Abstract
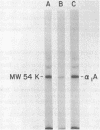
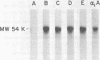
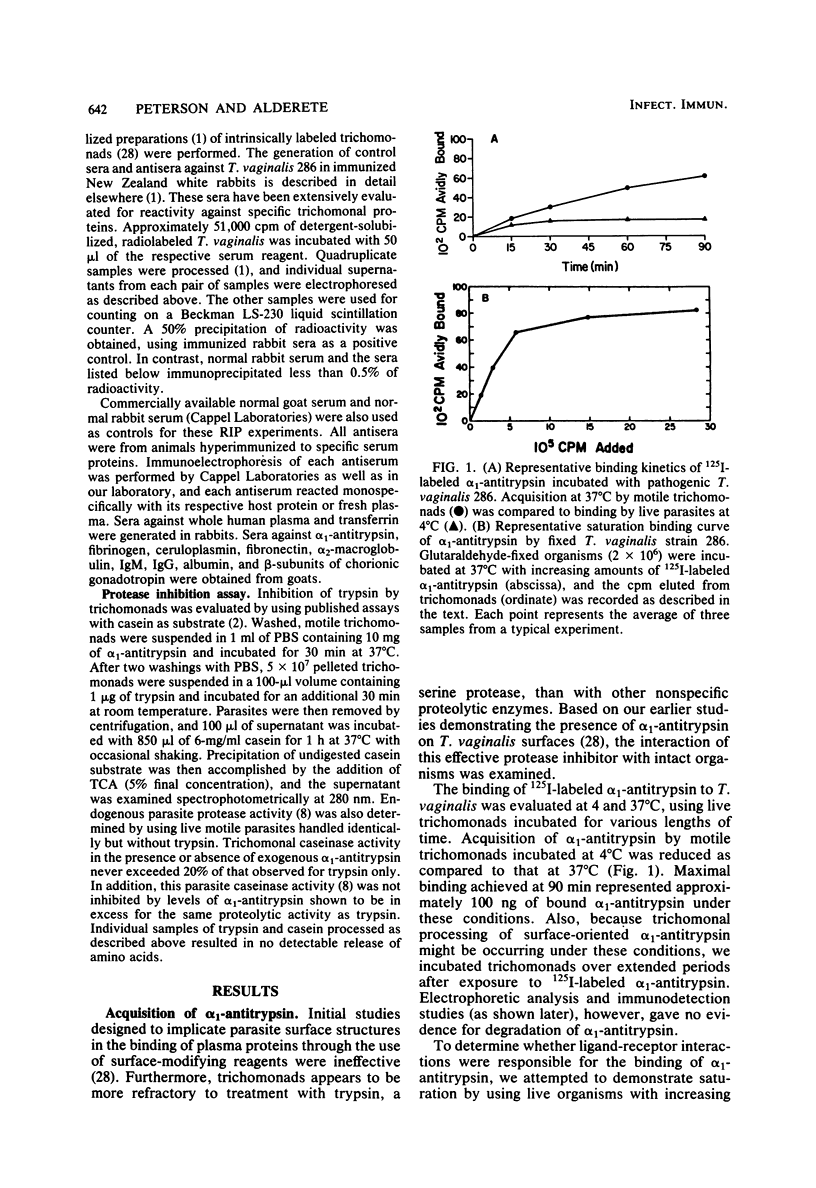
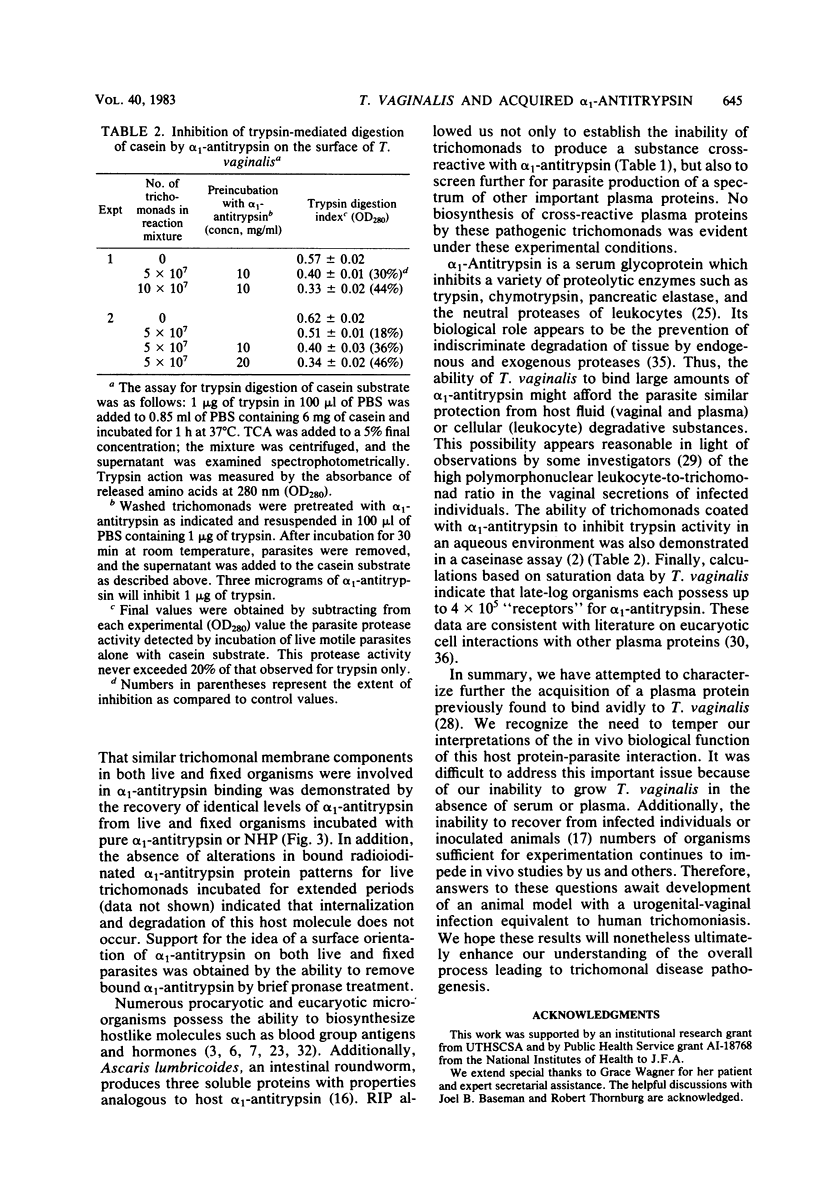
The interaction of alpha 1-Antitrypsin, the major serine protease inhibitor in plasma, with pathogenic Trichomonas vaginalis and the acquisition by trichomonads of this host protein from normal human plasma were investigated. alpha 1-Antitrypsin acquired by intact parasites could not be removed by repeated washings in phosphate-buffered saline. Saturation kinetics were observed after incubation of glutaraldehyde-fixed organisms with 125I-labeled alpha 1-antitrypsin. Evidence suggesting that specific parasite membrane sites were responsible for trichomonal acquisition of alpha 1-antitrypsin was obtained through competitive binding experiments using purified preparations of homologous versus heterologous plasma proteins. No evidence of degradation of bound antitrypsin by live parasites was observed. The avid binding of alpha 1-antitrypsin by pathogenic T. vaginalis after incubation in normal human plasma was demonstrated by using sensitive electrophoretic and immunodetection techniques. Radioimmunoprecipitation of intrinsically labeled, detergent-solubilized extracts of T. vaginalis incubated with monospecific antisera against alpha 1-antitrypsin and other human plasma proteins revealed the inability of parasites to biosynthesize any substance cross-reactive with host plasma proteins. Finally, T. vaginalis organisms pretreated with alpha 1-antitrypsin inhibited trypsin caseinase activity in an in vitro assay. The implications of these observations are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F. Antigen analysis of several pathogenic strains of Trichomonas vaginalis. Infect Immun. 1983 Mar;39(3):1041–1047. doi: 10.1128/iai.39.3.1041-1047.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes G. L., Kay R. Blood-groups in giardiasis. Lancet. 1977 Apr 9;1(8015):808–808. doi: 10.1016/s0140-6736(77)92999-3. [DOI] [PubMed] [Google Scholar]
- Bloom B. R. Games parasites play: how parasites evade immune surveillance. Nature. 1979 May 3;279(5708):21–26. doi: 10.1038/279021a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CAMERON G. L., STAVELEY J. M. Blood group P substance in hydatid cyst fluids. Nature. 1957 Jan 19;179(4551):147–148. doi: 10.1038/179147a0. [DOI] [PubMed] [Google Scholar]
- Coombs G. H. Proteinases of Leishmania mexicana and other flagellate protozoa. Parasitology. 1982 Feb;84(1):149–155. doi: 10.1017/s003118200005174x. [DOI] [PubMed] [Google Scholar]
- Culp L. A., Murray B. A., Rollins B. J. Fibronectin and proteoglycans as determinants of cell-substratum adhesion. J Supramol Struct. 1979;11(3):401–427. doi: 10.1002/jss.400110314. [DOI] [PubMed] [Google Scholar]
- DIAMOND L. S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957 Aug;43(4):488–490. [PubMed] [Google Scholar]
- Diffley P. Comparative immunological analysis of host plasma proteins bound to bloodstream forms of Trypanosoma brucei subspecies. Infect Immun. 1978 Aug;21(2):605–612. doi: 10.1128/iai.21.2.605-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval G., Welsh K. I., Wigzell H. A radioimmunoassay of cellular surface antigens on living cells using iodinated soluble protein A from Staphylococcus aureus. J Immunol Methods. 1975 Jun;7(2-3):237–250. doi: 10.1016/0022-1759(75)90021-6. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M. Immunologic and fine structure evidence of avidly bound host serum proteins in the surface coat of a bloodstream trypanosome. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1222–1226. doi: 10.1073/pnas.73.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espersen F., Clemmensen I. Isolation of a fibronectin-binding protein from Staphylococcus aureus. Infect Immun. 1982 Aug;37(2):526–531. doi: 10.1128/iai.37.2.526-531.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. B., Peanasky R. J. Isolation of the trypsin inhibitors in Ascaris lumbricoides var. suum using affinity chromatography. Anal Biochem. 1982 Mar 1;120(2):387–393. doi: 10.1016/0003-2697(82)90362-1. [DOI] [PubMed] [Google Scholar]
- Krieger J. N. Urologic aspects of trichomoniasis. Invest Urol. 1981 May;18(8):411–417. [PubMed] [Google Scholar]
- Kronvall G., Simmons A., Myhre E. B., Jonsson S. Specific absorption of human serum albumin, immunoglobulin A, and immunoglobulin G with selected strains of group A and G streptococci. Infect Immun. 1979 Jul;25(1):1–10. doi: 10.1128/iai.25.1.1-10.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maruo T., Cohen H., Segal S. J., Koide S. S. Production of choriogonadotropin-like factor by a microorganism. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6622–6626. doi: 10.1073/pnas.76.12.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse J. O. alpha1-antitrypsin deficiency (first of two parts). N Engl J Med. 1978 Nov 9;299(19):1045–1048. doi: 10.1056/NEJM197811092991905. [DOI] [PubMed] [Google Scholar]
- Müller M., Meingassner J. G., Miller W. A., Ledger W. J. Three metronidazole-resistant strains of Trichomonas vaginalis from the United States. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 1):808–812. doi: 10.1016/s0002-9378(16)32741-7. [DOI] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Host plasma proteins on the surface of pathogenic Trichomonas vaginalis. Infect Immun. 1982 Aug;37(2):755–762. doi: 10.1128/iai.37.2.755-762.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein M. F., Sullivan J. A., Mandell G. L. Trichomonacidal activity of human polymorphonuclear neutrophils: killing by disruption and fragmentation. J Infect Dis. 1980 Oct;142(4):575–585. doi: 10.1093/infdis/142.4.575. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L., Marshak-Rothstein A., Rup D., Lodish H. F. Identification and quantification of the rat hepatocyte asialoglycoprotein receptor. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3348–3352. doi: 10.1073/pnas.78.6.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. A., Beachey E. H. Adherence of group A streptococci to fibronectin on oral epithelial cells. Infect Immun. 1983 Jan;39(1):275–279. doi: 10.1128/iai.39.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence M. R., Hollander D. H., Smith J., McCaig L., Sewell D., Brockman M. The clinical and laboratory diagnosis of Trichomonas vaginalis infection. Sex Transm Dis. 1980 Oct-Dec;7(4):168–171. doi: 10.1097/00007435-198010000-00004. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turino G. M., Rodriguez J. R., Greenbaum L. M., Mandl I. Mechanisms of pulmonary injury. Am J Med. 1974 Sep;57(3):493–505. doi: 10.1016/0002-9343(74)90142-9. [DOI] [PubMed] [Google Scholar]
- Van Bockxmeer F. M., Yates G. K., Morgan E. H. Interaction of transferrin with solubilized receptors from reticulocytes. Eur J Biochem. 1978 Dec 1;92(1):147–154. doi: 10.1111/j.1432-1033.1978.tb12732.x. [DOI] [PubMed] [Google Scholar]
- Whitnack E., Beachey E. H. Antiopsonic activity of fibrinogen bound to M protein on the surface of group A streptococci. J Clin Invest. 1982 Apr;69(4):1042–1045. doi: 10.1172/JCI110508. [DOI] [PMC free article] [PubMed] [Google Scholar]