The best known type of inherited hyperferritinemia not related to iron overload is the hyperferritinemia-cataract syndrome (OMIM #600886), caused by a mutation in the iron-responsive element in the 5-prime non-coding region of the ferritin light chain gene (FTL). This study describes a novel missense mutation of FTL responsible for genetic hyperferritinemia without iron overload. See related perspective article on page 307.
Keywords: hyperferritinemia, glycosylated ferritin, L ferritin, iron overload
Abstract
Background
Elevated serum ferritin levels are frequently encountered in clinical situations and once iron overload or inflammation has been ruled out, many cases remain unexplained. Genetic causes of hyperferritinemia associated to early cataract include mutations in the iron responsive element in the 5’ untranslated region of the L ferritin mRNA, responsible for the hereditary hyperferritinemia cataract syndrome.
Design and Methods
We studied 91 probands with hyperferritinemia comprising 25 family cases belonging to families with at least two cases of unexplained hyperferritinemia, and 66 isolated cases. In the families, we also analyzed 30 relatives. Hyperferritinemia was considered as unexplained when transferrin saturation was below 45% and/or serum iron below 25 μmol/L and/or no tissue iron excess was detected, when inflammation had been ruled out and when iron responsive element mutation was absent. We carried out sequencing analysis of the FTL gene coding the L ferritin.
Results
A novel heterozygous p.Thr30Ile mutation in the NH2 terminus of L ferritin subunit was identified in 17 probands out of the cohort. The mutation was shown to cosegregate with hyperferritinemia in all the 10 families studied. No obvious clinical symptom was found associated with the presence of the mutation. This unique mutation is associated with an unusually high percentage of ferritin glycosylation.
Conclusions
This missense mutation of FTL represents a new cause of genetic hyperferritinemia without iron overload. We hypothesized that the mutation increases the efficacy of L ferritin secretion by increasing the hydrophobicity of the N terminal “A” α helix.
Introduction
Ferritin is the major iron storage protein in the body. It is made of a 24-subunit protein shell surrounding a cavity where the iron core is formed. The subunits are of two types, named H (Heavy, 21 kDa) and L (Light, 19 kDa), coded by two different genes, and ferritin molecules are heteropolymers containing various proportions of these two subunits. The two subunits share 55% identity at the amino acid level and adopt similar 3D structure, allowing them to co-assemble into the same ferritin molecule.1 Ferritin synthesis is regulated by iron through interaction between a stem-loop structure called iron responsive element (IRE) present in the 5’ untranslated region of the L or H ferritin mRNA and iron regulatory proteins (IRPs) acting as intracellular iron sensor.2 When cellular iron content increases, inactivation of the IRPs induces a rapid increase in ferritin mRNAs translation. Ferritin molecules are found in the cell cytosol or in vesicular bodies, although small concentrations of ferritin are also found in plasma. Serum ferritin has wide clinical utility primarily as an indicator of intracellular iron stores and hyperferritinemia is found in patients with hereditary or secondary iron overload.3,4 But serum ferritin levels are also increased in patients without significant iron overload as in inflammatory conditions, metabolic syndrome, cytolysis3,4 and in the Hereditary Hyperferritinemia Cataract Syndrome (HHCS). This syndrome is characterized by the presence of elevated serum ferritin levels, early onset bilateral cataract, and normal to low serum iron and transferrin saturation.5 HHCS is due to the presence of heterozygous point mutations in the L ferritin IRE which impair the negative feed-back regulation of L ferritin synthesis and lead to abnormal accumulation of ferritin in tissues and serum in the absence of iron overload.6–8 However, with the increasing frequency of serum ferritin determination in clinical settings or during routine health check-ups, it appears that high serum ferritin levels is a relatively common finding and a high proportion of these cases remain frequently unexplained.9
In this paper we report a new mutation located near the NH2 terminus of the L ferritin subunit that cosegregates with hyperferritinemia at the heterozygote state. This unique mutation is associated with an unusually high percentage of ferritin glycosylation and represents a new cause of genetic hyperferritinemia without iron overload.
Design and Methods
Patients and control population
Families in which at least two relatives presented with hyperferritinemia (degree 1), or isolated patients who presented with hyperferritinemia, were offered genetic testing in Bichat Hospital (Paris) or Rennes Hospital. Serum ferritin levels were considered elevated when they were above 300 μg/L in men and 200 μg/L in women.
We studied 91 probands with hyperferritinemia comprising 25 family cases belonging to families with at least two cases of unexplained hyperferritinemia, and 66 isolated cases. In the families, we also analyzed 30 relatives. Out of these 121 subjects, there were 36 women and 85 males, aged from eight to 83 years. None of the subjects was homozygous for the p.Cys282Tyr mutation of the HFE gene. Hyperferritinemia was considered as unexplained when transferrin saturation was below 45% and/or serum iron below 25 μmol/L, and when inflammation had been ruled out.
From the 66 isolated patients without family history who had unexplained hyperferritinemia, 47 of them had genetic testing for ferroportin (methods as previously described)9 and no mutation was found. Metabolic syndrome and inflammation were not considered responsible for hyperferritinemia on the basis of bio-clinical evaluation. Twenty-seven of them had liver iron (LIC) content evaluation by MRI or liver biopsy. Twenty-four patients had normal or mildly elevated LIC (<100 μmol/g dry liver weight, normal values <36) 3 patients had elevated LIC (120, 140 and 210 μmol/g) but these values were considered to not fully account for their plasma ferritin concentration (1800, 2000 and 760 μg/L respectively).
Although some of the patients were referred to us for the presence of early onset cataract, none of them had IRE mutation in the L ferritin exon 1. Informed consent was obtained for all patients.
Our control population consisted of 528 DNA samples prepared from the lymphocytes of healthy patients with normal iron status. These persons had given their informed written consent for the study of iron metabolism genes after approval of the protocol by the local ethical committee (98/35–197).
Sequencing of the L ferritin
The promoter, the four exons and three introns of the FTL gene were sequenced on both strands using an ABI sequencing kit on ABI 3130 sequencer (Applied Biosystem). The primers used for amplification were previously described for exon 19 and were as follows for the other exons: exon 2 (forward, 2F: 5’-GGTAAACAGAGGGCGGAGTC, reverse, 2R: 5’-GACACCTAC GCCCTCAAATC); exon 3 (forward, 3F: 5’-AACGACTCTTGGGAAATGTAGG, reverse, 3R: 5’-AGGTGTGAAATGAGGCTCTGA); exon 4 (forward, 4F: 5’-CATTTTAATCTGCAACTGGCT G, reverse, 4R: 5’-GAGGGAGAGGCTTAGGCAGA). Amplification of intron 1 was obtained with primers 1F and 2R, intron 2 with primers 2F and 3R, and intron 3 with primers 3F and 4R) 1Kb of the promoter was amplified with the following primers: the proximal promoter with (forward 5’-AACACCTCACAGCCTTCCAA, reverse 5’-TCTGTTCCGTCCAAACACTG) and the distal promoter with (forward 5’-CTTCTCTTTGTGGGCCTGAA, reverse 5’-CGCTGCGAGATAAGGAGTCT).
Genotyping for the p.Thr30Ile mutation was performed using a Taqman assay Custom by design (Applied Biosystem). PCR amplification was carried out using the following primer pair:
forward: CCGCCCTAGCCACGTC, reverse: GCGCAGCTGGAGGAAATTAG and allelic discrimination was achieved using two allele specific probes:VIC:CCTCCTACACCTACCTC for the wild type allele and FAM:CCTCCTACATCTACCTC for the mutated allele. Real time PCR was performed on an ABI 7500 instrument (Applied Biosystem).
Measurement of ferritin glycosylation
Glycosylated ferritin was determined according to the method of Worwood et al. with minor modifications.10 Serum was incubated for two hours at room temperature with Concanavalin A Sepharose 4B on a roller mixer. The sample was then centrifuged at 3000 rpm for 15 min and unbound ferritin was recovered in the supernatant and assayed using an immunoassay (DadeBehring). Glycosylated ferritin was obtained from the difference between total ferritin and unbound ferritin.
Results
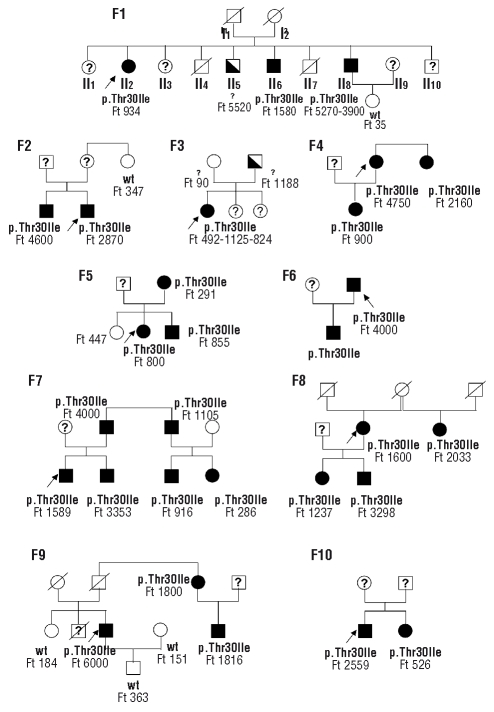
In the 25 subjects that were investigated on the basis of the presence of one or more first degree relatives with hyperferritinemia, we found that 12 of them were heterozygous for a c.89C>T mutation (NM_000146.3) in the FTL gene, leading to p.Thr30Ile amino-acid change. A co-segregation study was performed for 10 families carrying the FTL mutation (Figure 1). The heterozygous mutation was found in the 20 relatives with hyperferritinemia and was absent in the 10 other relatives with normal serum ferritin levels. When the results of 10 families were analyzed together assuming that the Thr30Ile marked the mutated allele and an autosomal dominant transmission, the calculated Lod Score for the implication of the FTL gene was 6.6 at θ=0.
Figure 1.
Family trees of 10 probands with hyperglycosylated serum ferritin. Families are numbered F1 to F10. Black symbols denote the presence of both hyperferritinemia and the heterozygous FTL mutation (p.Thr30Ile). The half-filled black symbols denote hyperferritinemia but no information on the FTL sequence. A question mark in the symbols indicates that the value of serum ferritin and the genotype are unknown. The genotype is indicated as p.Thr30Ile for the presence of the heterozygous FTL mutation, wild type (w.t.) for the normal sequence and a question mark for absence of genotyping. Serum ferritin values (Ft) are indicated in μg/L. The probands are indicated with an arrow.
We then searched for this mutation in the remaining cohort of 66 isolated cases of hyperferritinemia and found 5 additional subjects with the heterozygous c.89C>T mutation.
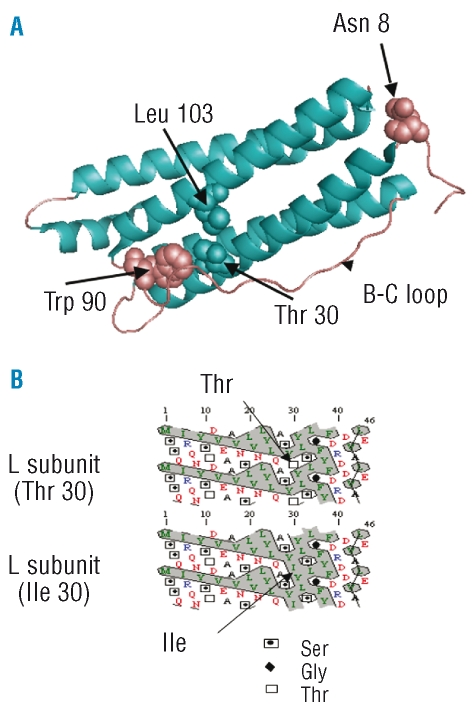
This p.Thr30Ile mutation has never been reported in the literature and was not found in 528 individuals of our control population. The threonine at position 30 of the L ferritin subunit is situated in the A α helix, nearest the N terminus of the molecule (Figure 2A). It is highly conserved throughout evolution in L subunits while the homologous amino acid residue in H subunits of most mammalian species is a valine.
Figure 2.
Position of the Thr30 in the L ferritin subunit crystal model and consequences of the mutation on the hydrophobicity of the N terminus. (A) Position of the Thr30 in the L ferritin crystal and details of its environment. Aminoacid residues Thr 30 is on the A helix, Trp90 is on the loop between the B and the C helices (B-C loop) and Leu 103 is on helix C. The glycosylated Asn is also indicated. The crystallographic data of L ferritin subunit from reference 18 were analyzed using the Pymol software. (B) Hydrophobicity cluster analysis of the A helix in the wild type L and the p.Thre30Ile mutant L ferritin subunits was carried out using the HCA software.23 (http://bioserv.rpbs.jussieu.fr/RPBS/cgi-bin/Ressource.cgi?chzn_lg=fr&chzn_rsrc=HCA). The replacement of the threonine residue by isoleucine at position 30 extends the hydrophobic cluster (hydrophobic residues are indicated in green and the hydrophobic cluster is encircled).
The serum ferritin values for the 37 subjects carrying the FTL mutation ranged from 400 to 6000 μg/L (see the values for the family cases on Figure 1). There were important fluctuations of the serum ferritin levels, either with time for the same individual (Figure 1, Family 1, patient II8), or between different individuals within the same family (Figure 1). One individual (Family 1, II8) has undergone six phlebotomies of 400 mL each over a nine month period and serum ferritin dropped from 3900 μg/L to 676 μg/L. No characteristic clinical symptoms could be underlined in the 37 individuals carrying the mutation, although 4 complained of joint pains, 3 of asthenia, one had bilateral cataract. The proband’s brother in Family 3 happened to be compound heterozygote for two HFE mutations (C282Y/H63D) and to have true liver iron overload, with liver iron concentration of 277 μmol/g dry weight liver (N<36).
Interestingly, serum ferritin glycosylation was evaluated in proband II2, in Family 1 and found to be close to 100%. Hyperglycosylation ranging from 90–99% was subsequently found in 8 individuals carrying the p.Thr30Ile mutations for whom serum was available for testing. The usual range of ferritin glycosylation is between 50% and 80%11 except for patients with Still’s disease who display much lower percentages of glycosylated ferritin (<20%).12 In patients with HHCS, ferritin glycosylation has not been extensively measured but in one family, 3 individuals with the same IRE mutation were found to have 10–30% glycosylated ferritin (Beaumont C. et al., unpublished data, 2008).
Discussion
Serum ferritin is a commonly measured parameter that is part of biological investigations in many circumstances. Several causes provoke elevated serum ferritin levels including not only primary and secondary iron overload, but also many pathological situations where hyperferritinemia has no direct relationship with body iron stores such as inflammatory syndromes, cytolysis, hemophagocytosis, Still’s disease, and some other etiologies.3 Genetically determined hyperferritinemia without iron overload is found in HHCS, an autosomal dominant inherited disease, associated with early onset cataract.13 Among 91 unrelated individuals referred to us with unexplained hyperferritinemia, we discovered a c.89C>T base change leading to a Thr30Ile amino acid change present at the heterozygous state in 17 of them, thereby identifying a new cause of isolated hyperferritinemia. However, this mutation accounts for about 20% of these hyperferritinemia cases since 74 subjects had no mutation and the cause of their hyperferritinemia remains to be determined. In these subjects with the p.Thr30Ile mutation, iron overload had been ruled out on the basis of transferrin saturation below 45%, or by MRI or liver biopsy in about half of the cases. Only one of these probands had cataract. Actually, no reproducible symptoms but hyperferritinemia could be identified in these patients with the FTL mutation. In the 10 families studied for cosegregation, relatives with normal ferritinemia had no sequence variant and a perfect cosegregation was observed between the mutated allele and the hyperferritinemia. Formally, the finding of the same p.Thr30Ile mutation in all family members with hyperferritinemia and neither in non-affected relatives nor in controls does not prove that this mutation is responsible for the observed hyperferritinemia. Indeed, it is conceivable that the p.Thr30Ile variant is in linkage disequilibrium with the causal mutation. However, we have verified that the Thr30Ile is the only significant base change in 2 unrelated patients for whom the L ferritin gene and the promoter region have been entirely sequenced.
In HHCS, the high serum ferritin levels reflect an increased synthesis of the L ferritin subunit in tissues, as shown by increased ferritin contents found in lymphoblastoid cell lines14 and in the lens15 of HHCS patients. On the contrary, EBV transformed cells from patients with the Thr30Ile variant had normal ferritin content (data not shown). Only a few mutations have been described in the FTL coding sequence. Dominantly inherited late-onset basal ganglia disease has been described resulting from frameshift mutations. These result in modifications of the C-terminal part of the molecules and are thought to affect protein folding and stability.16 Rare variants have been found, one in a patient with Parkinson disease (His133Pro) and one in 2 normal controls (Lys 54 Arg) whose possible functional consequences have not been explored.17 From three dimensional models of the assembled 24-mer ferritin molecule, it is likely that the FTL mutation that we describe here has little impact on the structure of the polymer. Indeed, the side chain of the mutated threonine is located on the external surface of the A alpha helix18 facing the rather hydrophobic residues Tryptophane 90 on the BC loop and Leucine 130 on helix C of the BC loop (Figure 2A). Furthermore, the homologous residue on the H subunit from many species is a valine. Accordingly, the mutated subunits probably do not alter the ferritin function in keeping with the observation that the carriers of the mutation do not present any specific symptoms.
An interesting feature is the very high percentage of glycosylated ferritin in subjects with hyperferritinemia and the Thr30Ile variant. Serum ferritin is thought to result from the secretion of a small fraction of cellular ferritin through the classical secretory pathway. Indeed, serum ferritin consists mostly in L ferritin subunits N-linked glycosylated in the Golgi apparatus (called G subunits)19,20 and the L ferritin subunit has a consensus glycosylation site (NYST) with the asparagine (N) at position 8 of the polypeptide chain. However, L ferritin lacks a typical amino-terminal, hydrophobic signal sequence and the mechanism by which it enters the secretory pathway is not known. It has been shown in rat hepatocytes that cytosolic L ferritin is targeted to the secretion pathway during translation despite the absence of conventional signal sequence.21 In this respect, a similar example is that of Plasminogen Activator Inhibitor type 2 (PAI-2) that also exists in two forms: a non-glycosylated cytoplasmic form and a glycosylated extracellular form. This dual localization was shown to result from a relatively inefficient routing into the secretory pathway. Like L ferritin, PAI-2 has no classical signal peptide and the secretion signal consists of 2 mildly hydrophobic sequences near the N terminal of the polypeptide.22 Interestingly, when this signal was made more hydrophobic by site directed mutagenesis, the secretion of PAI-2 became more efficient.22 The A helix of the L ferritin subunit may play a similar role in addressing a fraction of the molecules to the endoplasmic reticulum during translation of the mRNA. Interestingly, the A alpha helix of L ferritin has a higher hydrophobicity than the homologous helix of the H subunit that is not secreted and the substitution of the polar threonine at position 30 of L ferritin by a hydrophobic isoleucine increases the hydrophobicity of this helix (Figure 2B). Therefore, we suggest that the Thr30Ile amino acid change that we report here modifies a signal sequence resulting in an increase of ferritin secretion. The observed high level of glycosylation may simply reflect the enhanced proportion of mutated serum ferritin that is secreted as compared to the fraction that is released via cell lysis. From a practical point of view, identification of a novel genetic cause of high serum ferritin in the absence of iron overload may prevent unnecessary investigations and sometimes detrimental treatments in subjects with this form of unexplained hyperferritinemia.
Acknowledgments
the authors gratefully acknowledge the help of Michèle Vernet (Croix Rousse Hospital, Lyon) and Monique Dehoux (Bichat Hospital, Paris) for their help with the determination of ferritin glycosylation as well as Drs C. Fourcade, C. Kulekci, D. Vital-Durand, C. Buffet, S. Si Ahmed, V. Loustaud-Ratti, N. Ferry, F. Joram, P.R. Mannant, M. Kaassis and D. Devesa for referring their patients to them. We thank J.J. Lacapere for help with the 3D analysis of the mutation and hydrophobicity cluster analysis.
Footnotes
Authorship and Disclosures
CK and AMJ co-ordinated the study, analyzed data and wrote the paper. GH identified the first mutation, AM, DH and EBJ performed sequencing and analyzed data, FM performed cell culture experiments. MM, YD and PB diagnosed patients and analyzed data. BG and CB initiated the study, analyzed data and wrote the paper. All the authors approved the final version to be published.
The authors reported no potential conflicts of interest.
References
- 1.Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 2.Hentze MW, Kuhn LC. Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc Natl Acad Sci USA. 1996;93:8175–82. doi: 10.1073/pnas.93.16.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worwood M. Serum ferritin. CRC Crit Rev Clin Lab Sci. 1979;10:171–204. doi: 10.3109/10408367909147133. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar-Martinez P, Schved JF, Brissot P. The evaluation of hyperferritinemia: an updated strategy based on advances in detecting genetic abnormalities. Am J Gastroenterol. 2005;100:1185–94. doi: 10.1111/j.1572-0241.2005.40998.x. [DOI] [PubMed] [Google Scholar]
- 5.Bonneau D, Winter-Fuseau I, Loiseau MN, Amati P, Berthier M, Oriot D, Beaumont C. Bilateral cataract and high serum ferritin: a new dominant genetic disorder? J Med Genet. 1995;32:778–9. doi: 10.1136/jmg.32.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaumont C, Leneuve P, Devaux I, Scoazec JY, Berthier M, Loiseau MN, et al. Mutation in the iron responsive element of the L ferritin mRNA in a family with dominant hyperferritinaemia and cataract. Nat Genet. 1995;11:444–6. doi: 10.1038/ng1295-444. [DOI] [PubMed] [Google Scholar]
- 7.Girelli D, Corrocher R, Bisceglia L, Olivieri O, De Franceschi L, Zelante L, Gasparini P. Molecular basis for the recently described hereditary hyperferritinemia-cataract syndrome: a mutation in the iron-responsive element of ferritin L-subunit gene (the "Verona mutation") Blood. 1995;86:4050–3. [PubMed] [Google Scholar]
- 8.Cazzola M, Foglieni B, Bergamaschi G, Levi S, Lazzarino M, Arosio P. A novel deletion of the L-ferritin iron-responsive element responsible for severe hereditary hyperferritinaemia-cataract syndrome. Br J Haematol. 2002;116:667–70. doi: 10.1046/j.0007-1048.2001.03310.x. [DOI] [PubMed] [Google Scholar]
- 9.Hetet G, Devaux I, Soufir N, Grandchamp B, Beaumont C. Molecular analyses of patients with hyperferritinemia and normal serum iron values reveal both L ferritin IRE and 3 new ferroportin (slc11A3) mutations. Blood. 2003;102:1904–10. doi: 10.1182/blood-2003-02-0439. [DOI] [PubMed] [Google Scholar]
- 10.Worwood M, Cragg SJ, Wagstaff M, Jacobs A. Binding of human serum ferritin to concanavalin A. Clin Sci (Lond) 1979;56:83–7. doi: 10.1042/cs0560083. [DOI] [PubMed] [Google Scholar]
- 11.Worwood M. Ferritin in human tissues and serum. Clin Haematol. 1982;11:275–307. [PubMed] [Google Scholar]
- 12.Vignes S, Le Moel G, Fautrel B, Wechsler B, Godeau P, Piette JC. Percentage of glycosylated serum ferritin remains low throughout the course of adult onset Still’s disease. Ann Rheum Dis. 2000;59:347–50. doi: 10.1136/ard.59.5.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cazzola M, Skoda RC. Translational pathophysiology: a novel molecular mechanism of human disease. Blood. 2000;95:3280–8. [PubMed] [Google Scholar]
- 14.Levi S, Girelli D, Perrone F, Pasti M, Beaumont C, Corrocher R, et al. Analysis of ferritins in lymphoblastoid cell lines and in the lens of subjects with hereditary hyperferritinemia-cataract syndrome. Blood. 1998;91:4180–7. [PubMed] [Google Scholar]
- 15.Brooks DG, Manova-Todorova K, Farmer J, Lobmayr L, Wilson RB, Eagle RC, Jr, et al. Ferritin crystal cataracts in hereditary hyperferritinemia cataract syndrome. Invest Ophthalmol Vis Sci. 2002;43:1121–6. [PubMed] [Google Scholar]
- 16.Levi S, Cozzi A, Arosio P. Neuroferritinopathy: a neurodegenerative disorder associated with L-ferritin mutation. Best Pract Res Clin Haematol. 2005;18:265–76. doi: 10.1016/j.beha.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Foglieni B, Ferrari F, Goldwurm S, Santambrogio P, Castiglioni E, Sessa M, et al. Analysis of ferritin genes in Parkinson disease. Clin Chem Lab Med. 2007;45:1450–6. doi: 10.1515/CCLM.2007.307. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Li C, Ellenburg M, Soistman E, Ruble J, Wright B, et al. Structure of human ferritin L chain. Acta Crystallogr D Biol Crystallogr. 2006;62:800–6. doi: 10.1107/S0907444906018294. [DOI] [PubMed] [Google Scholar]
- 19.Cragg SJ, Wagstaff M, Worwood M. Sialic acid and the microheterogeneity of human serum ferritin. Clin Sci (Lond) 1980;58:259–62. doi: 10.1042/cs0580259. [DOI] [PubMed] [Google Scholar]
- 20.Cragg SJ, Wagstaff M, Worwood M. Detection of a glycosylated subunit in human serum ferritin. Biochem J. 1981;199:565–71. doi: 10.1042/bj1990565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh S, Hevi S, Chuck SL. Regulated secretion of glycosylated human ferritin from hepatocytes. Blood. 2004;103:2369–76. doi: 10.1182/blood-2003-09-3050. [DOI] [PubMed] [Google Scholar]
- 22.von Heijne G, Liljestrom P, Mikus P, Andersson H, Ny T. The efficiency of the uncleaved secretion signal in the plasminogen activator inhibitor type 2 protein can be enhanced by point mutations that increase its hydrophobicity. J Biol Chem. 1991;266:15240–3. [PubMed] [Google Scholar]
- 23.Gaboriaud C, Bissery V, Benchetrit T, Mornon JP. Hydrophobic cluster analysis: an efficient new way to compare and analyse amino acid sequences. FEBS Lett. 1987;224:149–55. doi: 10.1016/0014-5793(87)80439-8. [DOI] [PubMed] [Google Scholar]