Abstract
The phytopathogenic actinomycete Rhodococcus fascians induces neoplastic shooty outgrowths on infected hosts. Upon R. fascians infection of Arabidopsis (Arabidopsis thaliana), leaves are formed with small narrow lamina and serrated margins. These symptomatic leaves exhibit reduced tissue differentiation, display more but smaller cells that do not endoreduplicate, and accumulate in the G1 phase of the cell cycle. Together, these features imply that leaf growth occurs primarily through mitotic cell division and not via cell expansion. Molecular analysis revealed that cell cycle gene expression is activated continuously throughout symptomatic leaf development, ensuring persistent mitotic cycling and inhibition of cell cycle exit. The transition at the two major cell cycle checkpoints is stimulated as a direct consequence of the R. fascians signals. The extremely reduced phenotypical response of a cyclind3;1-3 triple knockout mutant indicates that the D-type cyclin/retinoblastoma/E2F transcription factor pathway, as a major mediator of cell growth and cell cycle progression, plays a key role in symptom development and is instrumental for the sustained G1-to-S and G2-to-M transitions during symptomatic leaf growth.
The Gram-positive phytopathogenic bacterium Rhodococcus fascians secretes growth-modulating hormones, such as cytokinins and auxins, and typically induces neoplastic, shooty outgrowths on a wide range of plant hosts (Goethals et al., 2001; Putnam and Miller, 2007). Whereas the role of auxin production in the symptomatology remains to be determined (Vandeputte et al., 2005), the central position of cytokinin secretion has been clearly established (Crespi et al., 1992, 1994; Temmerman et al., 2000; Pertry et al., 2009). The proliferation of shooty tissue in tobacco (Nicotiana tabacum) not only relies on activation of existing (dormant) meristems but also on de novo meristem formation in cortical tissues by reactivation of the cell cycle, a process that is accompanied by expression of the cyclins CYCB1;1 and CYCD3;2 (de O Manes et al., 2001). In Arabidopsis (Arabidopsis thaliana), only newly formed organs become symptomatic upon R. fascians infection. A strong induction of CYCA2;1 expression, followed by an enhanced transcription of CYCB1;1 and the cyclin-dependent kinase CDKA;1 upon infection, illustrates activation of the cell cycle in this host (Vereecke et al., 2000).
Plants can rapidly alter their growth rate and pattern in response to developmental and external conditions (Traas et al., 1998; Gendreau et al., 1999). During organ formation, primordium growth is initially driven by cell proliferation, whereas the second growth phase is primarily attributed to postmitotic cell expansion of the differentiating cells through endoreduplication and vacuolization (Donnelly et al., 1999; den Boer and Murray, 2000; Nath et al., 2003). Usually, ploidy levels and cell size are positively correlated, indicating that cell expansion and differentiation depend on the endocycle (Cionini et al., 1983; Melaragno et al., 1993; Gendreau et al., 1998; Joubès et al., 1999; Beemster et al., 2003; Tsukaya, 2006), but ploidy-independent growth mechanisms also exist (De Veylder et al., 2001; Beemster et al., 2002; Jasinski et al., 2002; Schnittger et al., 2003; Ferjani et al., 2007).
Cell cycle control and plant development are mainly integrated at the cell cycle checkpoints (G1/S and G2/M) and the molecular machineries involved (De Veylder et al., 2003; Inzé, 2005; Inzé and De Veylder, 2006). G1-to-S transition and S phase progression are controlled by a D-type cyclin/retinoblastoma-related (RBR)/E2F-DP transcription factor-mediated pathway (De Veylder et al., 2002; Kosugi and Ohashi, 2003; Boudolf et al., 2004b; Magyar et al., 2005; del Pozo et al., 2006). D-type cyclins have been proposed to be the primary sensors for external conditions (Dewitte and Murray, 2003), determining the cellular commitment to the mitotic cell cycle in response to several plant growth factors, such as cytokinin, auxin, gibberellin, brassinosteroid, and Suc (Soni et al., 1995; Riou-Khamlichi et al., 1999, 2000; Hu et al., 2000; Stals and Inzé, 2001; Oakenfull et al., 2002). Progression at the G2-to-M transition is mediated by the M phase-specific CDKs. CDKB1 is a plant-specific CDK dedicated to the G2-to-M transition (Boudolf et al., 2004b), whereas CDKA functions during both mitosis and the endocycle (Hemerly et al., 1995; Leiva-Neto et al., 2004; Verkest et al., 2005). Cell cycle exit and endoreduplication onset are accomplished through down-regulation of the mitotic CDK activity by inhibitors, such as KIP-related proteins (Wang et al., 1998; De Veylder et al., 2001; Verkest et al., 2005; Weinl et al., 2005; Nakai et al., 2006; Pettkó-Szandtner et al., 2006) and SIAMESE (SIM; Churchman et al., 2006). Other important control mechanisms for CDK activity are regulation of cyclin transcription and stability, and proteolysis by the anaphase-promoting complex-activating protein CELL CYCLE SWITCH52A (CCS52A; den Boer and Murray, 2000; Dewitte et al., 2003; Kondorosi and Kondorosi, 2004; Imai et al., 2006). Recently, in Arabidopsis, E2Fe/DEL1, a member of the E2F transcription factors present exclusively in mitotic cells, has been shown to repress the CCS52A2 activity, preventing cells from engaging in the endoreduplication program (Vlieghe et al., 2005; Lammens et al., 2008).
Here, we analyzed the development of newly formed symptomatic leaves in Arabidopsis upon infection with R. fascians and asked how modulation of the plant cell cycle is involved in symptom establishment. Our data show that the bacterial signals directly stimulate progression at the G1/S and G2/M checkpoints, which commits the leaf cells to mitotic cycling. Consequently, symptomatic leaves never reach maturity and are blocked in a physiological state that is relevant for niche establishment.
RESULTS
R. fascians Infection Inhibits Leaf Differentiation
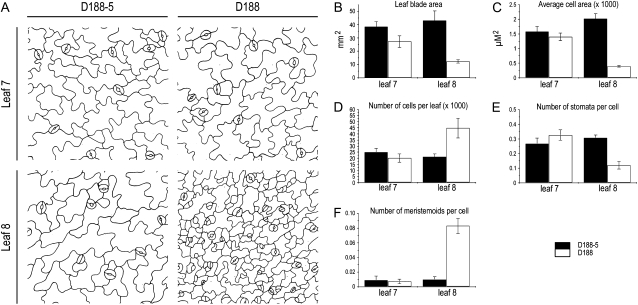
Typically, infection of Arabidopsis plants with the wild-type R. fascians strain D188 generates symptomatic leaves with small and narrow leaf blades and strongly serrated margins (Depuydt et al., 2008). To evaluate the leaf phenotype in greater detail, the youngest nonsymptomatic leaf (leaf 7) was compared with the first symptomatic leaf (leaf 8) of plants that were infected at developmental stage 1.05, when five leaves are visible (Boyes et al., 2001). Throughout this study, infections with the virulent strain D188 were compared with control infections with its nonvirulent linear plasmid-free derivative D188-5. At 28 d postinfection (dpi), the leaf surface area, the total cell number, the cell size of the pavement cells, and the stomatal index of the abaxial epidermis were quantified. Although the leaf surface area, the total cell number, and the average cell size for leaf 7 were somewhat smaller for plants infected with the virulent strain D188, these differences were not statistically significant at the time point of analysis (Fig. 1). By contrast, leaf 8 was 4-fold smaller due to an almost 5-fold reduced cell size (Fig. 1, A–C), but there were 2- to 3-fold more cells compared with the controls (Fig. 1, A and D). Moreover, the epidermal pavement cells of the responsive leaves did not have the typical sinusoid outline but remained rather polygon shaped (Fig. 1A). The stomatal index was lower in symptomatic leaves (Fig. 1E), but more meristemoids, the stomatal precursor cells, occurred per cell than in control infections (Fig. 1F).
Figure 1.
Epidermal pavement cell analysis of leaves 7 and 8 upon infection with R. fascians strain D188-5 (control) and D188. A, Drawing tube images. B, Leaf blade area. C, Average cell size. D, Total number of cells per leaf. E, Number of stomata per cell. F, Number of meristemoids per cell. All analyses were done at 28 dpi (n = 5). Error bars represent se.
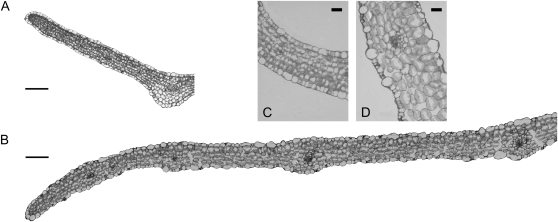
Altogether, these observations suggest that cell proliferation is the dominant mechanism for growth in symptomatic leaves and that differentiation is impaired. The latter was confirmed by microscopic analysis of cross-sections through the lamina (Fig. 2, A and B). Indeed, all of the parenchyma cells of D188-infected leaves were spherical with fewer intercellular air spaces than in control leaves, making it difficult to distinguish the palisade and spongy mesophyll layers (Fig. 2).
Figure 2.
Central cross-sections and close-ups through a R. fascians D188- (A and C) and a D188-5-infected leaf (B and D) at 28 dpi. Bars = 60 μm (A and B) and 20 μm (C and D).
R. fascians Prevents Endocycling during Leaf Development
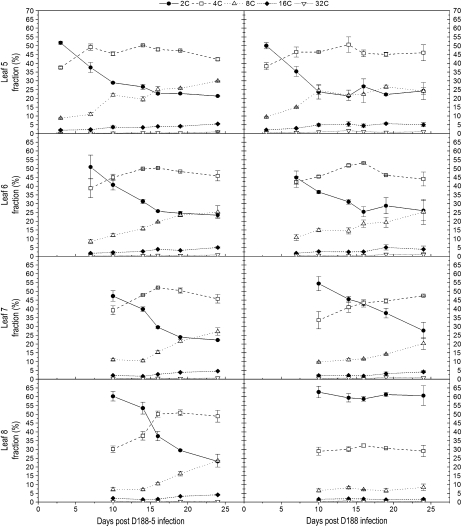
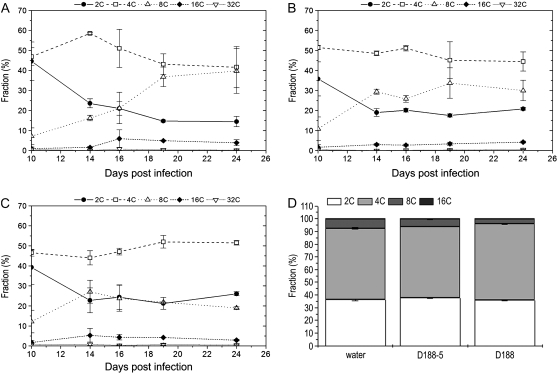
The data above indicate that the balance between cell proliferation and cell differentiation is disturbed during leaf maturation upon infection with R. fascians strain D188. In a time course experiment, we examined the endoreduplication kinetics as a marker for cell differentiation. Infections were carried out as described above, and together with leaf 5, the next three emerging leaves were analyzed at 3, 7, 10, 14, 16, 19, and 24 dpi (Fig. 3). Hence, four different stages in leaf development were considered: outgrowing primordia and young, expanding, and mature leaves. For leaves 5 and 6, no significant differences between the two conditions were measured, which was anticipated because of the lack of morphological alterations in these leaves (Depuydt et al., 2008). In leaf 7, the increase in cells with an 8C content started later upon D188 infection, suggesting a delay in the endoreduplication onset that possibly correlated with the moderate leaf phenotype (Fig. 1). Strikingly, throughout the experiment, the ploidy levels of leaf 8 did not change. Since the cell numbers strongly increased, this observation does not reflect a cell cycle stop but rather indicates that endoreduplication did not occur, confirming that growth of symptomatic leaves is driven by proliferative divisions and not by expansion via endoreduplication.
Figure 3.
DNA ploidy level distribution of leaves 5, 6, 7, and 8 upon infection with R. fascians D188-5 (left) and D188 (right). The data represent averages of two independent biological repeats with two technical replicates each. The error bars depict sd.
Maintenance of High Transcription Levels of Mitotic Cell Cycle Genes throughout Symptomatic Leaf Development Imposes a Commitment to Mitotic Cycling
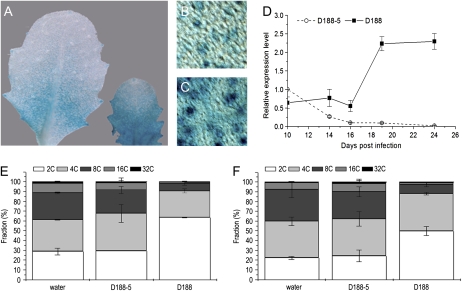
Most cells (60%–65%) in symptomatic leaves have a 2C content, implying that infection with R. fascians promotes the G2-to-M transition. Since CDKB1;1 expression typically correlates with proliferating cells and plays a key role at the G2/M checkpoint, the expression pattern of CDKB1;1 was analyzed upon infection with R. fascians. Histochemical analyses of CDKB1;1:GUS marker lines revealed promoter activity throughout the lamina of symptomatic leaves. The reported typical stomatal expression (Boudolf et al., 2004a) was higher than that in control leaves (Fig. 4, A–C). Quantitative reverse transcription (qRT)-PCR of the CDKB1;1 transcript level over 2 weeks showed a progressive decrease as the control leaf 8 matured and started to endoreduplicate (Fig. 4D). In contrast, upon D188 infection, CDKB1;1 expression in leaf 8 initially remained constant and after 16 dpi it increased drastically (Fig. 4D). The significance of this high CDKB1;1 expression upon D188 infection was illustrated by the complete phenotypical responsiveness of a CDKB1;1.N161 line, in which a dominant negative allele of CDKB1;1 is expressed, resulting in decreased CDK activity and increased ploidy levels (Boudolf et al., 2004b; Fig. 4, E and F). The typically high proportion of cells with a 2C content upon infection suggests that the continuous activation of the transcription of the wild-type allele in this mutant background largely compensates for the negative effect of the mutant allele. The latter was not completely overruled, however, because the 2C content was 15% lower than that of infected wild-type plants (Fig. 4, E and F).
Figure 4.
Role of CDKB1;1 in the stimulated G2-to-M transitions upon R. fascians infection. A, CDKB1;1:GUS expression in a young developing leaf of a control plant (left) and a R. fascians D188-infected plant (right). B and C, CDKB1;1:GUS expression at the cellular level in stomata of control and D188-infected leaves, respectively. D, Time course expression profile of CDKB1;1 upon infection. E and F, DNA ploidy level distribution of the Col-0 wild type and of CDKB1;1.N161 in control plants and upon infection at 24 dpi, respectively.
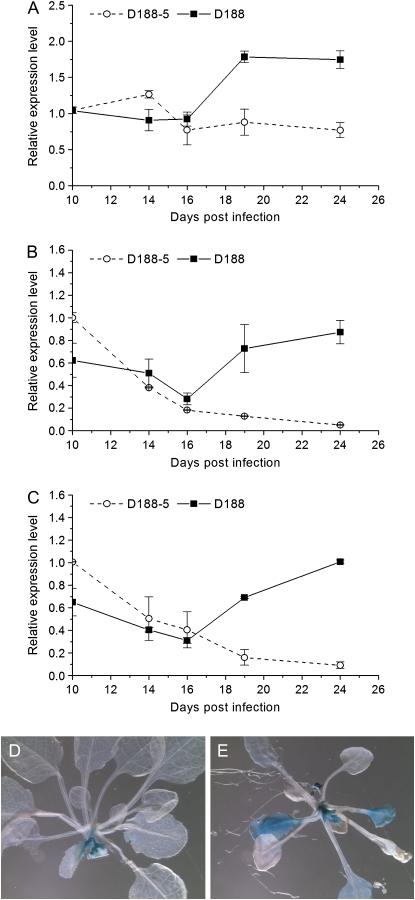
Expression of CDKA;1 and CYCA2;3, as a complex involved in the negative regulation of endocycling (Imai et al., 2006), was also specifically down-regulated as control leaves differentiated (Fig. 5, A and B). Interestingly, upon infection with D188, a biphasic expression pattern reminiscent of that of CDKB1;1 was observed for both genes (Fig. 5, A and B), confirming the proliferative status of symptomatic leaves. A similar differential expression in control versus infected leaves of E2Fe/DEL1 (Fig. 5C), exclusively present in mitotically dividing cells (Vlieghe et al., 2005; Lammens et al., 2008), together with the expansion of the expression zone in infected E2Fe/DEL1:GUS leaves beyond the leaf base into the leaf blade (Fig. 5, D and E), further support that leaf growth is only driven by mitotic cell divisions upon R. fascians infection.
Figure 5.
Assessment of the role of endocycle regulators during symptomatic leaf development. A to C, Expression kinetics of CDKA;1 (A), CYCA2;3 (B), and E2Fe/DEL1 (C) in leaf 8 upon infection. D and E, E2Fe/DEL1:GUS plants after infection with R. fascians D188-5 and strain D188, respectively.
Infection and subsequent determination of the disease phenotype and ploidy level distribution in symptomatic leaves of ccs52a2 and del1-1 knockout plants and in plants overexpressing E2Fe/DEL1 or SIM showed that these regulators do not play a significant role in the increased CDK activity and the stimulated progression at the G2-to-M transition during symptom development (Supplemental Fig. S1), suggesting that CDK expression and/or activity might be directly targeted by the R. fascians signals.
R. fascians Infection Recruits the CYCD3/RBR Pathway to Stimulate G1-to-S Transition and to Promote Proliferation over Differentiation
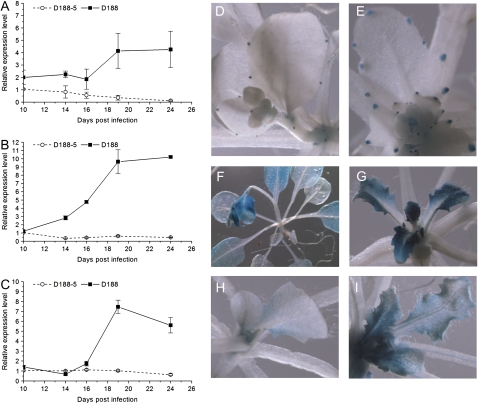
The continuation of mitotic cell divisions during symptomatic leaf development implies that cells not only efficiently pass the G2-to-M transition but subsequently cross the G1/S checkpoint and enter the S phase of the cell cycle. Entry into the S phase involves RBR phosphorylation and subsequent activation of S-phase gene expression in a CYCD3-dependent way (De Veylder et al., 2002; Dewitte et al., 2003). Although RBR activity is mainly controlled at the posttranscriptional level, transcription of RBR was on average 2-fold lower upon infection with strain D188 than that of controls (Table I), suggesting that G1-to-S transition is modulated during symptom development. Interestingly, the small size of epidermal pavement cells, their polygonic shape, the reduced tissue differentiation, and the prevention of endoreduplication in symptomatic leaves nearly phenocopy the leaf phenotype of transgenic CYCD3;1-overexpressing (CYCD3;1OE) Arabidopsis plants (Dewitte et al., 2003). Nevertheless, while leaf expansion was compromised upon D188 infection, this is not the case in the CYCD3;1OE plants under the tested conditions. The expression of all three CYCD3 genes of Arabidopsis was down-regulated as control leaves matured, but their expression during symptomatic leaf development exhibited the typical biphasic pattern, albeit with different kinetics. CYCD3;1 responded first (the differential expression started between 4 and 7 dpi when assayed on complete shoot tissue [data not shown]) and was up-regulated 4-fold at the end of the experiment (Fig. 6A). CYCD3;2 up-regulation only started between 10 and 14 dpi, but the final expression level was 10-fold higher than the control level (Fig. 6B). Finally, CYCD3;3 expression responded last and reached a 6-fold higher level than that of the control (Fig. 6C). Histochemical analysis confirmed the up-regulation of all three genes and showed that the expression zone in the leaf was not extended upon infection (Fig. 6, D–I).
Table I.
Phenotypical and molecular alterations in Col-0, Ler, cycd3;1-3, and CYCD3;1OE upon R. fascians D188 infection
Col-0, Columbia; Ler, Landsberg erecta; ++, strong response; +, intermediate response; (+), weak response; −, no response.
| Phenotypic Trait | Col-0/Ler | cycd3;1-3 | CYCD3;1OE |
|---|---|---|---|
| Anthocyanin production | ++ | + | + |
| Pale color | + | − | + |
| Small, newly formed leaves | ++ | + | ++ |
| Serrated margins of newly formed leaves | ++ | − | − |
| Activation of axillary meristems | ++ | (+) | + |
| Flowering induction | + | + | + |
| Inhibited elongation of flower stalks | + | + | + |
| Endoreduplication upon D188 infection | − | (+) | − |
| Expression in leaf tissue: fold changes at 24 dpi (R. fascians D188 versus D188-5 infection) | |||
| STM | >1,000a | 1 | 1 |
| ARR5 | >12a | 2.69 | 0.40 |
| E2Fe/DEL1 | 10 | 1.73 | 1 |
| RBR | 0.52 | 0.46 | 1 |
| CDKB1;1 | 20 | 2.39 | 0.66 |
Data derived from Depuydt et al. (2008).
Figure 6.
CYCD3 expression during R. fascians symptomatology. A to C, Expression kinetics of CYCD3;1 (A), CYCD3;2 (B), and CYCD3;3 (C) in leaf 8 upon infection with R. fascians strains D188-5 and D188. D to I, Histochemical localization of CYCD3;1 (D and E), CYCD3;2 (F and G), and CYCD3;3 (H and I) upon R. fascians D188-5 (D, F, and H) and D188 (E, G, and I) infection at 24 dpi.
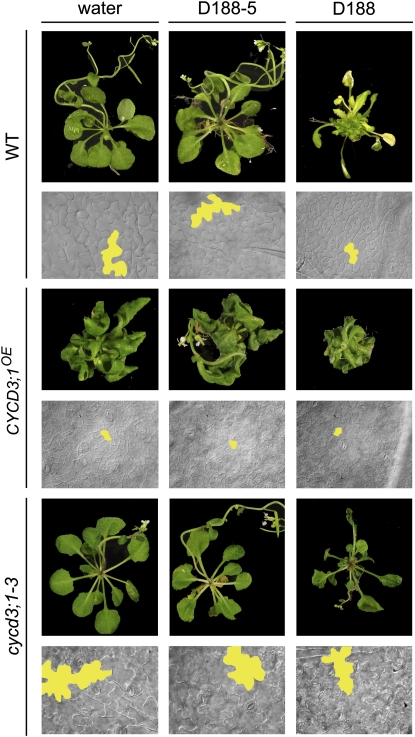
These data suggested that the CYCD3/RBR/E2F-DP pathway might be modulated upon R. fascians infection by direct targeting of the CYCD3 genes. The strongly reduced response of a cycd3;1-3 triple mutant line confirmed this hypothesis (Table I; Fig. 7). Importantly, in the triple mutant, the most typical aspects of the symptomatology were much less pronounced: activation of axillary meristems was only observed in 7% of the infected plants (versus 67% in wild-type plants), and leaves with serrated margins were not observed (versus 95% in wild-type plants). Leaf expansion was impaired upon infection, but not to the same extent as in infected wild-type plants (Fig. 7). The epidermal pavement cells of the infected triple mutant were sinusoid rather than polygon shaped, and their size was intermediate between the cycd3;1-3 controls and infected wild-type plants (Fig. 7). Flow cytometric analysis during development of leaf 8 of the noninfected cycd3;1-3 triple mutant revealed an initially higher level of cells with a 4C content and an earlier and steeper increase of cells with an 8C content (Fig. 8A) than in noninfected wild-type leaves (Fig. 3), confirming the premature onset of endoreduplication (Dewitte et al., 2007). In contrast to the effect on wild-type plants (Fig. 3), infection with D188 did not result in the typically high proportion of cells with a 2C content during development of leaf 8 of the cycd3;1-3 mutant, indicating that the activation of CYCD3 expression upon infection is required for the preclusion of endocycle onset. Interestingly, the number of cells with an 8C content decreased over time and was finally half that of the controls, suggesting that the R. fascians signals can still interfere with endoreduplication in the cycd3;1-3 mutant plants. The intermediate response of cycd3;1-3 plants to R. fascians infection could also be demonstrated at the molecular level by assessing the expression of marker genes (Depuydt et al., 2008). In agreement with the absence of leaf margin serrations, no ectopic expression of SHOOT MERISTEMLESS (STM), a meristem-associated class I KNOX gene, could be observed (Table I), but cytokinin signaling was still activated, as indicated by enhanced ARABIDOPSIS RESPONSE REGULATOR5 (ARR5) expression. Moreover, induced expression of E2Fe/DEL1 and CDKB1;1 and down-regulation of RBR were maintained in the infected cycd3;1-3 triple mutants. Nevertheless, the differential expression levels were much lower than those of infected wild-type controls (Table I).
Figure 7.
Functional analysis of the involvement of CYCD3 during R. fascians symptomatology. Complete plant phenotypes and epidermal pavement cell size of wild type (WT), CYCD3;1OE (Ler background), and cycd3;1-3 (Col-0 background) plants upon infection. As the wild type, Col-0 is shown; Ler behaved similarly upon infection (data not shown).
Figure 8.
DNA ploidy level distribution in cycd3;1-3 and CYCD3;1OE plants. A to C, Leaf 8 of cycd3;1-3 plants upon mock (A), R. fascians D188-5 (B), and D188 (C) infection. D, DNA ploidy level distribution of CYCD3;1OE upon infection at 24 dpi. Error bars represent sd (n = 4).
As described above, CYCD3;1OE plants had features that resembled R. fascians-induced phenotypes. Interestingly, these transgenic lines were only partially responsive to infection. Whereas axillary meristems were activated as in wild-type plants, leaf expansion was only partially compromised, no leaf serrations were observed (Fig. 7A), and the very small epidermal pavement cell size of the CYCD3;1OE plants was not further reduced upon infection (Fig. 7B). Moreover, the DNA ploidy level distribution did not change upon infection, no high population of cells with a 2C content could be established (Fig. 8D), and no differential expression of the marker genes was detected (Table I).
DISCUSSION
Arabidopsis leaves that develop upon infection with R. fascians strain D188 have a typical small and narrow leaf blade and serrated margins. The latter is accomplished by ectopic expression of meristem-associated class I KNOX genes, but the cause for the strongly reduced leaf expansion had not been addressed (Depuydt et al., 2008). Here, we determined that the small lamina size is caused by an almost 5-fold reduction in cell size that could not be compensated for by the 2- to 3-fold increase in cell number. The increased number of meristemoids, the reduced tissue differentiation, and the very high level of cells with a 2C content all illustrate that cells in D188-infected leaves hardly differentiate and remain in the proliferative state. These data imply that the time window in which mitotic cell division drives leaf growth is greatly extended upon infection and, moreover, indicate that, in symptomatic leaves, the subsequent stage of leaf growth via cell expansion, driven by endoreduplication, is never attained.
An increase in cell cycle gene expression upon infection with R. fascians had been reported previously in tobacco and Arabidopsis plants. Outer cortical cells of infected tobacco plants reenter the cell cycle, and subsequent divisions result in the formation of shoot primordia. The reiteration of this process together with the activation of axillary meristems ultimately culminates in the typical leafy gall symptom on tobacco and is correlated with the up-regulation of CYCD3;2 and CYCB1;1 (de O Manes et al., 2001). Also in Arabidopsis, activation of axillary meristems and de novo meristem formation contribute to symptom development (de O Manes et al., 2004; Simón-Mateo et al., 2006) and are associated with elevated expression of CYCA2;1, CYCB1;1, and CDKA;1 (Vereecke et al., 2000). Here, with the developing Arabidopsis leaf as a model system, we found that during infected leaf development the transcriptional profiles of the tested cell cycle genes all showed a typically biphasic pattern: initial expression was mostly constitutive but increased steadily from 16 dpi onward. In contrast, in control leaves, during maturation, the expression of the tested cell cycle genes steadily declined. Interestingly, at 16 dpi, the sampled leaf is approximately 8 d old, the age at which the switch from mitotic cycling to endocycling occurs in control leaves (Beemster et al., 2002). Consequently, the data suggest that already at the early stages of leaf development, infection activates the cell cycle. However, instead of a developmental switch into endocycling, proliferative cell division is strongly stimulated in infected leaves, representing the single mode of organ growth.
The persistent expression of the mitotic CDKs, CDKB1;1 and CDKA;1, and of CYCA2;3 continues to promote the G2-to-M transition. A fast progression through mitosis had been observed in tobacco Bright Yellow 2 cell cultures infected with R. fascians that was accompanied with transcriptional activation of genes associated with the G2/M phase (Vandeputte et al., 2007). Interestingly, CDKB1;1.N161 plants, expressing a dominant negative version of CDKB1;1, were phenotypically fully responsive, suggesting that in infected plants, the induced expression of the wild-type CDKB1;1 allele was sufficiently high to overcome the negative effect of the mutant allele. Expression of E2Fe/DEL1, an indirect activator of CDKB1;1 activity, was induced upon infection, but functional analysis revealed that the E2Fe/DEL1/CCS52A2 pathway was not involved in the increased CDK activity. Altogether, these data support the role of CDKB1;1 in the progression over the G2/M checkpoint during the R. fascians-induced pathology and imply that CDKB1;1 is possibly a direct target for the R. fascians signals. The main virulence factors of R. fascians are the fas-encoded cytokinins (Crespi et al., 1992, 1994; Vereecke et al., 2000; Pertry et al., 2009). Because cytokinins are known to directly regulate cell division at the G2-to-M transition by dephosphorylating CDKs (Zhang et al., 1996, 2005; Laureys et al., 1998; Spíchal et al., 2007), besides stimulating CDKB1;1 expression, the direct activation of CDKs by the bacterial cytokinins might represent a second level of stimulation at the G2/M checkpoint.
The transcriptional analysis indicated that the CYCD3/RBR/E2F-DP pathway that controls the G1-to-S transition was also modulated. Indeed, RBR expression was down-regulated upon infection, and all three CYCD3 genes were significantly up-regulated throughout development of the infected leaves. The CYCD3 proteins function as growth factor sensors (Riou-Khamlichi et al., 1999; Sorrell et al., 1999; Francis and Sorrell, 2001; Stals and Inzé, 2001), so the transcriptional induction by the bacterial cytokinins is probably directly responsible for the promotion of the G1-to-S transition. Importantly, a cycd3;1-3 triple knockout mutant had a strongly reduced response toward infection. Infected plants were overall smaller, leaf expansion was partially impaired, and epidermal pavement cell size was reduced, but strikingly, the typical serrations of the leaf margins and the activation of axillary meristems were nearly absent and the characteristic ploidy level distribution was not attained. Moreover, the differential expression level of STM, ARR5, E2Fe/DEL1, RBR, and CDKB1;1, used as marker genes, was much lower than that of infected wild-type plants. The loss of function of all three CYCD3 genes did not prevent the development into adult plants; therefore, CYCD3 is not essential for mitosis or morphogenesis during regular plant development but determines cell number, cell size, and endoreduplication in lateral shoot organs (Dewitte et al., 2007). Nevertheless, our data indicate that the CYCD3 proteins are instrumental for the developmental alterations that are triggered by the exogenous signals provided by R. fascians. Interestingly, CYCD3 has also been implicated in G2-to-M transition, because CYCD3 transcription and CYCD3;3-associated kinase activity peak at this checkpoint (Sorrell et al., 1999; Schnittger et al., 2002). Moreover, the occurrence of an E2F/DP-binding site in the promoter of CDKB1;1 directly places CYCD3 at the G2-to-M transition (Menges et al., 2005; Inzé and De Veylder, 2006).
Although transgenic CYCD3;1OE plants exhibit features that are reminiscent of R. fascians-induced symptoms, the overall phenotype was very different from that of infected wild-type plants. Indeed, expression of CYCD3;1 from the 35S promoter does not affect the overall organ size. More importantly, in contrast to R. fascians-infected plants in which cells accumulate in the G1 phase, the strongly enhanced G1-to-S transition is not accompanied with a promotion of the G2-to-M transition; hence, cells accumulate in the G2 phase (Dewitte et al., 2003; Menges et al., 2006). Unexpectedly, CYCD3;1OE plants were phenotypically much less responsive to R. fascians, an observation supported by the lack of differential expression of the marker genes and the absence of the typical ploidy distribution. Possibly, cells in G2 phase are much less sensitive to the R. fascians signals, or, alternatively, in the CYCD3;1OE plants, the leaf tissue is already pushed to its mitotic limit.
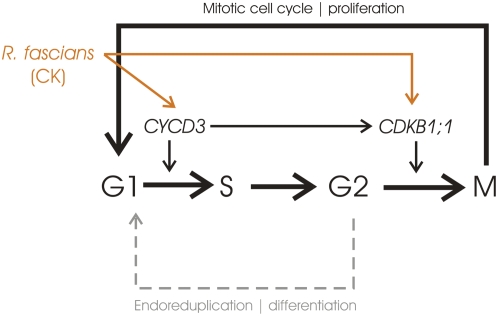
In conclusion, our data demonstrate that R. fascians modulates plant development by manipulating both checkpoints of the cell cycle (Fig. 9). Upon infection, the fast G1-to-S transition is accompanied by a fast G2-to-M transition; thus, most cells of symptomatic leaves are in the G1 phase. CYCD3 activity plays a central role in symptom development and potentially mediates both transitions, although direct targeting of CDKB1;1 might also contribute to the persistent mitotic cycling of the infected tissues. As a consequence of the action of the bacterial morphogens, newly formed organs only grow through mitotic cell divisions, hardly differentiate, and never reach maturity. It is hypothesized that young dividing tissues would provide the bacteria with specific nutrients that give a selective advantage over other plant-inhabiting microorganisms (Vereecke et al., 2002a, 2002b). Metabolic profiling experiments in which control and infected Arabidopsis leaves were compared indeed demonstrated that symptomatic leaves are sink tissues and represent an established niche for R. fascians. Importantly, the altered physiological status of infected tissues allows full manifestation of the bacterial virulence potential (Depuydt et al., 2009).
Figure 9.
Schematic representation of the R. fascians action in disturbing the progression from cell proliferation into differentiation by manipulating the cell cycle at the G1-to-S and G2-to-M transitions, mainly via CYCD3 and possibly directly via CDKB1;1. Dashed lines represent inhibited actions. CK, Cytokinin. [See online article for color version of this figure.]
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana L. Heyhn.) seeds were germinated and grown on half-strength Murashige and Skoog medium in a growth chamber under a 16-h/8-h light/dark photoperiod at 21°C ± 2°C, after sterilization by submergence for 2 min in 70% ethanol (v/v) and 12 min in 5% (w/v) NaOCl supplemented with 0.1% (v/v) polyoxyethylenesorbitan 20, and rinsing at least five times with sterile water. Unless stated otherwise, wild-type ecotype C24 was used for the infection experiments, obtained from the European Arabidopsis Stock Centre. CYCD3;1OE and cycd3;1-3 lines were obtained from Jim Murray (Dewitte et al., 2003, 2007); CDKB1;1.N161, del1-1, DEL1OE, ccs52a2, CDKB1;1:GUS, and E2Fe/DEL1:GUS were from Lieven De Veylder (Boudolf et al., 2004b; Vlieghe et al., 2005; Lammens et al., 2008); CYCD3;1:GUS, CYCD3;2:GUS, and CYCD3;3:GUS were from Tom Beeckman (Dewitte et al., 2007), and SIMOE lines were from John Larkin (Churchman et al., 2006).
Bacterial Strains, Growth Conditions, and Infection
The Rhodococcus fascians strains used were the pathogenic strain D188, containing the linear virulence plasmid pFiD188 and its plasmid-free nonpathogenic derivative D188-5 (Desomer et al., 1988). These strains were grown in liquid yeast extract broth for 2 d at 28°C under gentle agitation until late exponential phase. Prior to infection, these cultures were washed and concentrated 4-fold by resuspension of the bacterial pellets in sterile distilled water. At 16 d after germination, Arabidopsis plants were infected at stage 1.05 (Boyes et al., 2001) by local application of a drop of bacterial culture to the shoot apical meristem.
Analysis of Leaf Growth Parameters
The leaf area was determined by photographing the leaf with a binocular MZFLIII (Leica) and analyzing the picture with the public domain image-analysis program ImageJ (http://rsb.info.nig.gov/ij/; version 1.32j). For cell density and cell area, the leaves were cleared overnight in ethanol (70% [v/v]) and stored in lactic acid for analysis on a microscope stage fitted with differential interference contrast optics (DMLB; Leica). Images of at least 30 cells of the abaxial epidermis located at 25% and 75% from the leaf base, halfway between the midrib and the leaf margin, were drawn with a drawing tube mounted on the microscope. These images were scanned and processed with ImageJ. The total number of cells was estimated by dividing the leaf area with the average cell area. At least five independent biological repeats were analyzed, and average data are shown. The total numbers of stomata and meristemoids were derived by extrapolating the number of stomata and meristemoids, identified by cell shape and size, in the drawing tube images to the full leaf surface area.
Flow Cytometric Analysis
Depending on the experiment, leaf 5, 6, 7, and/or 8 was sampled (Figs. 3 and 8, A–C) or symptomatic leaves were pooled (Figs. 4, E and F, and 8D; Supplemental Fig. S1). Leaves were chopped with a razor blade in 300 μL of buffer (45 mm MgCl2, 30 mm sodium citrate, 20 mm MOPS, and 1% Triton X-100, pH 7; Galbraith et al., 1991). This mixture was filtered over a 30-μm mesh and, subsequently, 1 μL of 4,6′-diamidino-2-phenylindole from a 1 mg mL−1 stock was added. The nuclei were analyzed with the CyFlow flow cytometer with the accompanying FloMax software (Partec). Each sample was measured in two or three biological repeats and two technical replicates, unless stated otherwise.
Histochemical Analysis
The GUS assays were done as described (Beeckman and Engler, 1994). For microscopic analysis, samples were cleared by mounting in 90% lactic acid (Acros Organics).
qRT-PCR
For qRT-PCR, leaf 8 was sampled in a time course experiment and stored at −70°C until further processing. RNA was extracted from leaf tissue with the RNeasy kit (Qiagen). cDNA was prepared from 2 μg of total RNA with SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer's instructions. qRT-PCRs were quantified on a Lightcycler480 apparatus (Roche Diagnostics) with SYBR Green for detection in a 5-μL volume (2.5 μL of master, 0.25 μL of 5 μm of each forward and reverse primer, and 2 μL of cDNA) in triplicate on a 384-multiwell plate to allow determination of mean and SD of cycle threshold (CT) values. Data were analyzed with the 2−ΔΔCT method (Livak and Schmittgen, 2001), and values were normalized against those of ACTIN2, which was used as an internal standard. The mean expression level was calculated from six replicates of two biological repeats, obtained from two independent experiments. Primer sequences are given in Supplemental Table S1.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. DNA ploidy level distribution upon infection of del1-1, DEL1OE, ccs52a2, and SIMOE plants at 24 dpi.
Supplemental Table S1. Primers used in qRT-PCR amplification.
Supplementary Material
Acknowledgments
We thank Gerrit Beemster for fruitful discussions, Walter Dewitte for critical reading of the manuscript, Martine De Cock for help in preparing it, and Karel Spruyt for photographs.
This work was supported by the Bijzonder Onderzoeksfonds of Ghent University (predoctoral fellowship to S.D.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Danny Vereecke (danny.vereecke@psb.ugent.be).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Beeckman T, Engler G (1994) An easy technique for the clearing of histochemically stained plant tissue. Plant Mol Biol Rep 12 37–42 [Google Scholar]
- Beemster GTS, De Vusser K, De Tavernier E, De Bock K, Inzé D (2002) Variation in growth rate between Arabidopsis ecotypes is correlated with cell division and A-type cyclin-dependent kinase activity. Plant Physiol 129 854–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GTS, Fiorani F, Inzé D (2003) Cell cycle: the key to plant growth control? Trends Plant Sci 8 154–158 [DOI] [PubMed] [Google Scholar]
- Boudolf V, Barrôco R, de Almeida Engler J, Verkest A, Beeckman T, Naudts M, Inzé D, De Veylder L (2004. a) B1-type cyclin-dependent kinases are essential for the formation of stomatal complexes in Arabidopsis thaliana. Plant Cell 16 945–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudolf V, Vlieghe K, Beemster GTS, Magyar Z, Torres Acosta JA, Maes S, Van Der Schueren E, Inzé D, De Veylder L (2004. b) The plant-specific cyclin-dependent kinase CDKB1;1 and transcription factor E2Fa-DPa control the balance of mitotically dividing and endoreduplicating cells in Arabidopsis. Plant Cell 16 2683–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Görlach J (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchman ML, Brown ML, Kato N, Kirik V, Hülskamp M, Inzé D, De Veylder L, Walker JD, Zheng Z, Oppenheimer DG, et al (2006) SIAMESE, a novel plant-specific cell cycle regulator controls endoreplication onset in Arabidopsis thaliana. Plant Cell 18 3145–3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cionini PG, Cavallini A, Baroncelli S, Lercari B, D'Amato F (1983) Diploidy and chromosome endoreduplication in the development of epidermal cell lines in the first foliage leaf of durum wheat (Triticum durum Desf.). Protoplasma 118 36–43 [Google Scholar]
- Crespi M, Messens E, Caplan AB, Van Montagu M, Desomer J (1992) Fasciation induction by the phytopathogen Rhodococcus fascians depends upon a linear plasmid encoding a cytokinin synthase gene. EMBO J 11 795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi M, Vereecke D, Temmerman W, Van Montagu M, Desomer J (1994) The fas operon of Rhodococcus fascians encodes new genes required for efficient fasciation of host plants. J Bacteriol 176 2492–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JC, Diaz-Trivino S, Cisneros N, Gutierrez C (2006) The balance between cell division and endoreplication depends on E2FC-DPB, transcription factors regulated by the ubiquitin-SCFSKP2A pathway in Arabidopsis. Plant Cell 18 2224–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Boer BGW, Murray JAH (2000) Control of plant growth and development through manipulation of cell-cycle genes. Curr Opin Biotechnol 11 138–145 [DOI] [PubMed] [Google Scholar]
- de O Manes C-L, Beeckman T, Ritsema T, Van Montagu M, Goethals K, Holsters M (2004) Phenotypic alterations in Arabidopsis thaliana plants caused by Rhodococcus fascians infection. J Plant Res 117 139–145 [DOI] [PubMed] [Google Scholar]
- de O Manes C-L, Van Montagu M, Prinsen E, Goethals K, Holsters M (2001) De novo cortical cell division triggered by the phytopathogen Rhodococcus fascians in tobacco. Mol Plant Microbe Interact 14 189–195 [DOI] [PubMed] [Google Scholar]
- Depuydt S, Doležal K, Van Lijsebettens M, Moritz T, Holsters M, Vereecke D (2008) Modulation of the hormone setting by Rhodococcus fascians results in ectopic KNOX activation in Arabidopsis. Plant Physiol 146 1267–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt S, Trenkamp S, Fernie AR, Elftieh S, Renou J-P, Vuylsteke M, Holsters M, Vereecke D (2009) An integrated genomics approach to define niche establishment by Rhodococcus fascians. Plant Physiol 149: 1366–1386 [DOI] [PMC free article] [PubMed]
- Desomer J, Dhaese P, Van Montagu M (1988) Conjugative transfer of cadmium resistance plasmids in Rhodococcus fascians strains. J Bacteriol 170 2401–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GTS, de Almeida Engler J, Ormenese S, Maes S, Naudts M, Van Der Schueren E, Jacqmard A, Engler G, et al (2002) Control of proliferation, endoreduplication and differentiation by the Arabidopsis E2Fa/DPa transcription factor. EMBO J 21 1360–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GTS, Krols L, Terras F, Landrieu I, Van Der Schueren E, Maes S, Naudts M, Inzé D (2001) Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell 13 1653–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Joubès J, Inzé D (2003) Plant cell cycle transitions. Curr Opin Plant Biol 6 536–543 [DOI] [PubMed] [Google Scholar]
- Dewitte W, Murray JAH (2003) The plant cell cycle. Annu Rev Plant Biol 54 235–264 [DOI] [PubMed] [Google Scholar]
- Dewitte W, Riou-Khamlichi C, Scofield S, Healy JMS, Jacqmard A, Kilby NJ, Murray JAH (2003) Altered cell cycle distribution, hyperplasia, and inhibited differentiation in Arabidopsis caused by the D-type cyclin CYCD3. Plant Cell 15 79–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitte W, Scofield S, Alcasabas AA, Maughan SC, Menges M, Braun N, Collins C, Nieuwland J, Prinsen E, Sundaresan V, et al (2007) Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc Natl Acad Sci USA 104 14537–14542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG (1999) Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol 215 407–419 [DOI] [PubMed] [Google Scholar]
- Ferjani A, Horiguchi G, Yano S, Tsukaya H (2007) Analysis of leaf development in fugu mutants of Arabidopsis reveals three compensation modes that modulate cell expansion in determinate organs. Plant Physiol 144 988–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D, Sorrell DA (2001) The interface between the cell cycle and plant growth regulators: a mini review. Plant Growth Regul 33 1–12 [Google Scholar]
- Galbraith DW, Harkins KR, Knapp S (1991) Systemic endopolyploidy in Arabidopsis thaliana. Plant Physiol 96 985–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau E, Höfte H, Grandjean O, Brown S, Traas J (1998) Phytochrome controls the number of endoreduplication cycles in the Arabidopsis thaliana hypocotyl. Plant J 13 221–230 [DOI] [PubMed] [Google Scholar]
- Gendreau E, Orbovic V, Höfte H, Traas J (1999) Gibberellin and ethylene control endoreduplication levels in the Arabidopsis thaliana hypocotyl. Planta 209 513–516 [DOI] [PubMed] [Google Scholar]
- Goethals K, Vereecke D, Jaziri M, Van Montagu M, Holsters M (2001) Leafy gall formation by Rhodococcus fascians. Annu Rev Phytopathol 39 27–52 [DOI] [PubMed] [Google Scholar]
- Hemerly A, de Almeida Engler J, Bergounioux C, Van Montagu M, Engler G, Inzé D, Ferreira P (1995) Dominant negative mutants of the Cdc2 kinase uncouple cell division from iterative plant development. EMBO J 14 3925–3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Bao F, Li J (2000) Promotive effect of brassinosteroids on cell division involves a distinct CycD3-induction pathway in Arabidopsis. Plant J 24 693–701 [DOI] [PubMed] [Google Scholar]
- Imai KK, Ohashi Y, Tsuge T, Yoshizumi T, Matsui M, Oka A, Aoyama T (2006) The A-type cyclin CYCA2;3 is a key regulator of ploidy levels in Arabidopsis endoreduplication. Plant Cell 18 382–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzé D (2005) Green light for the cell cycle. EMBO J 24 657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzé D, De Veylder L (2006) Cell cycle regulation in plant development. Annu Rev Genet 40 77–105 [DOI] [PubMed] [Google Scholar]
- Jasinski S, Riou-Khamlichi C, Roche O, Perennes C, Bergounioux C, Glab N (2002) The CDK inhibitor NtKIS1a is involved in plant development, endoreduplication and restores normal development of cyclin D3;1-overexpressing plants. J Cell Sci 115 973–982 [DOI] [PubMed] [Google Scholar]
- Joubès J, Phan T-H, Just D, Rothan C, Bergounioux C, Raymond P, Chevalier C (1999) Molecular and biochemical characterization of the involvement of cyclin-dependent kinase A during the early development of tomato fruit. Plant Physiol 121 857–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondorosi E, Kondorosi A (2004) Endoreduplication and activation of the anaphase-promoting complex during symbiotic cell development. FEBS Lett 567 152–157 [DOI] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y (2003) Constitutive E2F expression in tobacco plants exhibits altered cell cycle control and morphological change in a cell type-specific manner. Plant Physiol 132 2012–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammens T, Boudolf V, Kheibarshekan L, Zalmas LP, Gaamouche T, Maes S, Vanstraelen M, Kondorosi E, La Thangue NB, Govaerts W, et al (2008) Atypical E2F activity restrains APC/CCCS52A2 function obligatory for endocycle onset. Proc Natl Acad Sci USA 105 14721–14726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys F, Dewitte W, Witters E, Van Montagu M, Inzé D, Van Onckelen H (1998) Zeatin is indispensable for the G2-M transition in tobacco BY-2 cells. FEBS Lett 426 29–32 [DOI] [PubMed] [Google Scholar]
- Leiva-Neto JT, Grafi G, Sabelli PA, Dante RA, Woo YM, Maddock S, Gordon-Kamm WJ, Larkins BA (2004) A dominant negative mutant of cyclin-dependent kinase A reduces endoreduplication but not cell size or gene expression in maize endosperm. Plant Cell 16 1854–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25 402–408 [DOI] [PubMed] [Google Scholar]
- Magyar Z, De Veylder L, Atanassova A, Bakó L, Inzé D, Bögre L (2005) The role of the Arabidopsis E2FB transcription factor in regulating auxin-dependent cell division. Plant Cell 17 2527–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melaragno JE, Mehrotra B, Coleman AW (1993) Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis. Plant Cell 5 1661–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges M, de Jager SM, Gruissem W, Murray JAH (2005) Global analysis of the core cell cycle regulators of Arabidopsis identifies novel genes, reveals multiple and highly specific profiles of expression and provides a coherent model for plant cell cycle control. Plant J 41 546–566 [DOI] [PubMed] [Google Scholar]
- Menges M, Samland AK, Planchais S, Murray JAH (2006) The D-type cyclin CYCD3;1 is limiting for the G1-to-S-phase transition in Arabidopsis. Plant Cell 18 893–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai T, Kato K, Shinmyo A, Sekine M (2006) Arabidopsis KRPs have distinct inhibitory activity toward cyclin D2-associated kinases, including plant-specific B-type cyclin-dependent kinase. FEBS Lett 580 336–340 [DOI] [PubMed] [Google Scholar]
- Nath U, Crawford BCW, Carpenter R, Coen E (2003) Genetic control of surface curvature. Science 299 1404–1407 [DOI] [PubMed] [Google Scholar]
- Oakenfull EA, Riou-Khamlichi C, Murray JAH (2002) Plant D-type cyclins and the control of G1 progression. Philos Trans R Soc Lond B Biol Sci 357 749–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertry I, Václavíková K, Depuydt S, Galuszka P, Spíchal L, Temmerman W, Stes E, Schmülling T, Kakimoto T, Van Montagu M, et al (2009) Identification of Rhodococcus fascians cytokinins and their modus operandi to reshape the plant. Proc Natl Acad Sci USA 106 929–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettkó-Szandtner A, Mészáros T, Horváth GV, Bakó L, Csordás-Tóth É, Blastyák A, Zhiponova M, Miskolczi P, Dudits D (2006) Activation of an alfalfa cyclin-dependent kinase inhibitor by calmodulin-like domain protein kinase. Plant J 46 111–123 [DOI] [PubMed] [Google Scholar]
- Putnam ML, Miller ML (2007) Rhodococcus fascians in herbaceous perennials. Plant Dis 91 1064–1076 [DOI] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JAH (1999) Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283 1541–1544 [DOI] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Menges M, Healy JMS, Murray JAH (2000) Sugar control of the plant cell cycle: differential regulation of Arabidopsis D-type cyclin gene expression. Mol Cell Biol 20 4513–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittger A, Schöbinger U, Bouyer D, Weinl C, Stierhof Y-D, Hülskamp M (2002) Ectopic D-type cyclin expression induces not only DNA replication but also cell division in Arabidopsis trichomes. Proc Natl Acad Sci USA 99 6410–6415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittger A, Weinl C, Bouyer D, Schöbinger U, Hülskamp M (2003) Misexpression of the cyclin-dependent kinase inhibitor ICK1/KRP1 in single-celled Arabidopsis trichomes reduces endoreduplication and cell size and induces cell death. Plant Cell 15 303–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón-Mateo C, Depuydt S, de Oliveira Manes CL, Cnudde F, Holsters M, Goethals K, Vereecke D (2006) The phytopathogen Rhodococcus fascians breaks apical dominance and activates axillary meristems by inducing plant genes involved in hormone metabolism. Mol Plant Pathol 7 103–112 [DOI] [PubMed] [Google Scholar]
- Soni R, Carmichael JP, Shah ZH, Murray JAH (1995) A family of cyclin D homologs from plants differentially controlled by growth regulators and containing the conserved retinoblastoma protein interaction motif. Plant Cell 7 85–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell DA, Combettes B, Chaubet-Gigot N, Gigot C, Murray JAH (1999) Distinct cyclin D genes show mitotic accumulation or constant levels of transcripts in tobacco Bright Yellow-2 cells. Plant Physiol 119 343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spíchal L, Kryštof V, Paprskářová Lenobel R, Stýskala J, Binarová P, Cenklová V, De Veylder L, Inzé D, Kontopidis G, Fischer PM, et al (2007) Classical anticytokinins do not interact with cytokinin receptors but inhibit cyclin-dependent kinases. J Biol Chem 282 14356–14363 [DOI] [PubMed] [Google Scholar]
- Stals H, Inzé D (2001) When plant cells decide to divide. Trends Plant Sci 6 359–364 [DOI] [PubMed] [Google Scholar]
- Temmerman W, Vereecke D, Dreesen R, Van Montagu M, Holsters M, Goethals K (2000) Leafy gall formation is controlled by fasR, an AraC-type regulatory gene, in Rhodococcus fascians. J Bacteriol 182 5832–5840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traas J, Hülskamp M, Gendreau E, Höfte H (1998) Endoreduplication and development: rule without dividing? Curr Opin Plant Biol 1 498–503 [DOI] [PubMed] [Google Scholar]
- Tsukaya H (2006) Mechanism of leaf-shape determination. Annu Rev Plant Biol 57 477–496 [DOI] [PubMed] [Google Scholar]
- Vandeputte O, Öden S, Mol A, Vereecke D, Goethals K, El Jaziri M, Prinsen E (2005) Biosynthesis of auxin by the Gram-positive phytopathogen Rhodococcus fascians is controlled by compounds specific to infected plant tissues. Appl Environ Microbiol 71 1169–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte O, Vereecke D, Mol A, Lenjou M, Van Bockstaele D, El Jaziri M, Baucher M (2007) Rhodococcus fascians infection accelerates progression of tobacco BY-2 cells into mitosis through rapid changes in plant gene expression. New Phytol 175 140–154 [DOI] [PubMed] [Google Scholar]
- Vereecke D, Burssens S, Simón-Mateo C, Inzé D, Van Montagu M, Goethals K, Jaziri M (2000) The Rhodococcus fascians-plant interaction: morphological traits and biotechnological applications. Planta 210 241–251 [DOI] [PubMed] [Google Scholar]
- Vereecke D, Cornelis K, Temmerman W, Holsters M, Goethals K (2002. a) Versatile persistence pathways for pathogens of animals and plants. Trends Microbiol 10 485–488 [DOI] [PubMed] [Google Scholar]
- Vereecke D, Cornelis K, Temmerman W, Jaziri M, Van Montagu M, Holsters M, Goethals K (2002. b) Chromosomal locus that affects the pathogenicity of Rhodococcus fascians. J Bacteriol 184 1112–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkest A, de O Manes C-L, Vercruysse S, Maes S, Van Der Schueren E, Beeckman T, Genschik P, Kuiper M, Inzé D, De Veylder L (2005) The cyclin-dependent kinase inhibitor KRP2 controls the onset of the endoreduplication cycle during Arabidopsis leaf development through inhibition of mitotic CDKA;1 kinase complexes. Plant Cell 17 1723–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlieghe K, Boudolf V, Beemster GTS, Maes S, Magyar Z, Atanassova A, de Almeida Engler J, De Groodt R, Inzé D, De Veylder L (2005) The DP-E2F-like DEL1 gene controls the endocycle in Arabidopsis thaliana. Curr Biol 15 59–63 [DOI] [PubMed] [Google Scholar]
- Wang H, Qi Q, Schorr P, Cutler AJ, Crosby WL, Fowke LC (1998) ICK1, a cyclin-dependent protein kinase inhibitor from Arabidopsis thaliana interacts with both Cdc2a and CycD3, and its expression is induced by abscisic acid. Plant J 15 501–510 [DOI] [PubMed] [Google Scholar]
- Weinl C, Marquardt S, Kuijt SJH, Nowack MK, Jakoby MJ, Hülskamp M, Schnittger A (2005) Novel functions of plant cyclin-dependent kinase inhibitors, ICK1/KRP1, can act non-cell-autonomously and inhibit entry into mitosis. Plant Cell 17 1704–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Diederich L, John PCI (2005) The cytokinin requirement for cell division in cultured Nicotiana plumbaginifolia cells can be satisfied by yeast Cdc25 protein tyrosine phosphatase: implications for mechanisms of cytokinin response and plant development. Plant Physiol 137 308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Z, Ravid K (1996) The cell cycle in polyploid megakaryocytes is associated with reduced activity of cyclin B1-dependent Cdc2 kinase. J Biol Chem 271 4266–4272 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.