Abstract
Many plants translocate sugar alcohols in the phloem. However, the mechanism(s) of sugar alcohol loading in the minor veins of leaves are debated. We characterized the loading strategies of two species that transport sorbitol (Plantago major and apple [Malus domestica]), and one that transports mannitol (Asarina scandens). Plasmodesmata are abundant at all interfaces in the minor vein phloem of apple, and in one of two types of phloem in the minor veins of A. scandens. Few plasmodesmata are present in the minor veins of P. major. Apple differs from the other two species in that sugar alcohol and sucrose (Suc) are present in much higher concentrations in leaves. Apple leaf tissue exposed to exogenous [14C]sorbitol, [14C]Suc, or 14CO2 did not accumulate radiolabel in the minor veins, as determined by macroautoradiography. P. major minor veins accumulated radiolabel from [14C]Suc, [14C]sorbitol, and 14CO2. A. scandens minor veins accumulated 14C from [14C]Suc and 14CO2, but not from [14C]mannitol. We conclude that the movement of sugar alcohol from the mesophyll into the phloem in apple and A. scandens is symplastic and passive, but in P. major it involves an apoplastic step and is energized. We also suggest that apple leaves transport sorbitol in high concentrations to avoid the feedback limitation of photosynthesis that would result from driving passive movement of solute into the phloem with high levels of Suc alone. The loading pathways and the mechanisms by which hydrostatic pressure is maintained in the minor vein phloem of these species are discussed.
Many species transport sugar alcohols in the phloem (Ziegler, 1975; Zimmermann and Ziegler, 1975; Loescher and Everard, 2000; Noiraud et al., 2001b). Sorbitol (glucitol) is transported in the Plantaginaceae and Rosaceae, mannitol in the Apiaceae, Combretaceae, Oleaceae, and Plantaginaceae, and dulcitol (galactitiol) in the Celastraceae. Several roles have been suggested for sugar alcohols, including osmoprotection, quenching of reactive oxygen species, facilitation of boron transport, storage of reducing power, tolerance to salinity or drought, and involvement in plant pathogen interactions (Loescher and Everard, 2000; Williamson et al., 2002; Pommerrenig et al., 2007). In some plants, sugar alcohol concentrations in phloem sap may considerably exceed those of Suc.
As with Suc, sugar alcohols are synthesized in the mesophyll and subsequently loaded into the minor vein phloem for delivery to sink tissues. Although it is reasonable to assume that sugar alcohols are loaded by the same species-specific strategies as Suc, these strategies have not been well documented. In particular, there is a debate over the possibility that sugar alcohols load through the symplast (Moing et al., 1997; Nadwodnik and Lohaus, 2008).
In general, solutes can enter the phloem either from the apoplast or through the symplast. Apoplastic loading is driven by the proton motive force and is capable of creating a steep uphill concentration gradient (Lalonde et al., 2004). Symplastic loading is necessarily passive since the cytosol is continuous through plasmodesmata (Schulz, 2005; Turgeon and Ayre, 2005). In willow (Salix spp.) leaves, Suc enters the minor vein phloem passively, through the symplast, and flux is driven by high Suc levels in the mesophyll (Turgeon and Medville, 1998). In other plants, such as the cucurbits, Suc loading is also symplastic, but the Suc is converted to raffinose and stachyose in the minor vein phloem, and these larger sugar molecules accumulate to high levels by polymer trapping, an active process (Turgeon and Gowan, 1990).
In this regard it is important to note that the term loading is sometimes used to signify the use of energy to transfer solute into the phloem against a thermodynamic gradient, and at other times to describe any route or mechanism of entry into the phloem, including an entirely passive one by diffusion or bulk flow through plasmodesmata. In this article, we use the term loading to indicate both active and passive modes of entry, making a distinction between them when necessary.
Several methods have been used to distinguish between symplastic and apoplastic loading pathways for sugar alcohols. Sugar alcohol transporters, including a mannitol transporter from celery (Apium graveolens) leaves (Noiraud et al., 2001a), and sorbitol transporters from Plantago (Ramsperger-Gleixner et al., 2004) and apple (Malus domestica) leaves (Watari et al., 2004) have been cloned and functionally characterized as proton symporters. Both the Plantago (Ramsperger-Gleixner et al., 2004) and apple (Watari et al., 2004) transporters are localized in the minor vein phloem. Furthermore, active uptake of mannitol has been demonstrated in isolated phloem strands of celery (Daie, 1987) and plasma membrane vesicles prepared from such strands (Salmon et al., 1995). As the respective authors have pointed out, these data are consistent with an energized, apoplastic loading mechanism. However, it must be emphasized that the presence of an active uptake mechanism for a solute in the phloem does not, in itself, prove that the phloem-loading route is apoplastic. Transporters are involved in the recovery of leaked solute from many cell types, including the phloem. This means that transporters are required in the phloem, even if the loading route from the mesophyll is symplastic. Note that Suc transporters are present in phloem in the petioles and stem, and even in sink tissues (Sauer, 2007).
A functional strategy often used to link Suc transporter activity to loading is to test the effects of p-chloromercuribenzenesulfonic acid (PCMBS), a membrane-impermeant sulfhydryl-modifying compound, on phloem transport (Giaquinta, 1976). PCMBS severely inhibits the function of Suc transporters. Unfortunately, sugar alcohol transporters tested to date are not affected by PCMBS (Flora and Madore, 1993; Noiraud et al., 2001a; Gao et al., 2003; Ramsperger-Gleixner et al., 2004; Watari et al., 2004; Juchaux-Cachau et al., 2007), with the exception of two sorbitol transporters (Ramsperger-Gleixner et al., 2004; McQueen et al., 2005).
In another approach to establishing the loading route, Moing et al. (1997) measured the sorbitol concentration in mesophyll cells of peach (Prunus persica) leaves and compared it to the concentration in phloem sap, obtained from severed aphid stylets. They reasoned that the lack of an uphill gradient would indicate passive loading. They found no significant differences in solute levels, but without information on intracellular compartmentation, which could considerably alter the local concentration in the cytosol of mesophyll cells, they were unable to distinguish between active and passive loading mechanisms.
To overcome this difficulty, Nadwodnik and Lohaus (2008) used the nonaqueous fractionation technique to study solute levels in various cellular compartments in three species, and compared them to phloem sap concentrations. In Plantago spp. and celery they found that Suc and sugar alcohol concentrations were substantially higher in the phloem than in the cytosol of mesophyll cells, with ratios of 4.5 to 40, indicating the presence of an energized, concentrating mechanism and strongly suggesting an apoplastic loading process. This conclusion is supported by the qualitative assessment of plasmodesmatal frequencies provided by Gamalei (1989), indicating low symplastic connectivity between the mesophyll and phloem in Plantago spp. and in the Apiaceae (celery).
Nadwodnik and Lohaus (2008) also studied peach leaves. Again they found that Suc and sorbitol levels were higher in phloem sap than in the mesophyll cytosol, but only by a factor of two, leaving the loading mechanism(s) open to question. Adding to the ambiguity is the fact that Prunus spp., according to Gamalei (1989), have intermediate numbers of plasmodesmata in minor veins. Furthermore, electron micrographs of minor veins in these species have not been published, so it is not known if plasmodesmata are abundant at all interfaces along the loading route.
In this study we compared phloem-loading mechanisms in apple (like Prunus, a member of the Rosaceae), Plantago major, and Asarina scandens. Asarina spp. transport Suc, mannitol, raffinose, and stachyose (Turgeon et al., 1993; Voitsekhovskaja et al., 2006). We conducted a thorough anatomical analysis of the minor veins to determine if there is a structural basis for a symplastic pathway. We also analyzed the capacity of minor veins to accumulate radiolabel when leaf tissue is exposed to 14CO2, or to exogenous [14C]Suc, [14C]sorbitol, or [14C]mannitol. Our results are consistent with an energized, apoplastic loading mechanism for sugar alcohol in Plantago and a passive, symplastic mechanism in apple and Asarina.
RESULTS
Apple
The phloem in the minor veins of apple leaves consists of sieve elements (SEs), companion cells (CCs), and phloem parenchyma cells (PPs; Fig. 1A). PP cells form a ring around the more internal SEs and CCs. This ring may be irregular, allowing direct contact of some CCs with bundle sheath (BS) cells. SEs, which are often in clusters of two or more, occupy the most central position in the veins and only occasionally abut the BS. In the smallest veins there is only a single cluster of SEs, but in larger veins there may be several, separate from one another and evidently derived from the confluence of smaller veins.
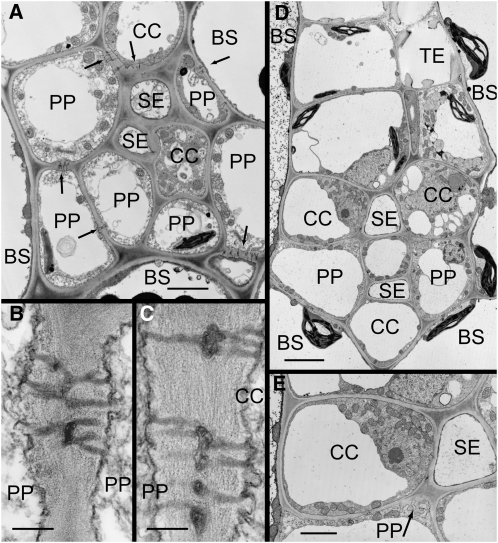
Figure 1.
Electron micrographs of apple (A and B) and P. major (C–E) minor veins in transverse sections. A, SEs and CCs are surrounded by PP cells. Numerous plasmodesmata are present at BS-PP and PP-CC interfaces (arrows), providing symplastic continuity into the phloem. B, Plasmodesmata between PP cells. C, Plasmodesmata between PP cells and CCs. D, SEs are surrounded by a ring of CCs and PP cells. Few plasmodesmata are visible at any interface. E, PP cells have transfer cell wall ingrowths (arrow) at the interfaces with SEs and CCs. Scale bars: A and E = 2 μm; B and C = 0.2 μm; D = 4 μm.
The ultrastructure of the SEs is as commonly described (Behnke and Sjolund, 1990), being almost devoid of internal contents and lined by parietal cytoplasm. Lateral sieve plates are commonly found between adjacent SEs. In some, but not all, SEs the walls are thick and convoluted internally (Schlag and Gal, 1996).
CCs and PP cells are much larger than SEs. CCs usually have denser cytoplasm, with numerous mitochondria and ribosomes. They have relatively small vacuoles, whereas PP cells are more highly vacuolated and have fewer mitochondria. The most consistent feature that distinguishes the two cell types is the nature of the plastids. PP cells have chloroplasts that are much smaller than those of mesophyll or BS cells, but have well-defined granal stacks. The plastids of CCs have few, if any, visible thylakoids, although plastoglobuli are common. Although these features often can be used to tell one cell type from another, in a given section the various distinguishing features may not be visible.
Plasmodesmata are a prominent feature of apple veins. They are found in electron micrographs with regularity (Fig. 1, B and C). The walls are generally thickened at the site of plasmodesmata. This thickening is less pronounced at BS:BS interfaces. The plasmodesmata at PP:PP and PP:CC interfaces are branched, usually bilaterally in a given section (Fig. 1, B and C), which means that they are more highly branched in three dimensions. The branches converge in a prominent central median cavity. The number of branches is approximately equal on either side of the median cavity (data not shown). Plasmodesmatal neck occlusions or constrictions were not observed. Where plasmodesmata-pore units link CCs and SEs, the walls are especially thick.
Plasmodesmata are most frequent at the BS:PP, PP:PP, and PP:CC interfaces (Table I). This is consistent with a role for PP cells in the distribution of photoassimilate within minor veins (Turgeon and Ayre, 2005) and a continuous symplastic pathway from the mesophyll to the minor vein SEs.
Table I.
Frequency of plasmodesmata in minor veins of apple and P. major
Data were acquired from images of transverse sections representing a total of seven veins in each species. When plasmodesmata were branched, each branch was counted as an individual channel, provided it extended at least halfway to the median cavity. The approximate section thickness for calculating plasmodesmatal frequencies was 70 nm. n/d, Interface not detected.
| Interface | Plasmodesmatal Channels
|
|
|---|---|---|
| Apple | P. major | |
| μm−2 | ||
| BS:PP | 11 | 2.0 |
| BS:CC | 0.6 | 2.4 |
| BS:BS | 7.5 | n/d |
| PP:PP | 8.5 | 1.8 |
| PP:CC | 7.0 | 1.6 |
| PP:SE | n/d | 0.8 |
| CC:CC | 3.6 | n/d |
| CC:SE | 6.0 | 2.7 |
| BS:SE | n/d | n/d |
Leaf discs of mature apple leaves were abraded with carborundum, exposed to solutions of either [14C]Suc, or [14C]sorbitol, washed, freeze dried, and autoradiographed (Fig. 2). Minor veins were not apparent in the autoradiographs, indicating that label did not accumulate in the phloem.
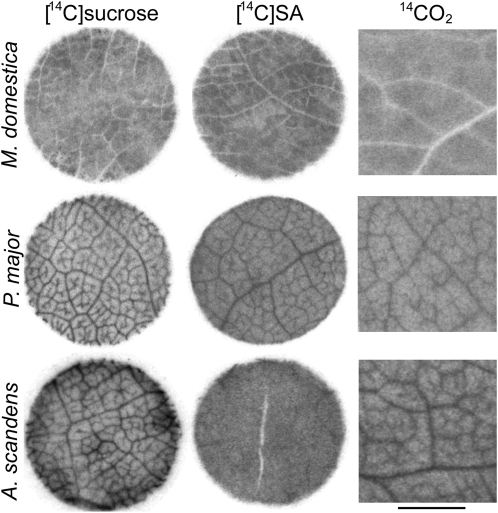
Figure 2.
Autoradiographs of leaf tissue from apple, P. major, and A. scandens. Abraded leaf discs were floated on either [14C]Suc or [14C]sugar alcohol for 1 h, then washed. Apple and P. major discs were floated on [14C]sorbitol, A. scandens discs on [14C]mannitol. Whole leaves were exposed to 14CO2 for 5 min, followed by a chase in room atmosphere for 55 min. Tissue was flash frozen, lyophilized, pressed thin, and autoradiographed. Leaf discs are 8 mm diameter. Scale bar for 14CO2 panels = 2 mm.
Since the absence of a vein image is a negative result, we considered the possibility that the radiolabeled solutions did not have adequate access to the interior of the leaf tissue. Therefore, a number of methods were used to facilitate uptake. Either the adaxial or abaxial surfaces were abraded with carborundum or sandpaper for different periods, resulting in a wide range of scouring levels from mild erosion of the cuticle to obvious disruption of leaf integrity. In other tests the adaxial epidermis, or the epidermis and one or more layers of mesophyll, was removed with a razor blade. Parallel experiments using the fluorescent tracer 6-carboxyfluorescein diacetate indicated that many of these treatments allowed access of solutions to the mesophyll and veins (data not shown). However, the results of all trials with radiolabeled solutions were uniformly negative: No dark vein images were obtained using either [14C]sorbitol or [14C]Suc. We also noted that no minor vein images were apparent at the cut edges of the discs although vein patterns are seen at the edges of leaf discs of apoplastically loading species, even if the tissue is unabraded, due to penetration of label into the cut surface (Eschrich and Fromm, 1994). Similarly negative results were obtained with abraded leaf tissue of several other members of the Rosaceae (data not shown).
A pattern of lesser radiolabel accumulation was observed in the autoradiographs that corresponded with larger veins (Fig. 2). In transverse sections of leaf tissue (Fig. 3) it can be seen that the larger veins have broad BS extensions, composed primarily of nonliving sclerenchyma cells that extend almost to the upper and lower epidermal layers (Nikolopoulos et al., 2002). Since the sclerenchyma cells do not accumulate radiolabel, the veins appear white in the autoradiographs. Detached apple leaves were exposed to 14CO2 in the light for 5 min and tissue was removed for autoradiography 1 h later. Again, no minor vein images were visible, only the negative images of large veins (Fig. 2).
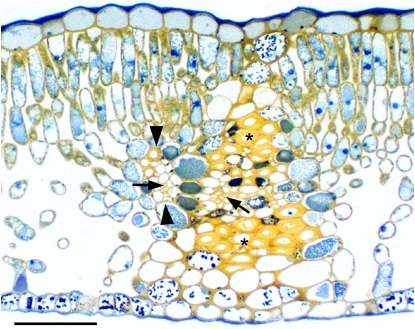
Figure 3.
Transverse section of an apple leaf. Note the minor vein (between arrowheads) converging with a large vein. The large vein has BS extensions above and below that reach almost to the epidermal layers and are composed primarily of sclerenchyma cells (asterisks). Arrows indicate the phloem of the minor and large veins. Scale bar = 100 μm. [See online article for color version of this figure.]
P. major
In P. major, the SEs are in the center of the vein and are surrounded by a single layer of alternating CC and PP cells that directly abut the BS (Fig. 1D). In larger minor veins, the SE-CC complexes are internal and are entirely surrounded by a layer of PP cells (data not shown). PP cells have relatively dense cytoplasm, numerous mitochondria, and small chloroplasts with starch grains. CCs have dense cytoplasm, numerous mitochondria, plastids with few internal membranes and no starch, and the vacuoles are usually smaller than those of PP cells. The abaxial PP cells, just below the CCs, are specialized as type B transfer cells (Gunning and Pate, 1969), i.e. wall ingrowths are present on the interior walls, adjacent to adjoining SEs and CCs (Fig. 1E). The ingrowths of type B transfer cells increase plasma membrane surface area and apparently aid in the efflux of solutes to the apoplast (Turgeon and Ayre, 2005). Between the cell types presumably involved in phloem loading (BS, PP, CC), the frequencies of plasmodesmata are approximately 20% of those in apple (Table I).
Autoradiographs of abraded leaf discs floated on solutions of [14C]Suc or [14C]sorbitol exhibited distinct vein-loading patterns (Fig. 2). Leaf tissue exposed to 14CO2 in the same manner as for apple also consistently produced minor vein images in autoradiographs (Fig. 2).
A. scandens
The concentrations of transport carbohydrates (Suc, mannitol, raffinose, and stachyose) and the minor vein anatomy of A. scandens (Turgeon et al., 1993) and Asarina barclaiana (Voitsekhovskaja et al., 2006) leaves have been described (see “Discussion”). To visualize accumulation of labeled compounds, tissue from mature leaves of A. scandens was abraded with carborundum and leaf discs were cut and randomized. Half the discs were floated on [14C]Suc and half on [14C]mannitol. Minor veins were readily apparent in all discs exposed to [14C]Suc but were not seen in any discs exposed to [14C]mannitol (Fig. 2). Minor veins were clearly apparent in autoradiographs of leaf tissue exposed to 14CO2 (Fig. 2).
DISCUSSION
Solute transport between plant cells occurs either through plasmodesmata or across the apoplast. Since Suc can enter the phloem in the minor veins of leaves by one or the other route, depending on species (Schulz, 2005; Turgeon and Ayre, 2005), it is reasonable to suspect that sugar alcohols have the same opportunities.
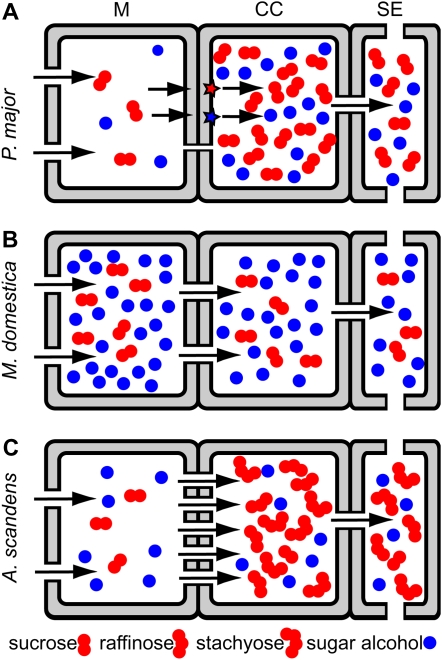
Of course, the issue of hydrostatic pressure must be addressed because long-distance transport requires the generation of sufficient pressure in the phloem to drive bulk flow of solution through sieve tubes. In the case of plants that transport sugar alcohols, this pressure can be produced in at least three ways (Fig. 4). The first is by a thermodynamically active mechanism that uses the proton motive force to load sugar alcohol into the phloem, from the apoplast, against a concentration gradient (Fig. 4A). Second, the combined concentrations of sugar alcohol and Suc, as well as other transported solutes, may be high enough in the cytosol of adjacent cells to generate the requisite osmotic potential in the phloem by passive transfer through plasmodesmata (Fig. 4B). Third, active loading of Suc by polymer trapping could provide the motivating force for long-distance transport, allowing sugar alcohol and other types of solutes to enter the phloem passively, through plasmodesmata, and to be carried along in the transport stream (Fig. 4C). The data presented here indicates that these three possibilities are realized in the three species under study.
Figure 4.
Schematic diagrams of hypothetical phloem-loading schemes. Relative concentrations of Suc and sugar alcohol are represented in mesophyll cells, CCs, and SEs. Solutes pass between cells through plasmodesmata (gaps in walls) or are transferred into the apoplast and are pumped into CCs by transporters (red and blue stars). A, Apoplastic loading of both sugar alcohol and Suc. B, Symplastic loading of both sugar alcohol and Suc, driven by their respective concentration gradients. C, Symplastic loading of sugar alcohol and Suc into intermediary cells, where Suc is converted into raffinose and stachyose. [See online article for color version of this figure.]
Several lines of evidence indicate that, in Plantago, loading is thermodynamically active and occurs from the apoplast, as in Figure 4A. Autoradiographs of leaf tissue exposed to [14C]Suc, [14C]sorbitol, or 14CO2 demonstrate radiolabel accumulation in minor veins. Compared to apple, the number of plasmodesmata between the cells of the minor veins is limited, consistent with the Gamalei (1989) designation of Plantago spp. as type 2, with relatively few plasmodesmata in the minor vein phloem. Although plasmodesmata are found at each interface along the loading route, this is true of all plant species, including those known to load Suc from the apoplast (Schulz, 2005; Turgeon and Ayre, 2005). It is reasonable to suspect that the plasmodesmata between the SE-CC complex and surrounding cells in apoplastic loading species are narrow enough to prevent Suc leakage back to the mesophyll. However, the size exclusion limit of plasmodesmata at this boundary in minor veins has not been measured in any species.
A thermodynamically active-loading mechanism in Plantago is also indicated by measurements of Suc and sorbitol concentrations. According to Nadwodnik and Lohaus (2008), the ratio of solute in the phloem sap (aphid stylet exudate) versus the cytosol of mesophyll cells in P. major is 3.2 for sorbitol and 40 for Suc, suggesting active accumulation, especially for Suc. In Plantago maritima the ratios are 24 for sorbitol and 18 for Suc (Nadwodnik and Lohaus, 2008).
In the case of apple, the anatomical evidence favors passive entry of Suc and sorbitol into the phloem, as in Figure 4B. Abundant plasmodesmata are present at every interface from mesophyll cells to the SEs, in numbers that greatly exceed those in the minor veins of P. major (Table I).
Apple leaf discs exposed to [14C]Suc or [14C]sorbitol do not accumulate radiolabel in minor veins. The appearance of white veins in the apple leaf autoradiographs (Fig. 2) corresponds to larger veins that have prominent BS extensions (Fig. 3). Since the sclerenchyma cells in the extensions are nonliving, they do not accumulate radiolabel. Radiolabel may be present in the phloem of these veins, but it constitutes such a small percentage of the vein volume (Fig. 3) that it does not produce an image. In any case, most loading occurs in minor veins and they have either small BS extensions or none at all. The minor veins occur in the areas of the autoradiographs between the white vein images. No dark vein images, which would indicate radiolabel accumulation, are visible in these areas even though all three sorbitol transporters cloned from apple leaves are localized to the phloem (Watari et al., 2004). It could be argued that the reason that exogenous [14C]Suc and [14C]sorbitol do not accumulate in the minor veins is that they do not have access to the minor vein phloem, even when several methods of abrasion are used and the epidermis of the leaf is removed. However, this argument does not apply when the radiolabel is introduced photosynthetically by exposing the leaves to 14CO2 since this is the physiological route of carbon entry. The most straightforward explanation is that plasmodesmata are sufficiently abundant, and sufficiently conductive, that radiolabel readily diffuses between cell types, precluding accumulation.
We suggest that the role of Suc and sorbitol transporters in apple leaves is to retrieve sorbitol that leaks from phloem cells into the apoplast. It is also possible that transporters transfer into the phloem a small amount of Suc and sorbitol that has leaked from the mesophyll into the apoplast. To a degree this could be considered phloem loading, but it is fundamentally different from a true apoplastic loading mechanism that relies primarily, if not entirely, on transporters and concentrates Suc in the phloem.
Solute concentrations in the mesophyll cells of apple leaves also indicate that passive loading is feasible. The concentration of sorbitol, based on leaf weight of 303 g m−2, and a water content of 62% fresh weight of leaf tissue (L. Cheng, unpublished data), is 269 mm in apple (Cheng et al., 2005), 28 times the concentration of sorbitol in P. major leaves (9.6 mm; Pommerrenig et al., 2007) and 112 times the concentration of mannitol in A. scandens leaves (2.4 mm; this study). Suc levels in apple leaves are also high (32 mm; Cheng et al., 2005) compared to P. major (2.1 mm; Pommerrenig et al., 2007) and A. scandens (6.3 mm; this study).
It is also important to note that flux through plasmodesmata is driven specifically by solute concentrations in the cytosolic compartment of cells. Studies of Suc and sugar alcohol compartmentation by nonaqueous fractionation indicate that disproportionately high concentrations are found in the cytosol compared to the vacuoles of mesophyll cells (Voitsekhovskaja et al., 2006; Nadwodnik and Lohaus, 2008, and refs. therein). This is the case in peach (Nadwodnik and Lohaus, 2008), which is in the same family as apple. Therefore, it seems likely that Suc and sorbitol levels in the cytosol of apple mesophyll cells are substantially higher than the concentrations measured by extraction of whole leaves.
Nonetheless, according to the data of Nadwodnik and Lohaus (2008) the concentrations of sorbitol and Suc, in peach, are twice as high in the phloem than in the mesophyll cytosol, which is not consistent with diffusion through plasmodesmata. As the authors pointed out, this concentration ratio (phloem/mesophyll cytosol) is lower than in other species studied. They concluded that, “the possibility of phloem loading of Suc by simple diffusion cannot be ruled out” (Nadwodnik and Lohaus, 2008, p. 1088). In this regard we note a striking coincidence in that the ratio was exactly the same for both sorbitol and Suc: 2.0 (Nadwodnik and Lohaus, 2008). If transporters load these two compounds independently, the ratios need not be the same. For example, in P. major, the phloem/mesophyll cytosol concentration ratio was 3.2 for sorbitol and 40 for Suc. On the other hand, if both compounds diffuse into the phloem, the phloem/mesophyll cytosol concentration ratios should be the same, as they are in peach. Perhaps the ratios are greater than 1.0 in peach due to some systematic problem(s) in the difficult and exacting procedures involved: nonaqueous fractionation and morphometry for cytosolic measurements, and the aphid stylet technique for analysis of phloem sap. As noted by the authors, measurements of Suc concentrations by the same methods in Alonsoa meridionalis, which has a symplastic mode of phloem loading, also suggest an apparent 2-fold concentration gradient from mesophyll cytosol to the phloem (Knop et al., 2001).
Considering the different types of data now available—autoradiography of 14C-labeled compounds, microscopic analysis of the symplastic pathway, and solute concentrations—we feel reasonably secure in proposing a symplastic and passive mode of phloem loading in apple and peach. We suggest that this offers an explanation for the high concentrations of sorbitol transported in these plants. Sugar alcohols serve several functions, including quenching of reactive oxygen species, improved boron mobility, storage of reducing power, and salt/drought tolerance (Loescher and Everard, 2000; Williamson et al., 2002; Pommerrenig et al., 2007). Indeed, apple leaves accumulate sorbitol under water stress conditions (Wang et al., 1995). However, in a species that loads passively, an additional solute in the mesophyll cytosol, such as sorbitol, will have another effect: it will increase the osmotic potential in the phloem, thereby increasing the efficiency of long-distance transport. There is a limit to the amount of Suc that can accumulate in the mesophyll, since high levels of Suc repress the expression of photosynthetic genes in source leaves (Rolland and Sheen, 2005). We suggest that this feedback limitation is avoided in the species of Rosaceae that transport sorbitol by diverting some of the carbon from the common Glc 6-P pool to sorbitol production, thus increasing the osmotic potential of the mesophyll cytosol, and the phloem, with an additional transport compound.
The evidence also strongly supports a passive mode of sugar alcohol entry into the minor vein phloem in A. scandens. Asarina spp. transport Suc, with smaller amounts of raffinose, stachyose, and mannitol. When abraded leaf discs are exposed to [14C]Suc, vein images are readily apparent in autoradiographs. However, when discs chosen randomly from the same abraded tissue samples are exposed to [14C]mannitol, the results are negative: no vein images are seen, even when autoradiograph exposure time is adjusted to account for differences in the amounts of radiolabel accumulated. The difference in autoradiographic results is not due to a trivial problem in the visualization of [14C]mannitol since leaf discs of celery floated on solutions of [14C]mannitol produce distinct vein images in autoradiographs (data not shown). The results on Asarina are consistent with those of Voitsekhovskaja et al. (2006) who found, on the basis of nonaqueous fractionation and analysis of aphid style exudate, that the concentration ratio between phloem and mesophyll cytosol is 22 for Suc, indicative of energized loading, but only 1.5 for mannitol. Again, the ratio is greater than 1.0, but the difference was considered insignificant by the authors, who suggested that mannitol is transferred passively from the mesophyll to the phloem.
It may seem unlikely that symplastic and apoplastic modes of loading can coexist in a single species given that symplastic transport requires plasmodesmatal continuity while that same continuity would result in a futile transport cycle in the apoplastic loading mode. However, Asarina spp. have two types of phloem in the minor veins (Turgeon et al., 1993; Voitsekhovskaja et al., 2006). The abaxial CCs are specialized as type A transfer cells, with extensive wall ingrowths and relatively few plasmodesmata (Gunning and Pate, 1969). Type A transfer cells in minor vein phloem increase the capacity for uptake of solute from the apoplast (Wimmers and Turgeon, 1991; Amiard et al., 2005). Thus, there is a strong structural correlate with apoplastic loading that suggests that Suc enters the minor vein phloem of Asarina spp. in an energized, transporter-mediated manner by cotransport with protons. Acanthus mollis also has transfer cells and intermediary cells in the same minor veins (van Bel et al., 1992).
Although sorbitol is loaded energetically into CCs in Plantago, mannitol is apparently not loaded the same way into the transfer cells of Asarina, otherwise a vein image would have been visible in autoradiographs when leaf tissue was floated on the radiolabeled compound. It is more likely that mannitol enters the adaxial CCs of Asarina. These CCs are not transfer cells. Rather, they resemble intermediary cells, with the asymmetrically branched plasmodesmata typical of this cell type. However, the plasmodesmata are not as numerous as in plants that transport large quantities of raffinose and stachyose, such as Alonsoa (Turgeon et al., 1993; Voitsekhovskaja et al., 2006). It seems likely that mannitol enters these modified intermediary cells symplastically, and passively, from the mesophyll. Indeed, if mannitol is present in the cytosol of mesophyll cells, it will necessarily diffuse into the modified intermediary cells through connecting plasmodesmata.
Symplastic continuity between the mesophyll and phloem would explain the failure of leaf discs of A. scandens to accumulate [14C]mannitol in the veins. Since mannitol has only half the mass of Suc, it diffuses through narrow pores more readily than Suc, perhaps explaining why the number of plasmodesmata is reduced. Since Asarina spp. also transport small amounts of raffinose and stachyose (Turgeon et al., 1993; Voitsekhovskaja et al., 2006), it is also reasonable to conclude that some Suc enters the modified intermediary cells and is converted there to these larger compounds, raising the overall solute level by polymer trapping. Thus, sugar alcohol loading into the modified intermediary cells appears to correspond to Figure 4C. The existence of multiple loading routes and mechanisms in the same veins raises interesting questions concerning the coordination of transport rates of the different compounds and the regulation of hydrostatic pressure in the phloem.
In conclusion, evidence is presented that sugar alcohols are loaded into the minor vein phloem of different species in different ways, taking advantage of the various pathways and mechanisms that are available for the entry of small molecules into SEs. Macroautoradiography of leaf tissue following abrasion and exposure to 14C-labeled exogenous compounds, measurement of solute levels, and analysis of plasmodesmatal frequencies are powerful tools in dissecting the pathways and mechanisms involved.
MATERIALS AND METHODS
Plant Material
Actively growing branches were collected from field-grown, fruiting cv Gala apple (Malus domestica Borkh.) trees at Cornell Orchards in Ithaca, New York. Branches were recut under water and transported to the lab. Leaves for two of the three leaf disc experiments were collected from potted Gala trees. Asarina scandens (Cav.) Penn. leaves were collected from plants grown in a greenhouse in Metromix 360 (E.C. Geiger). Plantago major leaves were collected in the field, except in the case of 14C-transport and leaf disc uptake assays, where the leaves were from plants cultivated in a growth chamber.
Microscopy
Leaf tissue was fixed in 2% (v/v) glutaraldehyde, 2% (v/v) paraformaldehyde, in 70 mm sodium cacodylate buffer, pH 7.0, for 1 h at room temperature, washed in the same buffer, and postfixed in 1% (v/v) osmium tetroxide. The tissue was dehydrated in an acetone series and embedded in Spurr's epoxy resin (Electron Microscopy Sciences). Thin sections were stained with uranyl acetate and lead citrate, and observed under a CM10 transmission electron microscope at 80 KV (Philips Electronic Instruments). For analysis of plasmodesmatal frequencies, individual plasmodesmatal channels were counted only if they extended one-half the distance from the plasma membrane to the median cavity. Plasmodesmatal channel frequencies were calculated by the formula of Gunning (1978), assuming a section thickness of 70 nm and a channel radius of 22.5 nm.
Radiolabeling
For studies of exogenous uptake of Suc or sugar alcohol, the adaxial surface of leaf tissue was abraded with carborundum powder (320 grit) or the adaxial epidermis was removed with a razor blade, as described in “Results.” Discs were cut with an 8-mm diameter cork borer under water and washed in MES buffer (20 mm MES plus 2 mm CaCl2, pH 5.5, with NaOH). Randomized discs were transferred, abraded side down, to the surface of fresh MES buffer in plastic dishes and the buffer was then removed and replaced with a solution containing MES buffer and either [14C]Suc, [14C]sorbitol, or [14C]mannitol (each at 1 mm; 40 kBq mL−1). After 1 h of radiolabel uptake at room temperature, with continuous, gentle agitation, the discs were washed for 1 h in ice-cold MES buffer, with several changes, and flash frozen in liquid N2 or powdered dry ice. The frozen leaf discs were lyophilized in a Virtis freeze dryer on a cold stage at −30°C with the condenser at −60°C. Dried discs were pressed thin between stainless steel plates in a bench vise, affixed to card stock with double-sided tape, and autoradiographed with Kodak BioMax MR film.
For studies of radiolabel distribution following photosynthetic incorporation of 14CO2, leaves were exposed to the radiolabeled gas as described (Turgeon and Medville, 1998) for 5 min. Following a 55-min chase period in room atmosphere, the leaves were flash frozen in dry ice, lyophilized, pressed flat, and autoradiographed as described above.
This work was supported by the U.S. Department of Agriculture (grant no. CSREES 2005–02485).
The author responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Robert Turgeon (ert2@cornell.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
Open access articles can be viewed online without a subscription.
References
- Amiard V, Mueh KE, Demmig-Adams B, Ebbert V, Turgeon R, Adams WW III (2005) Anatomical and photosynthetic acclimation to the light environment in species with differing mechanisms of phloem loading. Proc Natl Acad Sci USA 102 12968–12973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke HD, Sjolund RD (1990) Sieve Elements: Comparative Structure, Induction and Development. Springer-Verlag, New York
- Cheng L, Zhou R, Reidel EJ, Sharkey TD, Dandekar AM (2005) Antisense inhibition of sorbitol synthesis leads to up-regulation of starch synthesis without altering CO2 assimilation in apple leaves. Planta 220 767–776 [DOI] [PubMed] [Google Scholar]
- Daie J (1987) Sucrose uptake in isolated phloem of celery is a single saturable transport system. Planta 171 472–482 [DOI] [PubMed] [Google Scholar]
- Eschrich W, Fromm J (1994) Evidence for two pathways of phloem loading. Physiol Plant 90 699–707 [Google Scholar]
- Flora LL, Madore MA (1993) Stachyose and mannitol transport in olive (Olea europaea L.). Planta 189 484–490 [Google Scholar]
- Gamalei Y (1989) Structure and function of leaf minor veins in trees and herbs. Trees 3 96–110 [Google Scholar]
- Gao Z, Maurousset L, Lemoine R, Yoo SD, van Nocker S, Loescher W (2003) Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol 131 1566–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R (1976) Evidence for phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol 57 872–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning BES (1978) Age-related and origin-related control of the numbers of plasmodesmata in cell walls of developing Azolla roots. Planta 143 181–190 [DOI] [PubMed] [Google Scholar]
- Gunning BES, Pate JS (1969) “Transfer cells” plant cells with wall ingrowths specialized in relation to short distance transport of solutes—their occurrence structure and development. Protoplasma 68 107–133 [Google Scholar]
- Juchaux-Cachau M, Landouar-Arsivaud L, Pichaut JP, Campion C, Porcheron B, Jeauffre J, Noiraud-Romy N, Simoneau P, Maurousset L, Lemoine R (2007) Characterization of AgMaT2, a plasma membrane mannitol transporter from celery, expressed in phloem cells, including phloem parenchyma cells. Plant Physiol 145 62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop C, Voitsekhovskaja O, Lohaus G (2001) Sucrose transporters in two members of the Scrophulariaceae with different types of transport sugar. Planta 213 80–91 [DOI] [PubMed] [Google Scholar]
- Lalonde S, Wifp D, Frommer WB (2004) Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu Rev Plant Biol 55 341–372 [DOI] [PubMed] [Google Scholar]
- Loescher WH, Everard JD (2000) Regulation of sugar alcohol biosynthesis. In RC Leegood, TD Sharkey, S von Caemmerer, eds, Photosynthesis: Physiology and Metabolism. Kluwer Academic, Dordrecht, The Netherlands, pp 275–299
- McQueen JC, Minchin PEH, Thorpe MR, Silvester WB (2005) Short-term storage of carbohydrate in stem tissue of apple (Malus domestica), a woody perennial: evidence for involvement of the apoplast. Funct Plant Biol 32 1027–1031 [DOI] [PubMed] [Google Scholar]
- Moing A, Carbonne F, Zipperlin B, Svanella L, Gaudillere JP (1997) Phloem loading in peach: symplastic or apoplastic? Physiol Plant 101 489–496 [Google Scholar]
- Nadwodnik J, Lohaus G (2008) Subcellular concentrations of sugar alcohols and sugars in relation to phloem translocation in Plantago major, Plantago maritima, Prunus persica, and Apium graveolens. Planta 227 1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos D, Liakopoulos G, Drossopoulos I, Karabourniotis G (2002) The relationship between anatomy and photosynthetic performance of heterobaric leaves. Plant Physiol 129 235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiraud N, Maurousset L, Lemoine R (2001. a) Identification of a mannitol transporter, AgMaT1, in celery phloem. Plant Cell 13 695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiraud N, Maurousset L, Lemoine R (2001. b) Transport of polyols in higher plants. Plant Physiol Biochem 39 717–728 [Google Scholar]
- Pommerrenig B, Papini-Terzi FS, Sauer N (2007) Differential regulation of sorbitol and sucrose loading into the phloem of Plantago major in response to salt stress. Plant Physiol 144 1029–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsperger-Gleixner M, Geiger D, Hedrich R, Sauer N (2004) Differential expression of sucrose transporter and polyol transporter genes during maturation of common plantain companion cells. Plant Physiol 134 147–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Sheen J (2005) Sugar sensing and signalling networks in plants. Biochem Soc Trans 33 269–271 [DOI] [PubMed] [Google Scholar]
- Salmon S, Lemoine R, Jamai A, Bouché-Pilon S, Fromont JC (1995) Study of sucrose and mannitol transport in plasma-membrane vesicles from phloem and non-phloem tissues of celery (Apium graveolens L.) petioles. Planta 197 76–83 [Google Scholar]
- Sauer N (2007) Molecular physiology of higher plant sucrose transporters. FEBS Lett 581 2309–2317 [DOI] [PubMed] [Google Scholar]
- Schlag MG, Gal M (1996) The nacreous sieve-element wall in healthy and in MLO-infected apple trees. Int J Plant Sci 157 80–91 [Google Scholar]
- Schulz A (2005) Role of plasmodesmata in solute loading and unloading. In KJ Oparka, ed, Plasmodesmata, Vol Annual Plant Reviews, Vol 18. Blackwell, Oxford, pp 135–161
- Turgeon R, Ayre BG (2005) Pathways and mechanisms of phloem loading. In NM Holbrook, MA Zwieniecki, eds, Vascular Transport in Plants. Elsevier/Academic Press, Oxford, pp 45–67
- Turgeon R, Beebe DU, Gowan E (1993) The intermediary cell: minor-vein anatomy and raffinose oligosaccharide synthesis in the Scrophulariaceae. Planta 191 446–456 [Google Scholar]
- Turgeon R, Gowan E (1990) Phloem loading in Coleus blumei in the absence of carrier-mediated uptake of export sugar from the apoplast. Plant Physiol 94 1244–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R, Medville R (1998) The absence of phloem loading in willow leaves. Proc Natl Acad Sci USA 95 12055–12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bel AJE, Gamalei YV, Ammerlaan A, Bik LPM (1992) Dissimilar phloem loading in leaves with symplasmic or apoplasmic minor-vein configurations. Planta 186 518–525 [DOI] [PubMed] [Google Scholar]
- Voitsekhovskaja OV, Koroleva OA, Batashev DR, Knop C, Tomos AD, Gamalei YV, Heldt HW, Lohaus G (2006) Phloem loading in two Scrophulariaceae species: What can drive symplastic flow via plasmodesmata? Plant Physiol 140 383–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Quebedeaux B, Stutte GW (1995) Osmotic adjustment: effect of water stress on carbohydrates in leaves, stems and roots of apple. Aust J Plant Physiol 22 747–754 [Google Scholar]
- Watari J, Kobae Y, Yamaki S, Yamada K, Toyofuku K, Tabuchi T, Shiratake K (2004) Identification of sorbitol transporters expressed in the phloem of apple source leaves. Plant Cell Physiol 45 1032–1041 [DOI] [PubMed] [Google Scholar]
- Williamson JD, Jennings DB, Guo WW, Pharr DM, Ehrenshaft M (2002) Sugar alcohols, salt stress, and fungal resistance: polyols-multifunctional plant protection? J Am Soc Hortic Sci 127 467–473 [Google Scholar]
- Wimmers LE, Turgeon R (1991) Transfer cells and solute uptake in minor veins of Pisum sativum leaves. Planta 186 2–12 [DOI] [PubMed] [Google Scholar]
- Ziegler H (1975) Nature of transported substances in the phloem. In MH Zimmermann, JA Milburn, eds, Encyclopedia of Plant Physiology, NS Vol 1. Transport in Plants 1: Phloem Transport. Springer-Verlag, Berlin, pp 59–100
- Zimmermann MH, Ziegler H (1975) List of sugars and sugar alcohols in sieve-tube exudates. In MH Zimmermann, JA Milburn, eds, Encyclopedia of Plant Physiology, NS Vol 1. Transport in Plants 1: Phloem Transport. Springer, New York, pp 480–503