Abstract
Voltage-gated sodium channels initiate the rapid upstroke of action potentials in many excitable tissues. Mutations within intracellular C-terminal sequences of specific channels underlie a diverse set of channelopathies, including cardiac arrhythmias and epilepsy syndromes. The three-dimensional structure of the C-terminal residues 1777-1882 of the human NaV1.2 voltage-gated sodium channel has been determined in solution by NMR spectroscopy at pH 7.4 and 290.5 K. The ordered structure extends from residues Leu-1790 to Glu-1868 and is composed of four α-helices separated by two short anti-parallel β-strands; a less well defined helical region extends from residue Ser-1869 to Arg-1882, and a disordered N-terminal region encompasses residues 1777-1789. Although the structure has the overall architecture of a paired EF-hand domain, the NaV1.2 C-terminal domain does not bind Ca2+ through the canonical EF-hand loops, as evidenced by monitoring 1H,15N chemical shifts during aCa2+ titration. Backbone chemical shift resonance assignments and Ca2+ titration also were performed for the NaV1.5 (1773-1878) isoform, demonstrating similar secondary structure architecture and the absence of Ca2+ binding by the EF-hand loops. Clinically significant mutations identified in the C-terminal region of NaV1 sodium channels cluster in the helix I-IV interface and the helix II-III interhelical segment or in helices III and IV of the NaV1.2 (1777-1882) structure.
Voltage-gated sodium channels (VGSCs)5 are molecular assemblies that span the plasma membrane of excitable cells and conduct sodium current selectively in response to depolarizing stimuli. Mutations in VGSCs underlie a variety of diseases, including the cardiac arrhythmogenic Long-QT3 and Brugada syndromes (1, 2) and neurological syndromes, such as epilepsy (3, 4).
Known components of VGSCs include a pore-forming α subunit, auxiliary β subunits, and associated modulating proteins, such as calmodulin (5, 6). The α subunit is composed of four homologous six-transmembrane helical domains connected by inter-domain linkers and N-terminal and C-terminal cytoplasmic regions. Specific α subunit isoforms are expressed differentially in skeletal muscle (NaV1.4), cardiac muscle (NaV1.5) and the nervous system (NaV1.1, NaV1.2, NaV1.3, splice variants of NaV1.5, and NaV1.6-NaV1.9) and control the rapid upstroke of action potentials (7).
VGSC activity is characterized by two open states and several inactivated states (8). Kinetics of channel inactivation occur on timescales ranging from milliseconds to seconds and determine multiple aspects of action potentials (9, 10). The molecular mechanisms of VGSC inactivation are complex and involve the α subunit, the β subunits, and calmodulin (11-13). Specific contributions to α subunit inactivation have been localized to interhelical intra-domain regions (14-16), the linker region between domains III-IV, which forms the pore occluding inactivation gate (17, 18), and the C-terminal cytoplasmic domain (CTD) (19-21).
Specific disease-causing mutations within the CTD affect channel function by altering kinetics of channel inactivation (22). The CTD is predicted by sequence analysis (23, 24) and homology modeling (25-27) to contain a paired EF-hand domain and was observed to contain a distal calmodulin binding IQ motif (4, 12, 28-31). Structural modeling also predicts that specific interactions between helix I and helix IV control channel inactivation (27, 32). A recent model, based on NMR chemical shift perturbations, fluorescence spectroscopy, and electrophysiology, suggests that inactivation is regulated by Ca2+ binding to the proximal EF-hand, which is strongly influenced in turn by interactions with the distal IQ motif and calmodulin (33). Nevertheless, whether Ca2+ binds specifically to the putative CTD EF-hand and any resultant contribution to channel regulation is controversial (12, 26, 31, 34).
EXPERIMENTAL PROCEDURES
Constructs of the Nav1.2 CTD were designed by limited proteolysis and H/D exchange experiments. Briefly, the CTD of Nav1.2, residues 1777-1937 with the amino acid substitutions I1877A/Q1878A and an N-terminal His6 tag MGSSHHHHHHSSGLVPRGSHMAS (31), was subjected to proteolytic digestion with proteinase K at 4 °C for 15-60 min using a protein:protease ratio of 50:1-100:1. The termini of the protected proteolytic fragments were mapped by matrix-assisted laser desorption ionization time-of-flight time-of-flight mass spectrometry and N-terminal sequencing. H/D exchange experiments were performed by ExSAR (Monmouth Junction, NJ) and showed protection for proteolytic fragments extending from residues 1789 to 1879. The construct encompassing residues 1777-1882 of the Nav1.2 CTD defined by the above experiments, including the N-terminal His tag, was used for structure determination by solution NMR spectroscopy.
[U-13C,U-15N] NaV1.2 CTD (1777-1882) was overexpressed in Escherichia coli (BL21 DE3) transformed with a pET28 vector (EMD Biosciences) using M9 minimal media prepared with 15NH4 Cl and [13C6]glucose (35). Cultures were grown at 37 °C to A600 nm = 0.7, induced with 0.5 mm isopropyl β-d-1-thiogalactopyranoside, transferred to 16 °C, and harvested after 72 h. Cells were lysed using a French press, and the NaV1.2 CTD was purified with Ni+-affinity, gel-filtration (Superdex 200), and ion-exchange (Mono Q 5/50 GL) chromatography (GE Healthcare). The N-terminal tag was not removed. Sample buffer consisted of 20 mm d11-Tris (pH 7.4), 100 mm d5-glycine, 0.1 mm d16-EDTA, 1 mm d10-DTT, 0.02% NaN3, and 10% D2O. Proteins were exchanged into this buffer using centrifugal concentrators (Amicon Inc.), flash-frozen in liquid N2, and stored at -80 °C. Samples for calcium titrations were subsequently exchanged into 20 mm d11-Tris (pH 7.4), 100 mm d5-glycine, 10 μm d16-EDTA, 1 mm d10-dithiothreitol, 0.02% NaN3, and 10% D2O. Protein concentrations of 0.5 and 0.2 mm were used for structural experiments and calcium titrations, respectively. The NaV1.5 CTD construct, residues 1773-1878, was designed by sequence alignment to NaV1.2, using bl2seq (36), and protein samples were prepared by the same protocol. Sample temperatures were calibrated using 99.8% MeOD to a splitting of 1.616 ppm for NaV1.2 (290.5 K) and 1.545 ppm NaV1.5 (298.0 K) (37).
Backbone assignments for the NaV1.2 and NaV1.5 CTDs were obtained with HNCO, HNCA, HNCACB, HNCOCA, HNCACO, and CBCA(CO)NH experiments; side-chain assignments for NaV1.2 CTD were obtained with HBHA(CB-CACO)NH and HCCH-two-dimensional total correlation spectroscopy (TOCSY) experiments (38). A 10% 13C sample was used for stereospecific assignment of Leu and Val methyl groups (39). NOE connectivities were obtained with 15N-NOESY-HSQC (80-ms mixing time), 13Caliphatic-NOESY-HSQC (100 ms), and 13Caromatic-NOESY-HSQC (80 ms). Residual dipolar coupling constants were measured in a sample containing 15 mg/ml Pf1 phage (Asla Biotech) using two-dimensional In-phase/Antiphase 1H,15N HSQC for 1H,15N (40), three-dimensional HNCO for 13C′-13Cα (41), quantitative three-dimensional HNCO for 15N-13C′ (42), and HCACO for 1Hα-13Cα residual dipolar coupling constants (43). Fitting of the 15N-13C′ coupling constants was performed with Mathematica v5.2 (Wolfram Research, Inc.). Chemical shifts were referenced using DSS (44).
An initial structure of NaV1.2 CTD was calculated from dihedral angle and NOE distance restraints, with several iterations to resolve ambiguity using ARIA 2.2 (45) and CNS 1.2 (46). The structure was refined with XPLOR-NIH 2.18 using dihedral angle, NOE distance, and residual dipolar coupling constants restraints (47, 48). Dihedral angle restraints were derived from chemical shifts using TALOS (49). Distance restraints were obtained from NOE intensities corrected for multiplicity of the 1H spins. NOE connectivities were categorized into three classes (50). Class I contains all intra-residue HN-Hα and all intra-residue, sequential, and medium range Hβ-HX NOEs, where X is not a methyl proton. Class III contains all NOEs involving a methyl group, and class II includes all other NOEs. A calibration factor, kI, was obtained by equating the average class I intensity to 3.4 Å. The class II calibration factor kII = kI/2.42. The class III calibration factor kIII = kII/2. Class I was averaged with a 1/6 order exponent, whereas classes II and III were averaged using a 1/4 exponent (50, 51). A standard 10% error term was applied to the upper bound of each restraint. All distances were constrained to the range (1.8, 5.5 Å). Pseudo atom corrections were applied to upper distance restraints for geometric considerations (52).
The 1H,15N, 15N-13C′, and 1Hα-13Cα residual dipolar coupling constants were included in the structure calculations. The residual dipolar coupling magnitude and rhombicity were set to -12.5 Hz and 0.55, respectively, during the initial minimization and were refined in the final all-atom minimization. The final average residual dipolar coupling magnitude and rhombicity are -12.8 ± 0.23 Hz and Rh = 0.56 ± 0.01, respectively, for the 200 conformers.
Structural quality statistics refer to residues Leu-1790—Glu-1868 of the 15 lowest-energy structures of 200 total structures calculated. NOE completeness was determined with aqua3.2 (53). The Pearson correlation coefficient (R) and the quality factor (Q) were computed with PALES (54) from 64 C′-Cα dipolar couplings that were not included in the structure calculation. MolProbity scores were calculated for the lowest energy structure (55). Average root mean square deviation values were calculated to the average coordinates with VMD (56). Interhelical distances and angles (rounded to the nearest degree) were computed using interhlx.6 Structural alignments were performed with CE (57), and structure figures were prepared with VMD (56) and MOLMOL (58).
RESULTS
The isolated NaV1.2 CTD (1777-1882) and NaV1.5 CTD (1773-1878) constructs each contain the region just after their respective predicted IVS6 transmembrane helix and extend to a region highly conserved among all VGSCs just before the IQ motif. Assignments of 1H,15N resonances for the NaV1.2 CTD and the NaV1.5 CTD are, respectively, 99 and 97% complete. Notably, Asn-1835 could not be assigned in the 1H,15N HSQC of NaV1.2. The resonances for Asn-1831 (the homologue of Asn-1835) and Gln-1832 were not assigned, and the resonance for Ile-1833 appears broadened in 1H,15N HSQC of NaV1.5. Moreover, homologous resonances Leu-1855 in NaV1.2 and Met-1851 in NaV1.5 have liminal intensities in 1H,15N HSQC spectra. These observations suggest conserved dynamics between isoforms. For the Nav1.2 CTD (1777-1882), 13Cα and 13Cβ assignments are 100% complete, 13C′ assignments are 97.1% complete, 1H aromatic assignments are 89.1% complete, and non-aromatic 1H assignments are 97.7% complete. The NaV1.2 CTD construct contains six proline residues, of which Pro-1789, Pro-1807, Pro-1827, and Pro-1845 are in a trans conformation, whereas Pro-1828 and Pro-1834 are in a cis conformation. The cis conformation is evidenced by stronger X-Pro Hα-Hα than X-Pro Hα-Hδ NOE contacts and differences of Cβ-Cγ chemical shifts of 9.4 and 8.5 ppm, respectively (59, 60). Medium range 1H-1H NOEs, steady-state {1H}-15N NOE, and 13Cα secondary chemical shifts for NaV1.2 indicate that the CTD forms a well folded domain between residues Leu-1790 and Glu-1868, with a less well ordered region between residues Ser-1869 and Arg-1882 and a disordered N-terminal region between residues Gly-1777 and Pro-1789 (Fig. 1 and supplemental Fig. S1). Secondary chemical shifts indicate that the NaV1.5 CTD has a similar secondary structural architecture as NaV1.2 CTD (Fig. 1C).
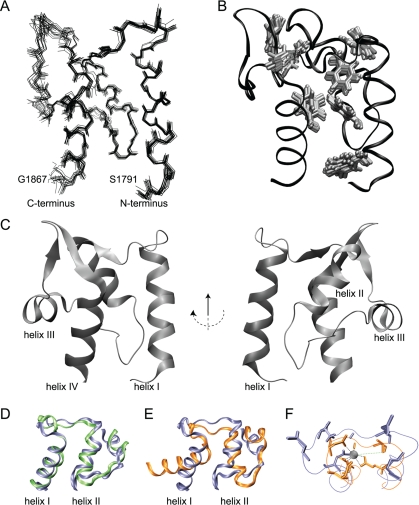
FIGURE 1.
Sequence alignments and NMR data for NaV1.2 and NaV1.5 CTDs. A, sequence alignment of NaV1.2 (1777-1882) and NaV1.5 (1773-1879) CTDs, with 83% identity and 93% similarity. Non-conservative substitutions are shown in bold type. B, medium range 1H-1H NOEs. C, secondary structure elements predicted from chemical shifts using TALOS (49) are shown as bars for α-helices and arrows for β-strands. {1H}-15N steady-state NOE (D) and secondary 13Cα chemical shifts for NaV1.2 CTD (E) indicate a well folded domain encompassing residues Leu-1790—Glu-1868. F, 1H,15N HSQC (right panel) with expansion of the central region (left panel) of NaV1.2 (1777-1882). The W1802ε1 resonance is aliased in the 15N dimension from 131.5 ppm.
The structure of NaV1.2 CTD is presented in Fig. 2 and supplemental Fig. S1 with statistical details of the calculation presented in Table 1. The structure contains four α-helices and two short anti-parallel β-strands, consistent with homology models based on structures of paired EF-hand domains (25, 26). Comparison of interhelical angles of helices I and II of NaV1.2 CTD and the N-terminal lobe of the prototypical EF-hand protein calmodulin suggests that the isolated NaV1.2 CTD most closely resembles the canonical apoEF-hand conformation (Fig. 3D and Table 2). The hydrophobic interface between helices I and IV predicted through mutational analysis (27) is observed with direct NOE contacts between residues Phe-1795, Phe-1798, and Tyr-1799 in helix I and Leu-1855, Ile-1857, and Leu-1858 in helix IV.
FIGURE 2.
Solution structure of the ordered core EF-hand motif of the NaV1. 2 CTD, residues Leu-1790-Glu-1868. Structural statistics are presented in Table 1; the structure of the construct from residues Gly-1777 to Arg-1882, including the N- and C-terminal regions, is shown in supplemental Fig. S1. Traces through the backbone heavy atoms of the 15 lowest energy conformers described in Table 1 are superposed in panel A. Phenylalanine side chains are superposed in panel B. Phe-1859 is not shown because the aromatic chain is not well ordered in the ensemble. A ribbon diagram of the lowest-energy structure is presented in panel C. The structural alignment of the N-terminal EF-hand motifs of NaV1.2 (blue ribbon) and apoCa2+ calmodulin PDB code 1CFD (green ribbon) is shown in panel D. The structural alignments to Ca2+-bound calmodulin PDB code 1EXR (orange ribbon) is shown in panel E. Pentagonal bipyrimidal coordination (dashed lines) of calcium by the N-terminal calmodulin (PDB code 1EXR) EF-Hand (orange) is shown with the corresponding residues of Nav1.2 in panel F. Coordination by T28 (green dashed line) occurs through a water molecule.
TABLE 1.
Structural statistics for Nav 1.2 CTD
Quality statistics are given for residues Leu-1790—Glyu-1868 of the 15 lowest energy structures out of 200 total structures calculated. Errors shown are S.D. for the ensemble. r.m.s.d., root mean square deviation.
| Quantity | Value |
|---|---|
| Unique NOE distance restraints | 1772 |
| Intra-residual | 699 |
| Sequential | 442 |
| Medium range (2 ≤ i ≤ 5) | 321 |
| Long range (i > 5) | 310 |
| Residual violations >0.3 Å per structure (n = 15) | 1.2 ± 1.1 |
| Maximum violation (Å) | 0.27 ± 0.2 |
| NOE completeness per shell | |
| 2.0-2.5 Å (%) | 87 |
| 2.5-3.0 Å (%) | 69 |
| 3.0-3.5 Å (%) | 57 |
| 3.5-4.0 Å (%) | 44 |
| TALOS dihedral restraints (ϕ, ψ) | 65, 65 |
| Residual dipolar coupling restraints | |
| H-N | 41 |
| N-C′ | 64 |
| Hα-Cα | 44 |
| R-value; Q-factor (64 C′-Cα couplings) | 0.93 ± 0.02; 0.38 ± 0.05 |
| PROCHECK | |
| Most favored (%) | 82.2 ± 1.6 |
| Allowed (%) | 15.8 ± 1.7 |
| Generously allowed (%) | 1.41 ± 0.8 |
| Disallowed (%) | 0.5 ± 0.9 |
| MolProbity score; all-atom clash score (percentiles) | 3.09 (20th); 20.33 (31th) |
| Average backbone r.m.s.d. (Å) | 0.80 |
| Average all atom r.m.s.d. (Å) | 1.29 |
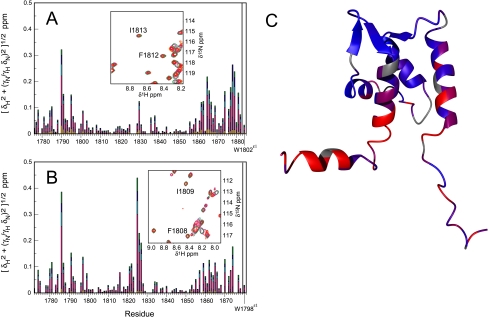
FIGURE 3.
Ca2+ titration of NaV1. 2 (1777-1882) (panel A) and NaV1.5 (1773-1878) (panel B). The plots show joint 1H,15N chemical shift deviations from resonance assignments in 0 mm Ca2+. The titration was performed by serial addition of Ca2+ obtaining the following concentrations: 0 (red), 0.1 (orange), 0.5 (maroon), 1.5 (magenta), 2.5 (cyan), 3.5 (blue), and 4.5 mm (green) for NaV1.2 (panel A) and (0 (red), 0.1 (orange), 0.5 (maroon), 2.5 (magenta), 3.5 (cyan), 4.5 (blue), and 5.5 mm (green) for NaV1.5. Insets show resonances Phe-1812—Ile-1813 and Phe-1808—Ile-1809 for NaV1.2 and NaV1.5, respectively. Titration curves are shown in supplemental Fig. S2. In panel C the joint 1H,15N chemical shift changes for NaV1.2 (1777-1882) at 4.5 mm Ca2+ are mapped onto the lowest energy structure, interpolated between 0 ppm (blue) and 0.1 ppm (red).
TABLE 2.
Comparison of helix orientations in EF-hand proteins
Interhelical angles are shown in degrees with interhelical distances shown in Å in parentheses. Calculations refer to the following structures. Ca-CaM is Ca2+ -loaded target-free calmodulin (PDB code 1EXR, 1.00 Å) (67), IQ-Ca-CaM is Ca2+ -loaded calmodulin bound to the voltage-gated Ca2+ channel Cav1.2 IQ-motif (PDB code 2F3Y, 1.45 Å) (81), and apoCaM is apoCa2+ target-free calmodulin (PDB code 1CFD, NMR) (82), with helix I defined as residues 6-18, helix II as residues 29-38, helix III as residues 45-54, and helix IV as residues 65-74. Interhelical angles for the Nav1.2 CTD are the averages with S.D. for the structural ensemble.
| Molecule | Helix II | Helix III | Helix IV |
|---|---|---|---|
| Helix I | |||
| Ca-CaM | 85 (20.4) | −134 (25.5) | 91 (14.6) |
| IQ-Ca-CaM | 92 (18.3) | −161 (21.9) | 117 (10.1) |
| ApoCaM | 136 (12.9) | −93 (21.2) | 126 (11.9) |
| Nav1.2 CTD | 152 ± 2 (10.9 ± 0.1) | −103 ± 6 (20.9 ± 0.3) | 143 ± 1 (15.7 ± 0.2) |
| Helix II | |||
| Ca-CaM | 83 (10.1) | −20 (16.7) | |
| IQ-Ca-CaM | 107 (11.2) | −41 (18.6) | |
| ApoCaM | 125 (11.9) | −49 (12.9) | |
| Nav1.2 CTD | 100 ± 5 (10.8 ± 0.4) | −46 ± 2 (13.4 ± 0.1) | |
| Helix III | |||
| Ca-CaM | 65 (15.3) | ||
| IQ-Ca-CaM | 80 (16.5) | ||
| ApoCaM | 129 (14.1) | ||
| Nav1.2 CTD | 98 ± 6 (14.4 ± 0.4) |
Helices I and IV contribute to the hydrophobic core of the protein, with a majority of aromatic side chains contributed from helix I. The segments between Gln-1811—Glu-1814 and Arg-1851—His-1853 participate in an anti-parallel β-sheet. An additional anti-parallel β-sheet contribution from residues Met-1846—Val-1847 is not present in all conformers of the structural ensemble. The helix II-III interhelical segment, delimited by two cis proline residues, Pro-1828 and Pro-1834, is well ordered in the structural ensemble. The conformation of residues Asp-1826—Leu-1829 is consistent with a type VI tight-turn (61), also called a βαR turn (62). The unique di-proline-leucine motif, Pro-1827—Leu-1829 extends the helix II-III interhelical segment by forming a small handle at the base of helix II (Figs. 3, D and E). The absence of long-range NOE contacts for residues Ser-1848 and Gly-1849 is represented by disorder of this region in the ensemble.
The segment from residues Ser-1869 to Arg-1882 is predicted to have residual helical content based on secondary 13C chemical shifts and characteristic dαN(i, i+3) and dαβ(i, i+3) NOEs (Fig. 1). A short helix V is observed in the final ensemble extending from Gly-1870 to Arg-1876 with a backbone root mean square deviation of 0.59 Å when superposed on itself (supplemental Fig. S1). However, the reduced magnitudes of the secondary 13C chemical shifts and the {1H}-15N NOEs for helix V compared with helices I to IV, suggest that the helical conformation is not fully populated in solution. Furthermore, helix V does not exhibit residual dipolar couplings or long-range NOE contacts and, hence, is not well defined relative to the core EF-hand domain structure (supplemental Fig. S1). Additional interactions present in longer constructs of the CTD or in complexes with other components of the VGSC may stabilize helix V.
Binding of Ca2+ by the NaV1.2 (1777-1882) and NaV1.5 (1773-1878) CTDs was assessed by monitoring 1H,15N chemical shifts as a function of Ca2+ concentration (0-4.5 mm). Chemical shift perturbations exhibit titration behavior suggesting that the interaction occurs on a fast-exchange timescale with equilibrium constants of 1.65 ± 0.03 mm for NaV1.2 CTD and 3.28 ± 0.13 mm for NaV1.5 CTD (Fig. 3 and supplemental Fig. S2), consistent with a previous report for the NaV1.5 CTD (33). However, resonance assignments were not obtained previously, and the structure of NaV1.2 CTD now reveals that chemical shift perturbations >0.05 ppm are localized to residues in the N terminus of helix I, the linker between helices II and III, the C terminus of helix IV and the partially structured helix V. Thus, this weak Ca2+ binding site is distal to the canonical EF-hand loop motifs. In contrast, the average chemical shift change between the end points of the titration is <0.01 ppm in the N-terminal EF-hand loop (residues 1806-1817) and in the C-terminal EF-hand loop (residues 1842-1853) for the NaV1.2 CTD. Respective values <0.02 ppm were obtained for corresponding residues 1802-1813 and 1832-1849 in the NaV1.5 CTD. In comparison, the average chemical shift changes of the N-terminal EF-hand loop between apoCa2+ and Ca2+-loaded calmodulin are 0.59 and 0.65 ppm in the N-terminal and C-terminal domains, respectively (63, 64). In particular, canonical Ca2+ binding by an EF-hand would require coordination of a Ca2+ atom by the backbone carbonyl atoms of Phe-1812 in NaV1.2 and Phe-1808 in NaV1.5, leading to significant chemical shift changes for inter-residual and sequential amide resonances (65, 66). In opposition, chemical shift changes less than 0.02 ppm were observed for backbone amide resonances for residues Phe-1812-Ile-1813 and Phe-1808—Ile-1809 of NaV1.2 and NaV1.5, respectively (Fig. 3). A structure-based sequence alignment of calmodulin and NaV1.2 and a comparison of Ca2+-induced chemical shift changes are shown in supplemental Fig. S3.
DISCUSSION
The solution structure determined by NMR spectroscopy for the NaV1.2 CTD (1777-1882) exhibits a core-ordered domain from residues Leu-1790 to Glu-1868, with four α-helices and two short anti-parallel β-strands arranged in tandem helix-sheet-helix motifs characteristic of paired EF-hand domains. Structural alignment of the NaV1.2 CTD and calmodulin reveals that the structure is more similar to apo-Ca2+ calmodulin than to peptide target and/or Ca2+-loaded calmodulin. The NaV1.5 CTD (1773-1878), which shares 83% identity with the NaV1.2 CTD, adopts a similar secondary structure and, likely, tertiary structure.
Titrations monitored by NMR chemical shift perturbations demonstrate that the canonical EF-hand loops of the NaV1.2 CTD (1777-1882) and NaV1.5 CTD (1773-1878) do not bind Ca2+; rather, Ca2+ binds weakly at a site distal to the canonical loops near the N terminus of helix I, the linker between helices II and III, the C terminus of helix IV, and the partially structured helix V. The high resolution crystal structure of calmodulin identified an additional Ca2+ binding site in the homologous region corresponding to the helix II-III linker, but the authors judged this site to be non-physiological (67).
A structure-based sequence alignment with calmodulin also suggests that the canonical EF-hand loops of NaV1.2 CTD do not bind Ca2+ (Table 3, Figs. 2, D and E, and supplemental Fig. S3). Chelation of Ca2+ requires an acidic residue, such as Glu or Asp, at sequence position 1817, corresponding to position 12 in a canonical EF-hand calcium binding motif (68), rather than the Lys residue present in NaV1.2. Mutation of the corresponding residue, Glu to Lys, in Drosophila melanogaster calmodulin abolishes Ca2+ binding, although this mutation may mimic a Ca2+-bound state in the context of certain targets (69, 70). Lys is found at position 12 in the non-canonical Ca2+ binding loop of scallop myosin essential light chain; however, coordination of Ca2+ is accomplished by an acidic residue at position -2, the backbone carbonyl group at position +2, and a water molecule (71). In NaV1.2 the residue at position +2 is Pro, and the residues at positions -3 and -2 are Glu and Lys. The latter two residues have chemical shift changes less than 0.05 ppm after the addition of 4.5 mm Ca2+.
TABLE 3.
Structure-based sequence alignment
N-terminal calcium binding loop of CaM aligned with the respective N-terminal EF-hand loop sequences from Nav1.2 and Nav1.5 CTD sequences, based on a structural alignment between apo-CaM and the Nav1.2 CTD.
| Ca2+ coordination | X | Y | Z | −Y | −X | −Z | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Nav1.2 | D | P | D | A | T | Q | F | I | E | F | A | K |
| Nav1.5 | D | P | E | A | T | Q | F | I | E | Y | S | V |
| CaM | D | K | D | G | D | G | T | I | T | T | K | E |
Higher affinity Ca2+ binding has been reported for longer constructs of NaV1.5 CTD, residues 1773-1920 and residues 1773-1925 that include the IQ motif, and binding is abolished by mutation of the IQ motif (33). However, the resonance assignments obtained for NaV1.5 indicate that chemical shift perturbations for key EF-hand canonical loop residues Phe-1808—Ile-1809 are not larger in these longer constructs (comparing the inset of Fig. 3B with supplemental Fig. 5D of Ref. 33), suggesting that higher affinity binding of Ca2+ also does not involve the canonical EF-hand loops.
The solution structure of NaV1.2 CTD can be used to predict the effect(s) of clinical mutations in VGSCs (Fig. 4) because of the high degree of homology between VGSC CTDs. Generally, clinically significant mutations that map in the CTD can be divided into two classes, with some overlap for several sites (supplemental Table SI). Mutations in Nav1.5 associated with the Long QT variant 3 (LQT3) cardiac arrhythmia phenotype and a subset of mutations in Nav1.1 associated with certain epilepsy syndromes lead to persistent current during maintained depolarization. A second set of mutations in Nav1.1 associated with multiple epilepsy syndromes and mutations in Nav1.5 associated with the Brugada syndrome cardiac arrhythmia led to decreased current, resulting from loss of function or enhanced inactivation kinetics.
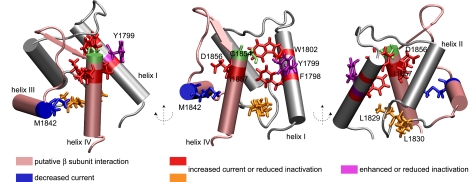
FIGURE 4.
NMR structure of NaV1. 2 (1777-1882) CTD with functionally significant mutations observed in Nav1.1 and Nav1.5 channels. The lowest energy structure of the calculated ensemble is shown. Mutations leading to persistent current cluster in helices I and IV (show in red) and the helix II-III segment (shown in orange), whereas a position (1842) at which mutation (M1852T) leads to decreased current is shown in blue. Position 1799 at which substitutions lead to increased or decreased inactivation is shown in violet, and residue Cys-1854 is shown in green. The putative β subunit interaction site is shown in pink.
Multiple mutations in NaV1.1 and NaV1.5 associated with an increased persistent current are observed at positions clustering in the corresponding helix I of the NaV1.2 CTD. The F1808L mutation associated with intractable childhood epilepsy with generalized tonic clonic seizures in NaV1.1 may destabilize the protein core because the aromatic ring of Phe-1798 in NaV1.2 contacts residues in helix IV and the helix II-III interhelical segment (4, 72). The insertion of an Asp residue at position 1795, Y1795insD, leads to both LQT3 and Brugada syndrome phenotypes in NaV1.5 and potentially disrupts helix I by shifting the register of helical interactions (73).
Substitution at position Tyr-1795 in NaV1.5 differentially leads to decreased inactivation for Y1795C in LQT3 or enhanced inactivation kinetics for Y1795H in Brugada syndrome, whereas both substitutions lead to sustained current during maintained depolarization and negative shift of voltage dependence of inactivation (27, 74). The Y1795C mutation has been suggested to form an intra-molecular disulfide bond with Cys-1850 in NaV1.5 (32). The average Cβ-Cβ distance of the corresponding residues in the NaV1.2 CTD structural ensemble is 9.6 ± 0.4 Å. The Cβ-Cβ distance in cysteine disulfide bonds ranges from 3.4 to 4 Å (75); thus, the proposed disulfide bond may be intermolecular or require structural rearrangement on the order of several angstroms between helix I and IV (Fig. 4) if it is formed. Furthermore, although Tyr-1795 in NaV1.5 was predicted to contribute to the hydrophobic interface between helices I and IV (27), the corresponding residue Tyr-1799 in NaV1.2 is found in a position closer to the surface; the total side-chain exposed surface area is 103 ± 10 Å2 for the conformers in Table 1. Hence, mutations at position Tyr-1799 may also affect interactions with other components of the intact channel. On the other hand, the conserved Trp-1802, corresponding to Trp-1798 in Nav1.5, is not completely accessible as observed previously (27); the total side-chain exposed surface area is 9 ± 5 Å2 for the conformers in Table 1.
The L1825P mutation associated with LQT3 and the R1826H mutation associated with sudden infant death syndrome in NaV1.5 occurs in the helix II-III interhelical segment (76, 77). The L1825P mutation results in significant persistent current and slows kinetics of inactivation. Interestingly, the L1825P mutation in NaV1.5 introduces a di-proline motif, as is observed in wild type NaV1.1, NaV1.2, NaV1.3, and NaV1.7, but shifted by one residue. The residue corresponding to Arg-1826 in NaV1.2 is Leu-1830, and some local difference in conformation probably exists. Like L1825P, the R1826H mutation leads to persistent current in NaV1.5, further suggesting that the helix II-III interhelical segment is critical to channel inactivation.
Two mutations implicated in interactions with other components of the sodium channel cluster in helices III and IV. The D1866Y mutation in NaV1.1, associated with generalized epilepsy and febrile seizures plus, leads to persistent current and decreased fast inactivation kinetics in the presence of the β subunit (78). The corresponding position Asp-1856 in NaV1.2 is at the start of helix IV and may disturb a putative surface for interaction with the β subunit, as interaction with the β1 subunit and the CTD is suggested to occur through the second helix-sheet-helix motif by yeast-two-hybrid analysis of residues Lys-1846—Arg-1886 in NaV1.1 (78). Additionally, the M1852T mutation in NaV1.1, also associated with generalized epilepsy and febrile seizures plus, results in decreased current (loss of function). This phenotype can be rescued by co-expression with β subunits or calmodulin (79). Proposed to be a folding/trafficking defect, this mutation may destabilize helix III, further suggesting that the second helix-sheet-helix motif may be important for interaction with other components of the sodium channel.
The notable exception to the above patterns is the LQT3 mutation D1790G in NaV1.5, resulting in a relative negative shift in the voltage dependence of inactivation in the presence of the β subunit (19, 80). D1790G corresponds to position D1794 in helix I of Nav1.2 and may disrupt the helix by introduction of a glycine residue, with the effect of propagating to helices III and IV.
The mechanisms and extent of NaV1 CTD function in binding the IQ motif and the specific role of calmodulin as well as Ca2+ in multiple phases of inactivation remains to be elaborated. Interactions with the IQ motif may be more complicated than present models and may involve additional components (33). Previous evidence shows that Ca2+-dependent regulation of VGSC is mediated by calmodulin (31), with the exact mode of interaction yet to be determined. The solution structure of the NaV1.2 C-terminal domain and chemical shift assignments of NaV1.5 (1773-1878) are initial steps in elucidating the mechanism of inactivation, extended to other isoforms by virtue of high degrees of homology. The current work provides a template to begin probing specific interactions between the C-terminal domain and other components that play a role in inactivation of voltage-gated sodium channels.
Supplementary Material
Acknowledgments
We thank Mary Ann Gawinowicz (Columbia University Protein Core Facility) for N-terminal sequencing.
The atomic coordinates and restraints list (code 2kav) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
Complete resonance assignments for NaV1.2 CTD (BMRB 16032) and backbone resonance assignments for NaV1.5 CTD (BMRB 16031) have been deposited in the BioMagResBank.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 HL71165 (to G. S. P.), MSTP 5T32 GM07367 (to V. Z. M.), and R01 GM59273 (to A. G. P.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table I and Figs. S1-S3.
Footnotes
The abbreviations used are: VSGC, voltage-gated sodium channel; NaV1, VSGC type 1; CTD, C-terminal domain; LQT3, Long QT syndrome type 3; CaM, calmodulin; HSQC, heteronuclear single quantum spectroscopy; NOESY, nuclear Overhauser effect (NOE) spectroscopy.
K. Yap, University of Toronto.
References
- 1.Brugada, J., Brugada, P., and Brugada, R. (1999) Europace 1 156-166 [DOI] [PubMed] [Google Scholar]
- 2.Terrenoire, C., Simhaee, D., and Kass, R. S. (2007) J. Cardiovasc. Electrophysiol. 18 900-905 [DOI] [PubMed] [Google Scholar]
- 3.Alekov, A. K., Rahman, M., Mitrovic, N., Lehmann-Horn, F., and Lerche, H. (2001) Eur. J. Neurosci. 13 2171-2176 [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara, T., Sugawara, T., Mazaki-Miyazaki, E., Takahashi, Y., Fukushima, K., Watanabe, M., Hara, K., Morikawa, T., Yagi, K., Yamakawa, K., and Inoue, Y. (2003) Brain 126 531-546 [DOI] [PubMed] [Google Scholar]
- 5.Abriel, H., and Kass, R. S. (2005) Trends Cardiovasc. Med. 15 35-40 [DOI] [PubMed] [Google Scholar]
- 6.Pitt, G. S. (2007) Cardiovasc. Res. 73 641-647 [DOI] [PubMed] [Google Scholar]
- 7.Goldin, A. L., Barchi, R. L., Caldwell, J. H., Hofmann, F., Howe, J. R., Hunter, J. C., Kallen, R. G., Mandel, G., Meisler, M. H., Netter, Y. B., Noda, M., Tamkun, M. M., Waxman, S. G., Wood, J. N., and Catterall, W. A. (2000) Neuron 28 365-368 [DOI] [PubMed] [Google Scholar]
- 8.The, Y. K., Fernandes, J., Popa, M. O., Alekov, A. K., Timmer, J., and Lerche, H. (2006) Biophys. J. 90 3511-3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldin, A. L. (2003) Curr. Opin. Neurobiol. 13 284-290 [DOI] [PubMed] [Google Scholar]
- 10.Tikhonov, D. B., and Zhorov, B. S. (2007) Biophys. J. 93 1557-1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu, Y. S., Isom, L. L., Westenbroek, R. E., Rogers, J. C., Tanada, T. N., McCormick, K. A., Scheuer, T., and Catterall, W. A. (1995) J. Biol. Chem. 270 25696-25701 [DOI] [PubMed] [Google Scholar]
- 12.Tan, H. L., Kupershmidt, S., Zhang, R., Stepanovic, S., Roden, D. M., Wilde, A. A. M., Anderson, M. E., and Balser, J. R. (2002) Nature 415 442-447 [DOI] [PubMed] [Google Scholar]
- 13.Ulbricht, W. (2005) Physiol. Rev. 85 1271-1301 [DOI] [PubMed] [Google Scholar]
- 14.Casini, S., Tan, H. L., Bhuiyan, Z. A., Bezzina, C. R., Barnett, P., Cerbai, E., Mugelli, A., Wilde, A. A. M., and Veldkamp, M. W. (2007) Cardiovasc. Res. 76 418-429 [DOI] [PubMed] [Google Scholar]
- 15.Hilber, K., Sandtner, W., Kudlacek, O., Glaaser, I. W., Weisz, E., Kyle, J. W., French, R. J., Fozzard, H. A., Dudley, S. C., and Todt, H. (2001) J. Biol. Chem. 276 27831-27839 [DOI] [PubMed] [Google Scholar]
- 16.Vilin, Y. Y., Makita, N., George, A. L., and Ruben, P. C. (1999) Biophys. J. 77 1384-1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuhmer, W., Conti, F., Suzuki, H., Wang, X. D., Noda, M., Yahagi, N., Kubo, H., and Numa, S. (1989) Nature 339 597-603 [DOI] [PubMed] [Google Scholar]
- 18.Rohl, C. A., Boeckman, F. A., Baker, C., Scheuer, T., Catterall, W. A., and Klevit, R. E. (1999) Biochemistry 38 855-861 [DOI] [PubMed] [Google Scholar]
- 19.An, R. H., Wang, X. L., Kerem, B., Benhorin, J., Medina, A., Goldmit, M., and Kass, R. S. (1998) Circ. Res. 83 141-146 [DOI] [PubMed] [Google Scholar]
- 20.Deschenes, I., Trottler, E., and Chahine, M. (1999) Circulation 100 278-279 [Google Scholar]
- 21.Motoike, H. K., Liu, H. J., Glaaser, I. W., Yang, A. S., Tateyama, M., and Kass, R. S. (2004) J. Gen. Physiol. 123 155-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, D. W., Makita, N., Kitabatake, A., Balser, J. R., and George, A. L. (2000) Circ. Res. 87 37-43 [DOI] [PubMed] [Google Scholar]
- 23.Babitch, J. A., and Anthony, F. A. (1987) J. Theor. Biol. 127 451-459 [DOI] [PubMed] [Google Scholar]
- 24.Babitch, J. (1990) Nature 346 321-322 [DOI] [PubMed] [Google Scholar]
- 25.Cormier, J. W., Rivolta, I., Tateyama, M., Yang, A. S., and Kass, R. S. (2002) J. Biol. Chem. 277 9233-9241 [DOI] [PubMed] [Google Scholar]
- 26.Wingo, T. L., Shah, V. N., Anderson, M. E., Lybrand, T. P., Chazin, W. J., and Balser, J. R. (2004) Nat. Struct. Mol. Biol. 11 219-225 [DOI] [PubMed] [Google Scholar]
- 27.Glaaser, I. W., Bankston, J. R., Liu, H. J., Tateyama, M., and Kass, R. S. (2006) J. Biol. Chem. 281 24015-24023 [DOI] [PubMed] [Google Scholar]
- 28.Mori, M., Konno, T., Ozawa, T., Murata, M., Imoto, K., and Nagayama, K. (2000) Biochemistry 39 1316-1323 [DOI] [PubMed] [Google Scholar]
- 29.Young, K. A., and Caldwell, J. H. (2005) J. Physiol. (Lond.) 565 349-370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biswas, S., Deschenes, I., DiSilvestre, D., Tian, Y., Halperin, V. L., and Tomaselli, G. F. (2008) J. Gen. Physiol. 131 197-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim, J., Ghosh, S., Liu, H. J., Tateyama, M., Kass, R. S., and Pitt, G. S. (2004) J. Biol. Chem. 279 45004-45012 [DOI] [PubMed] [Google Scholar]
- 32.Tateyama, M., Liu, H., Yang, A. S., Cormier, J. W., and Kass, R. S. (2004) Biophys. J. 86 1843-1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah, V. N., Wingo, T. L., Weiss, K. L., Williams, C. K., Balser, J. R., and Chazin, W. J. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 3592-3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pitt, B., and Pitt, G. S. (2007) Circulation 115 2976-2982 [DOI] [PubMed] [Google Scholar]
- 35.Maniatis, T., Fritsch, E. F., and Sambrook, J. (1989) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- 36.Tatusova, T. A., and Madden, T. L. (1999) FEMS Microbiol. Lett. 174 247-250 [DOI] [PubMed] [Google Scholar]
- 37.Findeisen, M., Brand, T., and Berger, S. (2007) Magn. Reson. Chem. 45 175-178 [DOI] [PubMed] [Google Scholar]
- 38.Cavanagh, J., Fairbrother, W., Palmer, A. I., Rance, M., and Skelton, N. (2007) Protein NMR Spectroscopy: Principles and Practice, 2nd Ed., Academic Press, Boston, MA
- 39.Neri, D., Szyperski, T., Otting, G., Senn, H., and Wüthrich, K. (1989) Biochemistry 28 7510-7516 [DOI] [PubMed] [Google Scholar]
- 40.Ottiger, M., Delaglio, F., and Bax, A. (1998) J. Magn. Reson. 131 373-378 [DOI] [PubMed] [Google Scholar]
- 41.Permi, P., Rosevear, P. R., and Annila, A. (2000) J. Biomol. NMR 17 43-54 [DOI] [PubMed] [Google Scholar]
- 42.Chou, J. J., Delaglio, F., and Bax, A. (2000) J. Biomol. NMR 18 101-105 [DOI] [PubMed] [Google Scholar]
- 43.Cicero, D. O., Contessa, G. M., Paci, M., and Bazzo, R. (2006) J. Magn. Reson. 180 222-228 [DOI] [PubMed] [Google Scholar]
- 44.Markley, J. L., Bax, A., Arata, Y., Hilbers, C. W., Kaptein, R., Sykes, B. D., Wright, P. E., and Wüthrich, K. (1998) J. Biomol. NMR 12 1-23 [DOI] [PubMed] [Google Scholar]
- 45.Rieping, W., Habeck, M., Bardiaux, B., Bernard, A., Malliavin, T. E., and Nilges, M. (2007) Bioinformatics 23 381-382 [DOI] [PubMed] [Google Scholar]
- 46.Brunger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J. S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T., and Warren, G. L. (1998) Acta Crystallogr. D Biol. Crystallogr. 54 905-921 [DOI] [PubMed] [Google Scholar]
- 47.Schwieters, C. D., Kuszewski, J. J., Tjandra, N., and Clore, G. M. (2003) J. Magn. Reson. 160 65-73 [DOI] [PubMed] [Google Scholar]
- 48.Schwieters, C. D., Kuszewski, J. J., and Clore, G. M. (2006) Prog. Nucl. Magn. Reson. Spectrosc. 48 47-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cornilescu, G., Delaglio, F., and Bax, A. (1999) J. Biomol. NMR 13 289-302 [DOI] [PubMed] [Google Scholar]
- 50.Guntert, P., and Wüthrich, K. (1991) J. Biomol. NMR 1 447-456 [DOI] [PubMed] [Google Scholar]
- 51.Mumenthaler, C., Guntert, P., Braun, W., and Wüthrich, K. (1997) J. Biomol. NMR 10 351-362 [DOI] [PubMed] [Google Scholar]
- 52.Fletcher, C. M., Jones, D. N. M., Diamond, R., and Neuhaus, D. (1996) J. Biomol. NMR 8 292-310 [DOI] [PubMed] [Google Scholar]
- 53.Doreleijers, J. F., Raves, M. L., Rullmann, T., and Kaptein, R. (1999) J. Biomol. NMR 14 123-132 [DOI] [PubMed] [Google Scholar]
- 54.Zweckstetter, M., and Bax, A. (2000) J. Am. Chem. Soc. 122 3791-3792 [Google Scholar]
- 55.Davis, I. W., Leaver-Fay, A., Chen, V. B., Block, J. N., Kapral, G. J., Wang, X., Murray, L. W., Arendall, W. B., Snoeyink, J., Richardson, J. S., and Richardson, D. C. (2007) Nucleic Acids Res. 35 375-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Humphrey, W., Dalke, A., and Schulten, K. (1996) J. Mol. Graph. 14 33-38 [DOI] [PubMed] [Google Scholar]
- 57.Shindyalov, I. N., and Bourne, P. E. (1998) Protein Eng. 11 739-747 [DOI] [PubMed] [Google Scholar]
- 58.Koradi, R., Billeter, M., and Wuthrich, K. (1996) J. Mol. Graph. 14 51-55 [DOI] [PubMed] [Google Scholar]
- 59.Wüthrich, K. (1986) NMR of Proteins and Nucleic Acids, Wiley, New York
- 60.Schubert, M., Labudde, D., Oschkinat, H., and Schmieder, P. (2002) J. Biomol. NMR 24 149-154 [DOI] [PubMed] [Google Scholar]
- 61.Chou, K. C. (2000) Anal. Biochem. 286 1-16 [DOI] [PubMed] [Google Scholar]
- 62.Wilmot, C. M., and Thorton, J. M. (1990) Protein Eng. 3 479-493 [DOI] [PubMed] [Google Scholar]
- 63.Ikura, M., Kay, L. E., and Bax, A. (1990) Biochemistry 29 4659-4667 [DOI] [PubMed] [Google Scholar]
- 64.Tjandra, N., Kuboniwa, H., Ren, H., and Bax, A. (1995) Eur. J. Biochem. 230 1014-1024 [DOI] [PubMed] [Google Scholar]
- 65.Biekofsky, R. R., Martin, S. R., Browne, J. P., Bayley, P. M., and Feeney, J. (1998) Biochemistry 37 7617-7629 [DOI] [PubMed] [Google Scholar]
- 66.Xu, X. P., and Case, D. A. (2002) Biopolymers 65 408-423 [DOI] [PubMed] [Google Scholar]
- 67.Wilson, M. A., and Brunger, A. T. (2000) J. Mol. Biol. 301 1237-1256 [DOI] [PubMed] [Google Scholar]
- 68.Gifford, J. L., Walsh, M. P., and Vogel, H. J. (2007) Biochem. J. 405 199-221 [DOI] [PubMed] [Google Scholar]
- 69.Maune, J. F., Klee, C. B., and Beckingham, K. (1992) J. Biol. Chem. 267 5286-5295 [PubMed] [Google Scholar]
- 70.Gao, Z. H., Krebs, J., Vanberkum, M. F. A., Tang, W. J., Maune, J. F., Means, A. R., Stull, J. T., and Beckingham, K. (1993) J. Biol. Chem. 268 20096-20104 [PubMed] [Google Scholar]
- 71.Houdusse, A., and Cohen, C. (1996) Structure 4 21-32 [DOI] [PubMed] [Google Scholar]
- 72.Rhodes, T. H., Vanoye, C. G., Ohmori, I., Ogiwara, I., Yamakawa, K., and George, A. L. (2005) J. Physiol. (Lond.) 569 433-445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bezzina, C., Veldkamp, M. W., van den Berg, M. P., Postma, A. V., Rook, M. B., Viersma, J. W., van Langen, I. M., Tan-Sindhunata, G., Bink-Boelkens, M. T. E., van der Hout, A. H., Mannens, M., and Wilde, A. A. M. (1999) Circ. Res. 85 1206-1213 [DOI] [PubMed] [Google Scholar]
- 74.Rivolta, I., Abriel, H., Tateyama, M., Liu, H. H., Memmi, M., Vardas, P., Napolitano, C., Priori, S. G., and Kass, R. S. (2001) J. Biol. Chem. 276 30623-30630 [DOI] [PubMed] [Google Scholar]
- 75.Hazes, B., and Dijkstra, B. W. (1988) Protein Eng. 2 119-125 [DOI] [PubMed] [Google Scholar]
- 76.Makita, N., Horie, M., Nakamura, T., Ai, T., Sasaki, K., Yokoi, H., Sakurai, M., Sakuma, I., Otani, H., Sawa, H., and Kitabatake, A. (2002) Circulation 106 1269-1274 [DOI] [PubMed] [Google Scholar]
- 77.Ackerman, M. J., Siu, B. L., Sturner, W. Q., Tester, D. J., Valdivia, C. R., Makielski, J. C., and Towbin, J. A. (2001) J. Am. Med. Assoc. 286 2264-2269 [DOI] [PubMed] [Google Scholar]
- 78.Spampanato, J., Kearney, J. A., de Haan, G., McEwen, D. P., Escayg, A., Aradi, I., MacDonald, B. T., Levin, S. I., Soltesz, I., Benna, P., Montalenti, E., Isom, L. L., Goldin, A. L., and Meisler, M. H. (2004) J. Neurosci. 24 10022-10034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rusconi, R., Scalmani, P., Cassulini, R. P., Giunti, G., Gambardella, A., Franceschetti, S., Annesi, G., Wanke, E., and Mantegazza, M. (2007) J. Neurosci. 27 11037-11046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abriel, H., Wehrens, X. H. T., Benhorin, J., Kerem, B., and Kass, R. S. (2000) Circulation 102 921-925 [DOI] [PubMed] [Google Scholar]
- 81.Fallon, J. L., Halling, D. B., Hamilton, S. L., and Quiocho, F. A. (2005) Structure 13 1881-1886 [DOI] [PubMed] [Google Scholar]
- 82.Kuboniwa, H., Tjandra, N., Grzesiek, S., Ren, H., Klee, C. B., and Bax, A. (1995) Nat. Struct. Biol. 2 768-776 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.