Abstract
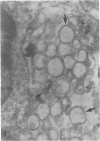
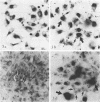
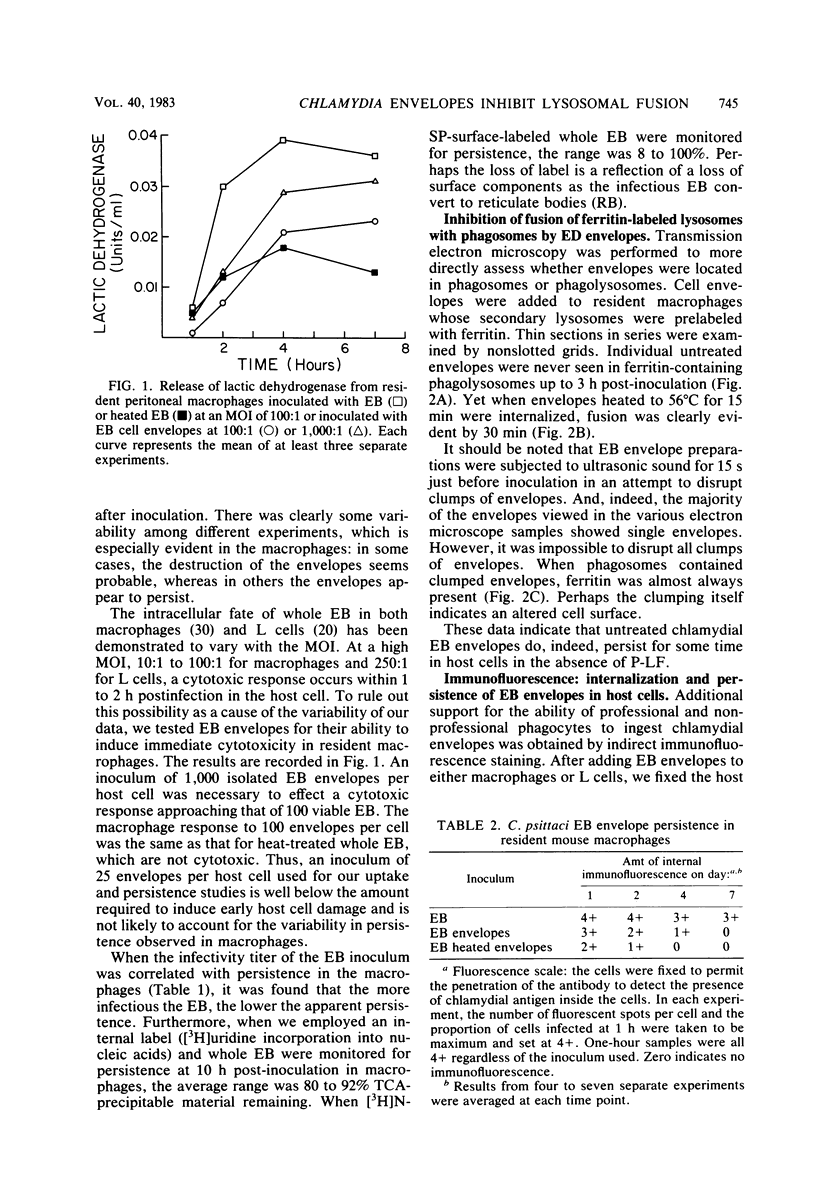
The cell surface of Chlamydia psittaci seems important for establishing infection since (i) UV-treated elementary bodies (EB) attach to and are ingested by L cells and (ii) heat or antibody treatment decreases attachment to L cells and promotes the fusion of chlamydiae-containing phagosomes with lysosomes in macrophages. In the studies reported here, [3H]uridine-labeled UV-treated EB also persisted in mouse resident peritoneal macrophages and L cells, suggesting that phagosome-lysosome fusion is inhibited. We therefore chose to investigate the ingestion and internal fate of isolated purified EB envelopes in both nonprofessional and professional phagocytic cells. EB envelopes are internalized by target host cells as efficiently as are whole EB. Transmission electron microscopy of macrophages whose lysosomes were marked with ferritin revealed the persistence of individual envelopes in phagosomes devoid of ferritin for the 3-h observation period. In contrast, EB envelopes heated to 56 degrees C for 15 min were consistently found in ferritin-labeled phagolysosomes as early as 30 min. As another index of persistence, isolated EB envelopes were radioisotopically labeled with a Bolton-Hunter analog, [3H]N-succinimidyl propionate, and their fate as trichloroacetic acid-precipitable material was followed. A third probe, employed to detect the persistence of non-biodegradable antigen, was indirect immunofluorescence. Fluorescein-positive antigens were brightly visible for 7 days in both macrophages and L cells when they were inoculated with untreated EB or EB maintained in penicillin. But L cells inoculated with EB envelopes or EB treated with UV or chloramphenicol, all of which prevent the conversion of infectious EB into the metabolically active reticulate bodies, displayed reduced internal fluorescence by 2 days and the appearance of fluorescent material on the cell surface. This release of EB envelope material occurred in the absence of phagolysosome fusion. The data add credence to the belief that the spontaneous breakdown or autolytic enzyme release of EB envelope components must occur preparatory to the conversion of EB to reticulate bodies.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownridge E., Wyrick P. B. Interaction of Chlamydia psittaci reticulate bodies with mouse peritoneal macrophages. Infect Immun. 1979 Jun;24(3):697–700. doi: 10.1128/iai.24.3.697-700.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Kinetics of phagocytosis of Chlamydia psittaci by mouse fibroblasts (L cells): separation of the attachment and ingestion stages. Infect Immun. 1978 Feb;19(2):607–612. doi: 10.1128/iai.19.2.607-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Moulder J. W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978 Feb;19(2):598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Requirements for ingestion of Chlamydia psittaci by mouse fibroblasts (L cells). Infect Immun. 1976 Sep;14(3):645–651. doi: 10.1128/iai.14.3.645-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg L. G., Wyrick P. B. Inhibition of phagolysosome fusion is localized to Chlamydia psittaci-laden vacuoles. Infect Immun. 1981 May;32(2):889–896. doi: 10.1128/iai.32.2.889-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink D. J., Gainer H. The use of a labeled acylating probe for the study of fast axonal transport, in vivo. Brain Res. 1979 Nov 9;177(1):208–213. doi: 10.1016/0006-8993(79)90934-x. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. H., Hart P. D., Young M. R. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature. 1980 Jul 3;286(5768):79–80. doi: 10.1038/286079a0. [DOI] [PubMed] [Google Scholar]
- Goren M. B., D'Arcy Hart P., Young M. R., Armstrong J. A. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Vance D. W., Jr, Al-Hossainy E. Attachment of Chlamydia psittaci to formaldehyde-fixed and unfixed L cells. J Gen Microbiol. 1981 Aug;125(2):273–283. doi: 10.1099/00221287-125-2-273. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lammert J. K. Cytotoxic cells induced after Chlamydia psittaci infection in mice. Infect Immun. 1982 Mar;35(3):1011–1017. doi: 10.1128/iai.35.3.1011-1017.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn A. M., Blyth W. A., Taverne J. Interactions of TRIC agents with macrophages and BHK-21 cells observed by electron microscopy. J Hyg (Lond) 1973 Sep;71(3):515–528. doi: 10.1017/s0022172400046507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N. J., Moulder J. W. Attachment of cell walls of Chlamydia psittaci to mouse fibroblasts (L cells). Infect Immun. 1982 Sep;37(3):1059–1065. doi: 10.1128/iai.37.3.1059-1065.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manire G. P., Tamura A. Preparation and chemical composition of the cell walls of mature infectious dense forms of meningopneumonitis organisms. J Bacteriol. 1967 Oct;94(4):1178–1183. doi: 10.1128/jb.94.4.1178-1183.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W., Hatch T. P., Byrne G. I., Kellogg K. R. Immediate toxicity of high multiplicities of Chlamydia psittaci for mouse fibroblasts (L cells). Infect Immun. 1976 Jul;14(1):277–289. doi: 10.1128/iai.14.1.277-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond S. J., Stirling P. Localization of chlamydial group Antigen in McCoy cell monolayers infected with Chlamydia trachomatis or Chlamydia psittaci. Infect Immun. 1981 Nov;34(2):561–570. doi: 10.1128/iai.34.2.561-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes G. V. Formation and destruction of internal membranes in L cells infected with Chlamydia psittaci. Infect Immun. 1973 Feb;7(2):173–177. doi: 10.1128/iai.7.2.173-177.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMURA A., HIGASHI N. PURIFICATION AND CHEMICAL COMPOSITION OF MENINGOPNEUMONITIS VIRUS. Virology. 1963 Aug;20:596–604. doi: 10.1016/0042-6822(63)90284-8. [DOI] [PubMed] [Google Scholar]
- Tamura A., Manire G. P. Effect of penicillin on the multiplication of meningopneumonitis organisms (Chlamydia psittaci). J Bacteriol. 1968 Oct;96(4):875–880. doi: 10.1128/jb.96.4.875-880.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd W. J., Storz J. Ultrastructural cytochemical evidence for the activation of lysosomes in the cytocidal effect of Chlamydia psittaci. Infect Immun. 1975 Sep;12(3):638–646. doi: 10.1128/iai.12.3.638-646.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Salari H. Control mechanisms governing the infectivity of Chlamydia trachomatis for hela cells: modulation by cyclic nucleotides, prostaglandins and calcium. J Gen Microbiol. 1982 Mar;128(3):639–650. doi: 10.1099/00221287-128-3-639. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Hoffstein S. Leukocytic proteases and the immunologic release of lysosomal enzymes. Am J Pathol. 1972 Sep;68(3):539–564. [PMC free article] [PubMed] [Google Scholar]
- Wyrick P. B., Brownridge E. A. Growth of Chlamydia psittaci in macrophages. Infect Immun. 1978 Mar;19(3):1054–1060. doi: 10.1128/iai.19.3.1054-1060.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrick P. B., Brownridge E. A., Ivins B. E. Interaction of Chlamydia psittaci with mouse peritoneal macrophages. Infect Immun. 1978 Mar;19(3):1061–1067. doi: 10.1128/iai.19.3.1061-1067.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]