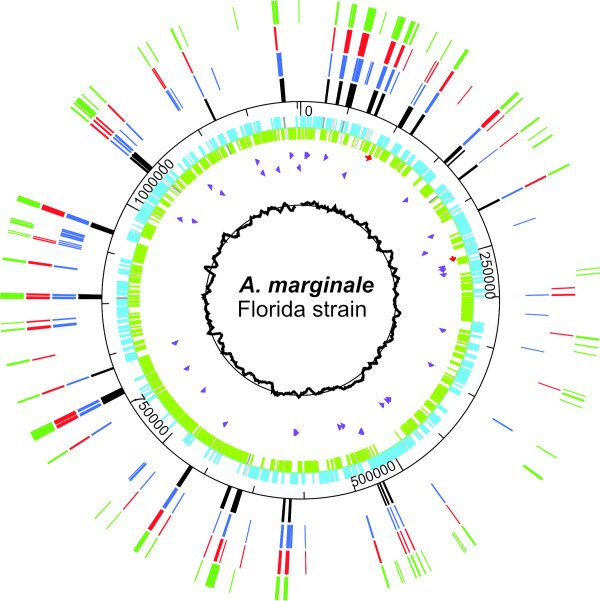
An additional two transmissible strains (Virginia and Puerto Rico) and one non-transmissible strain (Mississippi) were subjected to genome-scale pyrosequencing [24] (454 Life Sciences, Branford, CT), which provided at least 96% genome coverage when compared to either Florida or St. Maries (Table 3). Most of the missing sequences corresponded to repetitive regions (such as msp2 and msp3 pseudogenes and the aaap locus) (Figure 4) [also see additional files 1 and 2], and reflects the limitations of assembling short sequence reads (averaging approximately 250 bp per read) without additional scaffolding. No new genes were detected in the pyrosequenced contigs of any of the strains.
Table 3.
Pyrosequencing results for three strains of Anaplasma marginale
| Puerto Rico | Virginia | Mississippi | ||||
| St. Maries | Florida | St. Maries | Florida | St. Maries | Florida | |
| Number of large contigs | 75 | 59 | 81 | 70 | 78 | 82 |
| Bases in large contigs | 1,150,801 | 1,158,530 | 1,146,893 | 1,153,875 | 1,139,486 | 1,141,520 |
| % Genome coverage | 96.89% | 97.00% | 96.88% | 96.77% | 96.34% | 96.25% |
| High quality variations | 6,038 | 2,729 | 6,613 | 3,868 | 6,302 | 6,773 |
Figure 4.
Distribution of gaps in the three pyrosequenced genomes. In the outer rings, gaps in the Mississippi sequence are green, gaps in Puerto Rico are red, and gaps in Virginia are blue. Known repetitive genes are represented by black bars. The inner rings represent CDSs (blue and green) and functional pseudogenes (shades of grey) in the Florida strain, rRNAs (red) and tRNAs (purple), and the G-C skew (black graph).
Diversity of A. marginale strains
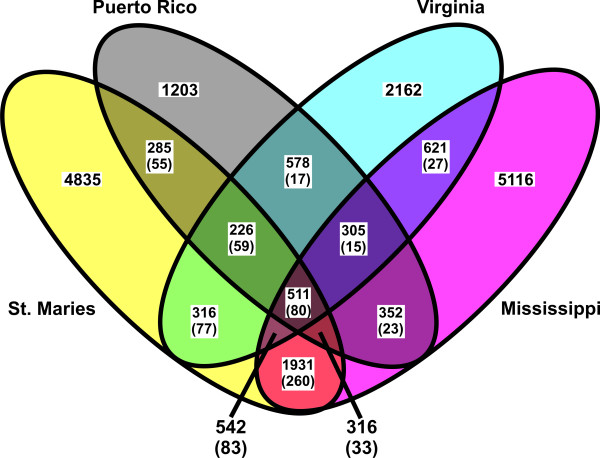
Global comparison of all strains with the Florida strain revealed 20,028 total sites with a single nucleotide polymorphism (SNP) in at least one of the compared strains. Of these, 511 (2.6%) were different in the Florida genome and identical in the other four strains, and 13,316 (66.5%) were unique to one of the four strains (Figure 5). The remaining 30.9% of SNPs represent those SNPs relative to Florida that were present in two or three of the strains. There were 9,609 SNPs between the Florida and St. Maries strains, comprising 0.80% of the larger Florida genome. The SNPs were distributed evenly throughout the genome, which is similar to both Ehrlichia ruminantium and Rickettsia bellii [see additional file 3], and are proportionally distributed throughout coding and non-coding regions. The numbers of polymorphisms in the Puerto Rico, Virginia, and Mississippi strains (2,729, 3,868, and 6,773, respectively) are minimums, as the gaps are regions predicted to have significant numbers of SNPs. When the genome size was corrected for the gaps in coverage, the SNP rates for the Puerto Rico, Virginia, and Mississippi genomes were 0.32%, 0.46%, and 0.73% of the Florida genome, respectively.
Figure 5.
Distribution of SNPs in four strains compared to the Florida strain. Numbers in parentheses show SNPs that are different from Florida in each strain in the subset, but are not the same in the compared strains.
Discussion
This study illustrates the dangers of drawing universal conclusions when strains are selected based on specific criteria, such as phenotypic differences. No two strains in this study are truly representative of the population as a whole. Additionally, the large number of differences in pair-wise comparisons of any two strains illustrates the difficulty of associating genes with phenotypic differences, and the utility of sequencing multiple strains to increase the power of these associations. While our initial selection of the Florida strain was based on a phenotypic difference – that of tick transmissibility, the selection of subsequent strains (PR, VA, and MS) were made to try and minimize the effect of bias based on that phenotype, as well as select a wider geographic range of isolates to increase interstrain diversity. Interestingly, when the pyrosequenced strains are compared to Florida, there are more high-quality polymorphisms (identified when four reads, each with at least 20 base pairs flanking the polymorphic site, contain the difference, with at least one read in each direction) between Florida and the Mississippi strain, despite the fact that neither is tick-transmissible by D. andersoni [19,25]. Further, St. Maries appears to be an outlier sequence, as there are at least 6,000 differences between St. Maries and all other sequenced strains.
The level of SNP diversity in these strains coupled with the high degree of gene content conservation also sheds an interesting light on the concept of the "core genome", described for Streptococcus agalactiae [10]. For S. agalactiae, approximately 90.5% of genes were considered part of the "core genome", or constant between strains, and each new strain added additional strain-specific genes to the "pan-genome". This is contrasted with Bacillus anthracis, which had no new strain-specific genes after four strains were compared. The strains of A. marginale sequenced here present an interesting data point, as A. marginale has not been hypothesized to be a clonal population derived from another organism (as has been postulated for B. anthracis), and yet has a closed core genome. The accumulation of large numbers of SNPs might indicate a greater evolutionary distance; however, the closed-core genome could be due to other factors. These could include the isolated nature of the intracellular niche occupied by A. marginale, causing the organism to undergo reductive evolution to the point it is approaching the minimal gene complement, or may be, despite our efforts, related to the strains selected for sequencing. However, if this is due to long-term reductive evolution, it calls into question the source of the six split ORFs between the Florida and St. Maries genomes, as these are thought to be early reductive changes. Another possibility is that transmission of the organism among animals in a relatively restricted geographic area (i.e., within a herd) promotes a relatively clonal population of organisms through isolation in a similar environment.
Analysis of the level of SNP diversity in several bacterial genomes brings into question previous conclusions about the variability of obligate intracellular pathogens. Previous studies [6] have found relatively large numbers of SNPs between intracellular organisms. It was therefore hypothesized that the relatively isolated intracellular niche limits opportunities for genetic exchange and increased numbers of SNPs provides a compensatory mechanism for providing diversity to drive evolution. Our results suggest this is unlikely, as there is no correlation between intracellular, facultative intracellular, and free-living organisms and the level of diversity. With few exceptions, there is a large range in the degree of variability in all the strains compared. Additionally, the organisms with the two highest rates of variability, Pseudomonas syringae and Rhodopseudomonas palustris, are both free-living. There is also significant variation at the genus and family level. These data suggest that the factors for retention of SNPs leading to bacterial diversity are likely multifactorial and complex.
While the composition of the gene content of the pan-genome is obviously important, this study reveals another characteristic that needs examination: the level of diversity in the pan-genome. The minimum of 20,028 variable sites found among these five genomes is approximately 1.67% of the estimated size of the pan-genome. The large number of unique SNPs in each strain (24.1% in the St. Maries genome, 6.0% in the Puerto Rico genome, 10.8% in the Virginia genome, and 25.5% in the Mississippi genome) suggests that while A. marginale has a closed core genome, the SNP profile of the core genome is moderately "open". When several strains of Streptococcus agalactiae (CJB111, COH1, A909, and 515) are compared to the 2603 VR strain, 99.18% of the 46,579 total detected SNPs are unique to an individual strain, while zero SNPs are common to all four strains. Similarly, 100% of SNPs between three strains of Bacillus anthracis (Ames, Ames Ancestor, and Sterne) and Mycobacterium tuberculosis (F11, H37Ra, and H37Rv), 98.8% of SNPs between three strains of Neisseria meningitides (FAM18, MC58, and Z2491), and 99.9% of SNPs between four strains of Chlamydophila pneumoniae (AR39, CWL029, J138, and TW-183) are unique to one strain. This suggests that these genomes have open SNP profiles regardless of being open or closed-core at the genome level. Further, there is no correlation between SNP diversity and lifestyle, with high levels of variation between strains and within genera, with limited exceptions. However, given that the majority of strains were selected based on phenotypic traits or previous work with each strain, it is unlikely that this represents the true diversity of these organisms. Additionally, the majority of organisms have only two sequenced strains, making analysis of variation within a species impossible to determine. Additional work will be required to build a picture of genomic diversity.
The genome of A. marginale is highly recombinogenic, which, in spite of the highly conserved gene content, leads to increased plasticity. There are between five and nine functional msp2 pseudogenes in the strains examined to date [11,22,26,27], and these can recombine in whole or in part into the msp2 expression site (or with each other) to generate new antigenic variants [26,27]. Symmetrical inversions around the origin are thought to be quite common in bacteria [28] and have been noted in Anaplasmataceae, often utilizing repeated genes such as msp2 to mediate the inversion. These inversions are highlighted by comparisons between A. marginale and Ehrlichia ruminantium [29] and Anaplasma phagocytophilum [30]. Many of these repetitive sequences flank ori, as does another duplicated gene, rho. While not around the origin, a smaller scale inversion was found between two strains of A. marginale flanked by msp3 pseudogenes close to ori. Another highly plastic genomic region is the AAAP locus [23] that appears to be expanding and contracting within and between strains. In addition to changes in gene number, the sequences are highly variable (Table 2). Further research will be needed to determine the significance of these differences, as well as the function of this locus.
Conclusion
Sequencing of multiple strains of bacteria, as well as sequencing multiple isolates from the same strain, will yield a tremendous amount of information about natural rates of variation in bacterial populations, which in turn will influence our views of bacterial evolution, epidemiology, and vaccine strategies. This study reveals that interstrain SNP diversity does not appear to be influenced by the environmental niche an organism occupies, nor is it generally consistent throughout a specific family or genera. Comparison of multiple strains of A. marginale finds few changes at the gene level, while there is robust diversity at the nucleotide level. Finally, multistrain SNP analysis appears to be a more powerful tool for A. marginale phylogenetic studies than genotyping of the major surface proteins [15], and this strategy should be useful for epidemiologic studies of other species of bacteria.
Methods
Experimental approval
All animal experiments described in this paper were approved by the Washington State University Institutional Animal Care and Use Committee (IACUC), with approval number 3386.
Strains of A. marginale used
The Florida strain [GenBank: CP001079] of A. marginale was originally isolated from a pool of blood samples collected from cattle in 1955 [16,31,32]. The Mississippi strain [GenBank: ABOP00000000] was isolated from an acute clinical case of anaplasmosis [25,33]. Both of these strains are virulent, and are not transmissible by D. andersoni ticks. The Virginia strain [GenBank: ABOR00000000] was isolated from a cow in Southern Virginia in 1972 [34]. The Puerto Rico strain [GenBank: ABOQ00000000] was received as a frozen stabilate after isolation from cattle in Puerto Rico in 1985 [35,36]. Both the Virginia and Puerto Rico strains are virulent, and are transmissible by D. andersoni. While passage histories are not well documented for these strains, all strains have been passaged multiple times in cattle since isolation. The Florida strain has the longest passage history, being passed continuously since isolation, and the Puerto Rico strain has only been passaged once since coming to our laboratory.
DNA isolation for genome sequencing
Blood stabilates from the Florida, Mississippi, Puerto Rico, and Virginia strains were inoculated into splenectomized calves, which were shown to be free of A. marginale infection via competitive enzyme-linked immunoabsorbent assay (cELISA) [37]. Blood samples were taken at peak parasitemia, washed seven times in phosphate buffered saline (137 mM NaCl, 10 mM Phosphate, and 2.7 mM KCl), and centrifuged at 1,500 × g for 10 minutes with the removal of the buffy coat after each spin. Erythrocytes not used immediately were diluted 1:1 in PBS and frozen for later use.
A. marginale preparation
After thawing, lysed erythrocytes were passed over a column containing loosely-packed CF-11 cellulose (Sigma-Aldrich Corporation, St. Louis, MO). The column eluate was washed repeatedly with PBS and centrifuged at 19,000 g for 20 minutes until all remaining hemoglobin was removed, leaving a pellet of A. marginale initial bodies and erythrocyte membranes.
Bacterial artificial chromosome (BAC) library construction and manipulation
The bacterial preparation was embedded into 1% agarose blocks (A-9539, Sigma Chemical Co., St. Louis, MO), and cells within the blocks were lysed using proteinase K and SDS [38]. A. marginale genomic DNA was partially digested with either HindIII or MboI, size selected on pulse-field gels, ligated into the pBELOBAC11 vector, and electroporated into Escherichia coli strain DH10B (Amplicon Express, Pullman, WA). A total of 3,072 clones (1,536 clones from each restriction enzyme) were arrayed into 384 well plates. The average insert size of the clones was 120 kb.
Genome sequencing
For the Florida strain, a BAC-based clone-by-clone strategy was adopted. BAC clones were screened using digoxigenin (DIG)-labeled (Roche Applied Science) probes to bovine genomic DNA and several A. marginale genes (including msp1α, msp1β, msp2, msp3, msp4, msp5, dnaK, recA, groEL, and sodB). Selected clones were end-sequenced and a minimum tiling path was constructed based on comparison with the previously-sequenced St. Maries strain. Sequencing of BACs, assembly of completed sequences, and genome annotation were as described [11].
Genomic DNA from the Mississippi, Puerto Rico, and Virginia strains was extracted from isolated bacteria (prepared as described above) using the Puregene Blood kit (Qiagen Corporation, Valencia, CA). DNA was then sequenced on a Genome Sequencer 20 instrument (454 Life Sciences Corporation, Branford, CT), using a pyrosequencing protocol [24]. The Newbler program was used with its default settings to assemble the sequence and to compare all contigs to the completed Florida and St. Maries genomes, which revealed the location of gaps in coverage. High-quality variations were called when four reads, each with at least 20 base pairs flanking the polymorphic site, contain the difference, with at least one read in each direction. The nucleotide sequences of assembled contigs were compared to the St. Maries genome using BLASTn. Any contigs without hits better than 1e-10 were then compared to the bovine whole genome shotgun sequence database, to screen for bovine DNA contamination. Any contigs with no hits to bovine sequence were then compared to the nt database. Large contigs assembled by Newbler were compared to the Florida genome using MUMmer v3.1 [39] after filling gaps in the assembly with the corresponding sequence from the Florida strain.
Genome comparisons
MUMmer v3.1 was used to compare the completed St. Maries and Florida genomes and the contigs of the Mississippi, Puerto Rico, and Virginia strains, as described [5]. Output from the SNP detection algorithm (show-snps) was processed using custom scripts (written with AutoIt v3.2.2.0) to determine the number of SNPs per ORF. Show-snps output was also processed in Excel (Microsoft Corporation, Redmond, WA) to graph the location of SNPs throughout the genome. FASTA sequences from single-chromosome genomes with multiple strains sequenced were downloaded from Genbank [see supplementary table 2 for the genomes compared, their sizes, and Genbank accession numbers]. All strains for a given species were compared to each other using MUMmer 3.1, as described above. The number of SNPs per comparison was then divided by the larger of the two compared genomes to yield the percent SNPs per genome. For species with more than two strains sequenced, all percentages were averaged to give the mean and standard deviation. The phylogenetic tree was inferred using the Maximum Parsimony method [40] of MEGA4 [41] comparing concatenated sequences from groEL, groES, atpA, and recA. The bootstrap consensus tree is inferred from 1000 replicates, and branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. There were a total of 458 positions in the final dataset, out of which 379 were parsimony informative.
Southern analysis
Genomic DNA from all five strains was digested with XbaI and HindIII (New England Biolabs Corporation, Ipswich, MA), as these enzymes cut within the conserved flanking genes. Resultant fragments were separated on a 0.8% agarose gel, and subsequently transferred to a charged nylon membrane and crosslinked with a Stratalinker UV apparatus (Stratagene Inc., La Jolla, CA) per the manufacturer's directions. The blots were prehybridized at 42°C for at least two hours in Dig Easy Hyb buffer (Roche Corporation, Indianapolis, IN). Digoxigenin-labeled probes to aaap were produced using the PCR DIG Probe Synthesis Kit (Roche Corporation) and hybridized overnight at 42°C in DIG Easy Hyb buffer. The membrane was washed three times for 15 minutes in 2 × SSC and 0.1% SDS, with the first two washes at room temperature and the third at 65°C. A final wash was performed in 0.2 × SSC and 0.1% SDS at 65°C. Chemiluminescent detection of the probes was performed using the DIG Wash and Block Buffer Kit (Roche Corporation) per the manufacturer's directions.
Authors' contributions
MJD, GHP, DPK, and KAB designed the study. DRH, LSK, KAB, and MJD participated in sequencing and assembly of the Florida genome. MPG, EN, and MJD carried out additional targeted sequencing. MJD isolated DNA for pyrosequencing, and performed computational analysis. MJD, KAB, LSK, DRH, EN, and MPG analyzed data. MJD, GHP, DPK, and KAB wrote and edited the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Pyrosequencing gaps. A listing of genes containing gaps in the pyrosequenced genomes (Puerto Rico [PR], Virginia [VA], and Mississippi [MS]).
Species compared in SNP analysis. A listing of bacterial species compared in the SNP analysis, with Genbank accession numbers for each genome analyzed.
SNP distribution in three species. Distribution of SNPs between the compared strains of A. marginale, E. ruminantium, and R. bellii.
Acknowledgments
Acknowledgements
The authors thank Beverly Hunter and Ralph Horn for their excellent technical assistance. Funding was provided by the USDA CREES NRI CGP 2004-35600-14175 (KAB), the Wellcome Trust GR075800M (GHP), and NIH Mentored Clinical Scientist Research Award K08-AI64162 (MJD).
Contributor Information
Michael J Dark, Email: darkmich@wsu.edu.
David R Herndon, Email: herndon@vetmed.wsu.edu.
Lowell S Kappmeyer, Email: lkapp@vetmed.wsu.edu.
Mikel P Gonzales, Email: mgonzales@westernu.edu.
Elizabeth Nordeen, Email: enordeen@vetmed.wsu.edu.
Guy H Palmer, Email: gpalmer@vetmed.wsu.edu.
Donald P Knowles, Jr, Email: dknowles@vetmed.wsu.edu.
Kelly A Brayton, Email: kbrayton@vetmed.wsu.edu.
References
- Mobius P, Luyven G, Hotzel H, Kohler H. High Genetic diversity among Mycobacterium avium subsp. paratuberculosis strains of German cattle herds shown by combination of IS900 RFLP analysis and MIRU-VNTR typing. J Clin Microbiol. 2008 doi: 10.1128/JCM.01801-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof SY, Solberg OD, Riley LW. Genotypic analyses of uropathogenic Escherichia coli based on fimH single nucleotide polymorphisms (SNPs) J Med Microbiol. 2007;56:1363–1369. doi: 10.1099/jmm.0.47262-0. [DOI] [PubMed] [Google Scholar]
- Wu T, Chellemi DO, Graham JH, Martin KJ, Rosskopf EN. Comparison of soil bacterial communities under diverse agricultural land management and crop production practices. Microb Ecol. 2008;55:293–310. doi: 10.1007/s00248-007-9276-4. [DOI] [PubMed] [Google Scholar]
- Bottos EM, Vincent WF, Greer CW, Whyte LG. Prokaryotic diversity of arctic ice shelf microbial mats. Environ Microbiol. 2008 doi: 10.1111/j.1462-2920.2007.01516.x. [DOI] [PubMed] [Google Scholar]
- Fleischmann RD, Alland D, Eisen JA, Carpenter L, White O, Peterson J, DeBoy R, Dodson R, Gwinn M, Haft D, et al. Whole-genome comparison of Mycobacterium tuberculosis clinical and laboratory strains. J Bacteriol. 2002;184:5479–5490. doi: 10.1128/JB.184.19.5479-5490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas I, Klasson L, Canback B, Naslund AK, Eriksson AS, Wernegreen JJ, Sandstrom JP, Moran NA, Andersson SG. 50 million years of genomic stasis in endosymbiotic bacteria. Science. 2002;296:2376–2379. doi: 10.1126/science.1071278. [DOI] [PubMed] [Google Scholar]
- Carlson JH, Porcella SF, McClarty G, Caldwell HD. Comparative genomic analysis of Chlamydia trachomatis oculotropic and genitotropic strains. Infect Immun. 2005;73:6407–6418. doi: 10.1128/IAI.73.10.6407-6418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson T, Busch JD, Ravel J, Read TD, Rhoton SD, U'Ren JM, Simonson TS, Kachur SM, Leadem RR, Cardon ML, Van Ert MN, Huynh LY, Fraser CM, Keim P. Phylogenetic discovery bias in Bacillus anthracis using single-nucleotide polymorphisms from whole-genome sequencing. Proc Natl Acad Sci USA. 2004;101:13536–13541. doi: 10.1073/pnas.0403844101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratilo CW, Lewis CT, Bryden L, Mulvey MR, Bader D. Single-nucleotide repeat analysis for subtyping Bacillus anthracis isolates. J Clin Microbiol. 2006;44:777–782. doi: 10.1128/JCM.44.3.777-782.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettelin H, Masignani V, Cieslewicz MJ, Donati C, Medini D, Ward NL, Angiuoli SV, Crabtree J, Jones AL, Durkin AS, Deboy RT, Davidsen TM, Mora M, Scarselli M, Margarit y Ros I, Peterson JD, Hauser CR, Sundaram JP, Nelson WC, Madupu R, Brinkac LM, Dodson RJ, Rosovitz MJ, Sullivan SA, Daugherty SC, Haft DH, Selengut J, Gwinn ML, Zhou L, Zafar N. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial "pan-genome". Proc Natl Acad Sci USA. 2005;102:13950–13955. doi: 10.1073/pnas.0506758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayton KA, Kappmeyer LS, Herndon DR, Dark MJ, Tibbals DL, Palmer GH, McGuire TC, Knowles DP., Jr Complete genome sequencing of Anaplasma marginale reveals that the surface is skewed to two superfamilies of outer membrane proteins. Proc Natl Acad Sci USA. 2005;102:844–849. doi: 10.1073/pnas.0406656102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and 'HGE agent' as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- Allred DR, McGuire TC, Palmer GH, Leib SR, Harkins TM, McElwain TF, Barbet AF. Molecular basis for surface antigen size polymorphisms and conservation of a neutralization-sensitive epitope in Anaplasma marginale. Proc Natl Acad Sci USA. 1990;87:3220–3224. doi: 10.1073/pnas.87.8.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente J, Ruybal P, Mtshali MS, Naranjo V, Shuqing L, Mangold AJ, Rodriguez SD, Jimenez R, Vicente J, Moretta R, Torina A, Almazan C, Mbati PM, de Echaide ST, Farber M, Rosario-Cruz R, Gortazar C, Kocan KM. Analysis of world strains of Anaplasma marginale using major surface protein 1a repeat sequences. Vet Microbiol. 2007;119:382–390. doi: 10.1016/j.vetmic.2006.09.015. [DOI] [PubMed] [Google Scholar]
- de la Fuente J, Bussche RA Van Den, Garcia-Garcia JC, Rodriguez SD, Garcia MA, Guglielmone AA, Mangold AJ, Friche Passos LM, Barbosa Ribeiro MF, Blouin EF, Kocan KM. Phylogeography of New World isolates of Anaplasma marginale based on major surface protein sequences. Vet Microbiol. 2002;88:275–285. doi: 10.1016/S0378-1135(02)00122-0. [DOI] [PubMed] [Google Scholar]
- McGuire TC, Palmer GH, Goff WL, Johnson MI, Davis WC. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984;45:697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh SM, Brayton KA, Knowles DP, Agnes JT, Dark MJ, Brown WC, Baszler TV, Palmer GH. Differential expression and sequence conservation of the Anaplasma marginale msp2 gene superfamily outer membrane proteins. Infect Immun. 2006;74:3471–3479. doi: 10.1128/IAI.01843-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriks IS, Stiller D, Goff WL, Panton M, Parish SM, McElwain TF, Palmer GH. Molecular and biological characterization of a newly isolated Anaplasma marginale strain. J Vet Diagn Invest. 1994;6:435–441. doi: 10.1177/104063879400600406. [DOI] [PubMed] [Google Scholar]
- Wickwire KB, Kocan KM, Barron SJ, Ewing SA, Smith RD, Hair JA. Infectivity of three Anaplasma marginale isolates for Dermacentor andersoni. Am J Vet Res. 1987;48:96–99. [PubMed] [Google Scholar]
- Frutos R, Viari A, Ferraz C, Morgat A, Eychenie S, Kandassamy Y, Chantal I, Bensaid A, Coissac E, Vachiery N, Demaille J, Martinez D. Comparative genomic analysis of three strains of Ehrlichia ruminantium reveals an active process of genome size plasticity. J Bacteriol. 2006;188:2533–2542. doi: 10.1128/JB.188.7.2533-2542.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata H, Audic S, Renesto-Audiffren P, Fournier PE, Barbe V, Samson D, Roux V, Cossart P, Weissenbach J, Claverie JM, Raoult D. Mechanisms of evolution in Rickettsia conorii and R. prowazekii. Science. 2001;293:2093–2098. doi: 10.1126/science.1061471. [DOI] [PubMed] [Google Scholar]
- Rodriguez JL, Palmer GH, Knowles DP, Jr, Brayton KA. Distinctly different msp2 pseudogene repertoires in Anaplasma marginale strains that are capable of superinfection. Gene. 2005;361:127–132. doi: 10.1016/j.gene.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Stich RW, Olah GA, Brayton KA, Brown WC, Fechheimer M, Green-Church K, Jittapalapong S, Kocan KM, McGuire TC, Rurangirwa FR, Palmer GH. Identification of a novel Anaplasma marginale appendage-associated protein that localizes with actin filaments during intraerythrocytic infection. Infect Immun. 2004;72:7257–7264. doi: 10.1128/IAI.72.12.7257-7264.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, Dewell SB, Du L, Fierro JM, Gomes XV, Godwin BC, He W, Helgesen S, Ho CH, Irzyk GP, Jando SC, Alenquer ML, Jarvie TP, Jirage KB, Kim JB, Knight JR, Lanza JR, Leamon JH, Lefkowitz SM, Lei M, Li J, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueti MW, Reagan JO, Jr, Knowles DP, Jr, Scoles GA, Shkap V, Palmer GH. Identification of midgut and salivary glands as specific and distinct barriers to efficient tick-borne transmission of Anaplasma marginale. Infect Immun. 2007;75:2959–2964. doi: 10.1128/IAI.00284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayton KA, Knowles DP, McGuire TC, Palmer GH. Efficient use of a small genome to generate antigenic diversity in tick-borne ehrlichial pathogens. Proc Natl Acad Sci USA. 2001;98:4130–4135. doi: 10.1073/pnas.071056298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayton KA, Palmer GH, Lundgren A, Yi J, Barbet AF. Antigenic variation of Anaplasma marginale msp2 occurs by combinatorial gene conversion. Mol Microbiol. 2002;43:1151–1159. doi: 10.1046/j.1365-2958.2002.02792.x. [DOI] [PubMed] [Google Scholar]
- Eisen JA, Heidelberg JF, White O, Salzberg SL. Evidence for symmetric chromosomal inversions around the replication origin in bacteria. Genome Biol. 2000;1:RESEARCH0011. doi: 10.1186/gb-2000-1-6-research0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins NE, Liebenberg J, de Villiers EP, Brayton KA, Louw E, Pretorius A, Faber FE, van Heerden H, Josemans A, van Kleef M, Steyn HC, van Strijp MF, Zweygarth E, Jongejan F, Maillard JC, Berthier D, Botha M, Joubert F, Corton CH, Thomson NR, Allsopp MT, Allsopp BA. The genome of the heartwater agent Ehrlichia ruminantium contains multiple tandem repeats of actively variable copy number. Proc Natl Acad Sci USA. 2005;102:838–843. doi: 10.1073/pnas.0406633102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotopp JC, Lin M, Madupu R, Crabtree J, Angiuoli SV, Eisen J, Seshadri R, Ren Q, Wu M, Utterback TR, Smith S, Lewis M, Khouri H, Zhang C, Niu H, Lin Q, Ohashi N, Zhi N, Nelson W, Brinkac LM, Dodson RJ, Rosovitz MJ, Sundaram J, Daugherty SC, Davidsen T, Durkin AS, Gwinn M, Haft DH, Selengut JD, Sullivan SA, et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2006;2:e21. doi: 10.1371/journal.pgen.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic M, Sibinovic S, Welter CJ. An attenuated Anaplasma marginale vaccine. Proc Annu Meet U S Anim Health Assoc. 1968;72:56–69. [PubMed] [Google Scholar]
- Ristic M, Carson CA. Methods of Immunoprophylaxis Against Bovine Anaplasmosis with Emphasis on use of the Attenuated Anaplasma marginale Vaccine. In: Miller LH, Pino JA, McKelvey JJJ, editor. Immunity to Blood Parasites of Animals and Man. New York: Plenum Press; 1977. pp. 151–188. [DOI] [PubMed] [Google Scholar]
- Hidalgo RJ, Palmer GH, Jones EW, Brown JE, Ainsworth AJ. Infectivity and antigenicity of Anaplasma marginale from tick cell culture. Am J Vet Res. 1989;50:2033–2036. [PubMed] [Google Scholar]
- Kuttler KL, Winward LD. Serologic comparisons of 4 Anaplasma isolates as measured by the complement-fixation test. Vet Microbiol. 1984;9:181–186. doi: 10.1016/0378-1135(84)90033-6. [DOI] [PubMed] [Google Scholar]
- Scoles GA, Ueti MW, Noh SM, Knowles DP, Palmer GH. Conservation of transmission phenotype of Anaplasma marginale (Rickettsiales: Anaplasmataceae) strains among Dermacentor and Rhipicephalus ticks (Acari: Ixodidae) J Med Entomol. 2007;44:484–491. doi: 10.1603/0022-2585(2007)44[484:COTPOA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Futse JE, Ueti MW, Knowles DP, Jr, Palmer GH. Transmission of Anaplasma marginale by Boophilus microplus: retention of vector competence in the absence of vector-pathogen interaction. J Clin Microbiol. 2003;41:3829–3834. doi: 10.1128/JCM.41.8.3829-3834.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torioni de Echaide S, Knowles DP, McGuire TC, Palmer GH, Suarez CE, McElwain TF. Detection of cattle naturally infected with Anaplasma marginale in a region of endemicity by nested PCR and a competitive enzyme-linked immunosorbent assay using recombinant major surface protein 5. J Clin Microbiol. 1998;36:777–782. doi: 10.1128/jcm.36.3.777-782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birren B, Green ED, Klapholz S, Myers RM, Roskams J. Genome analysis: a laboratory manual. Plainview, N.Y.: Cold Spring Harbor Laboratory Press; 1997. [Google Scholar]
- Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. Versatile and open software for comparing large genomes. Genome Biol. 2004;5:R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eck RV, Dayhoff MO. Atlas of protein sequence and structure. Silver Spring, Md.: National Biomedical Research Foundation; 1966. [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pyrosequencing gaps. A listing of genes containing gaps in the pyrosequenced genomes (Puerto Rico [PR], Virginia [VA], and Mississippi [MS]).
Species compared in SNP analysis. A listing of bacterial species compared in the SNP analysis, with Genbank accession numbers for each genome analyzed.
SNP distribution in three species. Distribution of SNPs between the compared strains of A. marginale, E. ruminantium, and R. bellii.