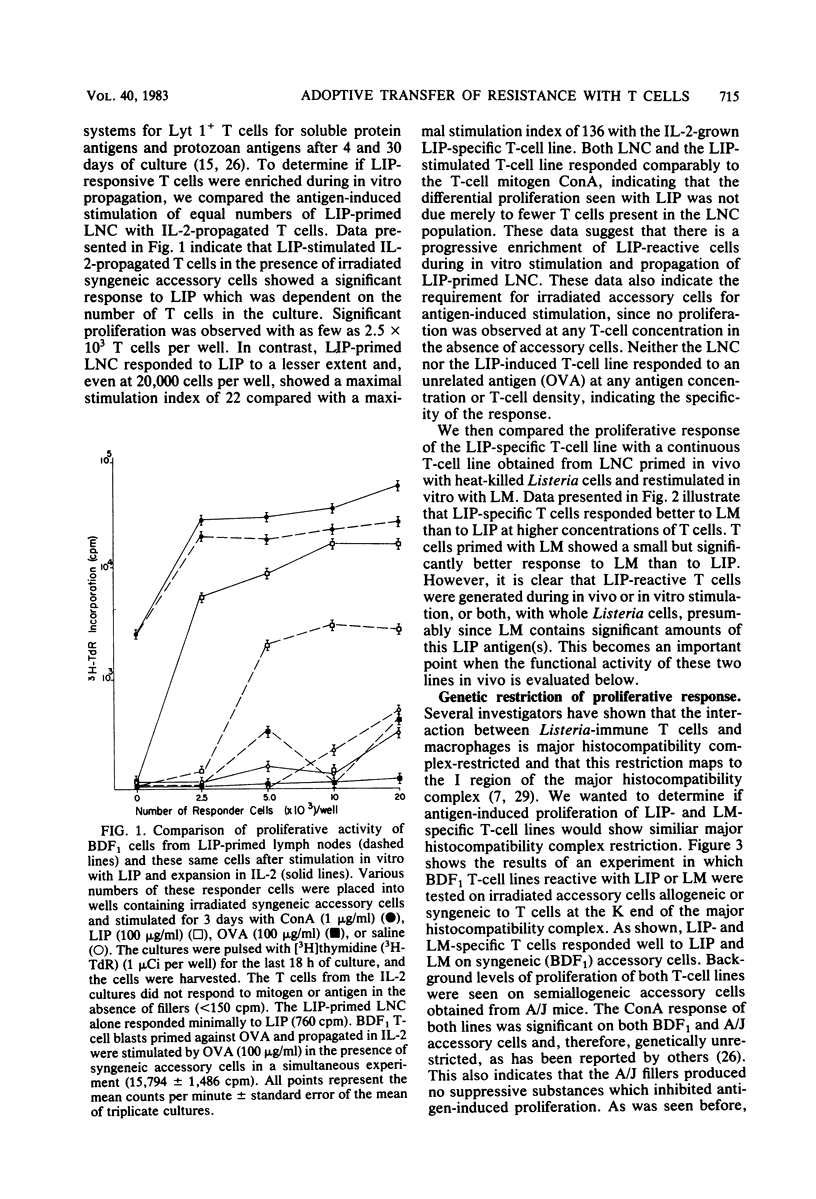
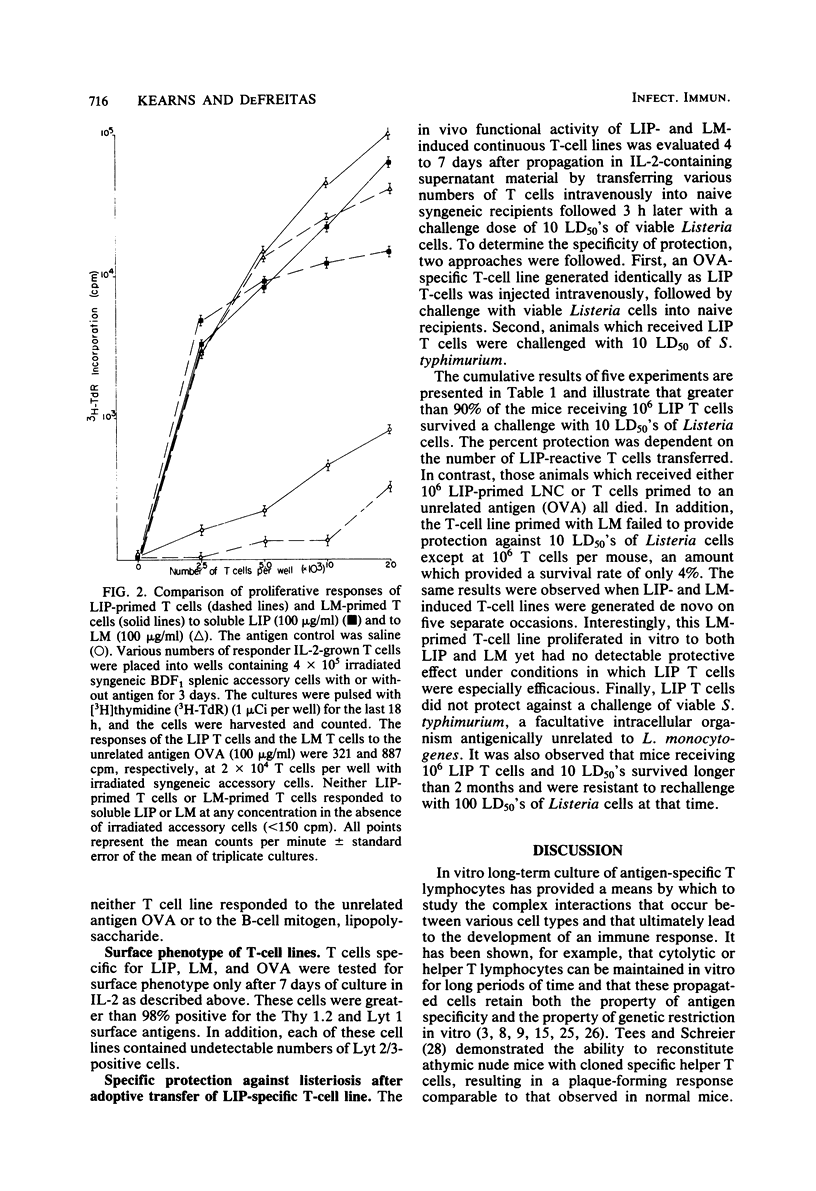
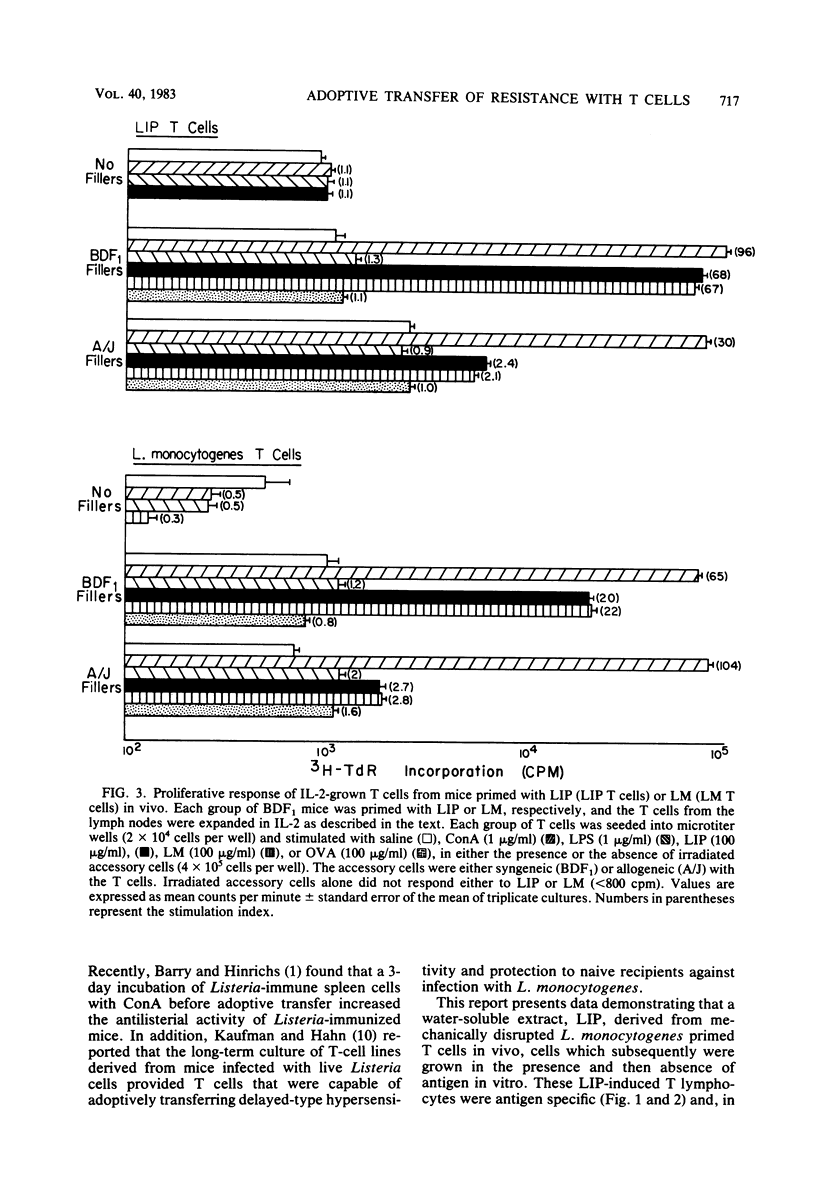
Abstract
Murine T cells generated against heat-killed Listeria monocytogenes or Listeria intracellular product (LIP) were propagated in a source of Interleukin 2. Both T-cell cultures were greater than 98% Lyt 1+, 2/3- and proliferated specifically against LIP and L. monocytogenes crude whole-cell antigen in vitro. Proliferation of both T-cell cultures required the presence of antigen and accessory cells syngeneic to the T cells at the left end of the major histocompatibility complex. The ability of these cultures to adoptively transfer protection against challenge with viable Listeria cells was dramatically different. As few as 10(6) LIP-specific T cells conferred significant protection against a lethal challenge of Listeria cells, whereas cultures induced against crude whole-cell antigen showed little or no protective function. The resistance conferred by LIP-specific T cells was specific in that the cells did not reduce the mortality seen after challenge with Salmonella typhimurium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry R. A., Hinrichs D. J. Enhanced adoptive transfer of immunity to Listeria monocytogenes after in vitro culture of murine spleen cells with concanavalin A. Infect Immun. 1982 Feb;35(2):560–565. doi: 10.1128/iai.35.2.560-565.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Langman R. E. Cell-mediated immunity to bacterial infection in the mouse. Thymus-derived cells as effectors of acquired resistance to Listeria monocytogenes. Scand J Immunol. 1972;1(4):379–391. doi: 10.1111/j.1365-3083.1972.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Corradin G., Etlinger H. M., Chiller J. M. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977 Sep;119(3):1048–1053. [PubMed] [Google Scholar]
- Davidson W. F., Parish C. R. A procedure for removing red cells and dead cells from lymphoid cell suspensions. J Immunol Methods. 1975 Jun;7(2-3):291–300. doi: 10.1016/0022-1759(75)90026-5. [DOI] [PubMed] [Google Scholar]
- Farr A. G., Kiely J. M., Unanue E. R. Macrophage-T cell interactions involving Listeria monocytogenes--role of the H-2 gene complex. J Immunol. 1979 Jun;122(6):2395–2404. [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Augustin A. A. Helper activity of T cells stimulated in long-term culture. Eur J Immunol. 1979 Sep;9(9):671–680. doi: 10.1002/eji.1830090904. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H. Biological functions of t cell lines with specificity for the intracellular bacterium Listeria monocytogenes in vitro and in vivo. J Exp Med. 1982 Jun 1;155(6):1754–1765. doi: 10.1084/jem.155.6.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns R. J., Hinrichs D. J. Heat-labile B-cell mitogen obtained from Listeria monocytogenes. Infect Immun. 1978 Dec;22(3):676–680. doi: 10.1128/iai.22.3.676-680.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasky S., Pickett M. J. Immunogenicity and specificity of Listeria monocytogenes cell walls. J Infect Dis. 1968 Feb;118(1):65–75. doi: 10.1093/infdis/118.1.65. [DOI] [PubMed] [Google Scholar]
- Krahenbuhl J. L., Remington J. S. In vitro induction of nonspecific resistance in macrophages by specifically sensitized lymphocytes. Infect Immun. 1971 Oct;4(4):337–343. doi: 10.1128/iai.4.4.337-343.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis J., Moedder E., Behin R., Engers H. Recognition of protozoan parasite antigens by murine T lymphocytes. I. Induction of specific T lymphocyte-dependent proliferative response to Leishmania tropica. Eur J Immunol. 1979 Nov;9(11):841–847. doi: 10.1002/eji.1830091103. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKI K., MACKANESS G. B. THE PASSIVE TRANSFER OF ACQUIRED RESISTANCE TO LISTERIA MONOCYTOGENES. J Exp Med. 1964 Jul 1;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. C., Unanue E. R. Effects of bacterial products on lymphocytes and macrophages: their possible role in natural resistance to listeria infetion in mice. J Immunol. 1974 Sep;113(3):984–992. [PubMed] [Google Scholar]
- Rodriguez G. E., McClatchy J. K., Campbell P. A. Induction of Resistance by Listeria monocytogenes Cell Wall Fraction. Infect Immun. 1974 Nov;10(5):1163–1169. doi: 10.1128/iai.10.5.1163-1169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Tees R. Clonal induction of helper T cells: conversion of specific signals into nonspecific signals. Int Arch Allergy Appl Immunol. 1980;61(2):227–237. doi: 10.1159/000232437. [DOI] [PubMed] [Google Scholar]
- Schrier R. D., Skidmore B. J., Kurnick J. T., Goldstine S. N., Chiller J. M. Propagation of antigen-specific T cell helper function in vitro. J Immunol. 1979 Dec;123(6):2525–2531. [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Enhancement of macrophage bactericidal capacity by antigenically stimulated immune lymphocytes. Cell Immunol. 1972 Jun;4(2):163–174. doi: 10.1016/0008-8749(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Tees R., Schreier M. H. Selective reconstitution of nude mice with long-term cultured and cloned specific helper T cells. Nature. 1980 Feb 21;283(5749):780–781. doi: 10.1038/283780a0. [DOI] [PubMed] [Google Scholar]
- Van Der Meer C., Hofhuis F. M., Willers J. M. Killed Listeria monocytogenes vaccine becomes protective on addition of polyanions. Nature. 1977 Oct 13;269(5629):594–595. doi: 10.1038/269594a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A., Adler B., Blanden R. V., Davidson W. F., Kees U., Dunlop M. B., Shreffler D. C. H-2 restriction of cell-mediated immunity to an intracellular bacterium: effector T cells are specific for Listeria antigen in association with H-21 region-coded self-markers. J Exp Med. 1977 May 1;145(5):1353–1367. doi: 10.1084/jem.145.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H., Hofhuis F. M., Berns E. M., van der Meer C., Willers J. M. Killed Listeria monocytogenes vaccine is protective in C3H/HeJ mice without addition of adjuvants. Nature. 1980 Aug 14;286(5774):713–714. doi: 10.1038/286713a0. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Hengartner H., Nabholz M., Lernhardt W., Schreier M. H., Haas W. Fine specificity of a continuously growing killer cell clone specific for H-Y antigen. Eur J Immunol. 1979 Aug;9(8):592–597. doi: 10.1002/eji.1830090804. [DOI] [PubMed] [Google Scholar]


