Abstract
Calcium modulates the 5-HT3 receptor response by reducing peak current amplitude and increasing rates of activation, deactivation and desensitisation, but the binding site(s) and mechanism(s) of this modulation are unknown. Here we study residues that may be involved in calcium binding in two partially overlapping regions of the extracellular domain (E213-E215-E218 and D204-E218-V219). The modulatory effects of calcium were assessed by radioligand binding and whole-cell patch-clamp. Comparisons of [3H]granisetron binding showed an increase in Kd in 10 mM calcium that was abolished by the substitutions E213Q, E215Q, D204N and V219L. E218Q mutant receptors displayed no specific binding or function, and immunofluorescence showed that they did not reach the cell surface. E213Q increased inherent rates of desensitisation, but the relative effects of calcium on these rates, and on the reduction in current amplitude, were similar to wild type receptors. Current responses and calcium-mediated effects at E215Q mutant receptors were indistinguishable from wild type. D204N and V219L mutants were non-functional. A calcium impermeable mutant (E277A/S297R) revealed no changes in peak amplitude or kinetics with increased calcium. Our results are consistent with residues D204, E218 and V219 participating in receptor assembly, structure and/or trafficking to the plasma membrane, and we speculate that this might rely upon the stabilising effect of bound calcium. E213, E215, D204 and V219 may contribute to a calcium binding site that is responsible for the calcium-mediated effects on ligand binding. However, the major site for calcium-dependent modulation of the 5-HT3 current is located within the ion channel or cell interior.
Keywords: Serotonin, Cys-loop, Channel, Pore, Extracellular domain, Modulation, Calcium, 5-HT3
1. Introduction
The 5-HT3 receptor is a member of a therapeutically important group of ligand gated ion channels known as the Cys-loop family. Members of this family are responsible for fast chemical neurotransmission, and they include the nicotinic acetylcholine (nACh), glycine and GABAA receptors (Thompson et al., 2006a). All of these receptors are composed of five subunits that are symmetrically arranged around a central ion-conducting pore and each subunit contains three domains. The extracellular N-terminal domain contains the ligand binding sites which are located between two adjacent subunits. Amino acids responsible for ligand binding arise from the convergence of three loops from a principal subunit and three loops from a complementary subunit (Thompson et al., 2006a; Fig. 1A). The energy from ligand binding opens an integral ion channel which is located in the transmembrane domain. This domain consists of four transmembrane α-helices (M1-M4). M2 lines the ion permeable channel and hydrophobic residues close to its centre are thought to act as the channel gate. The intracellular domain is formed by a loop of approximately 110 residues between M3 and M4, and plays a role in channel conductance and receptor modulation by intracellular messengers (Kelley et al., 2003; Thompson et al., 2006a). Activation of the 5-HT3 receptor elicits an inward current, which is predominantly carried by the monovalent cations, sodium and potassium, but to a lesser extent is also permeable to divalent ions (Jackson and Yakel, 1995; Peters et al., 1988).
Fig. 1.
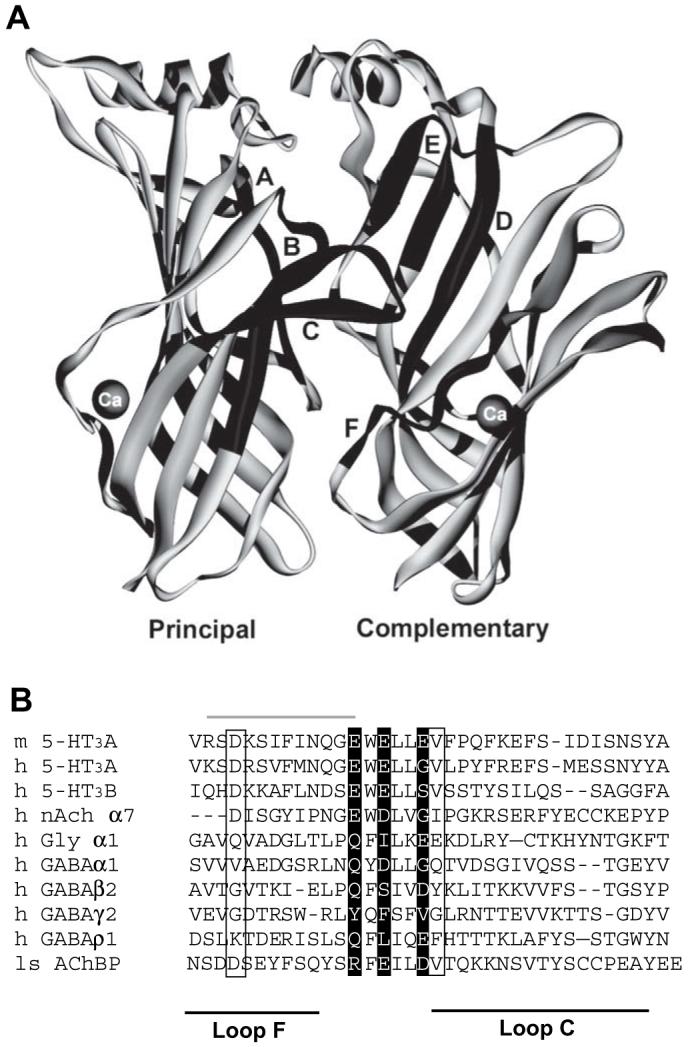
Location of two potential calcium binding sites in the 5-HT3 extracellular domain. (A) The original AChBP structure (1i9b) with Ca2+ coordinated by the side chains of D161 and D175, and the backbone carbonyl of V176. Only two of the five subunits are shown for clarity. The six loops (A-F) that constitute the binding site are shown in black. (B) An alignment of the AChBP loops C and F with members of the Cys-loop LGIC family. The three glutamate residues of the 5-HT3 consensus calcium binding site are shown as white text on a black background. The three residues from the calcium binding site identified in AChBP are indicated by a box. Note that the two potential binding sites overlap at E218. The region of the 5-HT3 receptor that was exchanged for the corresponding α7 nACh sequence by Galzi et al. (1996) is shown by a grey line above the text.
All the receptors in this family are affected by a range of divalent cations, including calcium, magnesium and zinc, but their actions can vary according to the receptor type (Hubbard and Lummis, 2000; Peters et al., 1988). For example, in the α7 nACh receptor, divalent ions potentiate the response, while these same ions reduce peak currents and increasing the rate of activation, deactivation and desensitisation at 5-HT3 receptors (Hu and Lovinger, 2005). In α7 nACh receptors, binding sites in both the extracellular domain and the ion channel have been characterised, and it has also been suggested that similar sites might exist in the 5-HT3 receptor (Bertrand et al., 1993; Eddins et al., 2002a, b; Gill et al., 1995; Hu and Lovinger, 2005; Quirk et al., 2004; Van Hooft and Wadman, 2003).
Here we examine amino acids that contribute to two potential calcium binding sites in the 5-HT3 receptor extracellular domain, and also use a calcium impermeant mutant 5-HT3 receptor, to determine the roles of these residues on calcium-mediated effects on binding and modulation of the current response.
2. Methods
2.1. Materials
All cell culture reagents were obtained from Gibco BRL (Paisley, UK), except foetal calf serum which was from Labtech International (Ringmer, UK). [3H]granisetron (81 Ci/mmol) was from PerkinElmer (Boston, MA, USA). All other reagents were of the highest obtainable grade.
2.2. Cell culture
Human embryonic kidney (HEK) 293 cells were maintained on 90 mm tissue culture plates in DMEM:F12 (Dulbecco’s Modified Eagle Medium/Nutrient Mix F12 (1:1)) with GlutaMAX I™ containing 10% foetal calf serum, at 37 °C and 7% CO2 in a humidified atmosphere. Cells in 90 mm dishes were transfected using calcium phosphate precipitation at 70-80% confluency (Chen and Okayama, 1988; Jordan et al., 1996).
2.3. Receptor expression and mutagenesis
Mouse 5-HT3A receptor subunit cDNA (Accession: AY605711) was cloned into pRc/CMV (Invitrogen Ltd., Paisley, UK) and mutagenesis was performed using the method described by Kunkel (Kunkel, 1985). Oligonucleotide primers were designed according to the recommendations of Sambrook et al. (1989) and some suggestions of the Primer Generator (Turchin and Lawler, 1999); http://www.med.jhu.edu/medcenter/primer/primer.cgi). A silent restriction site was incorporated into each primer to assist identification of mutants.
2.4. Electrophysiology
HEK 293 cells were either transiently transfected using standard commercial reagents or stable cell lines were selected using antibiotic resistance (Geneticin) encoded within the pRc/CMV plasmid. Electrophysiological measurements were performed in the whole-cell configuration using an Axopatch 200 amplifier (Axon Instruments, Union City, CA, USA), Lab-PC + A/D board (National Instruments Inc., TX, USA) and Strathclyde Electrophysiology Software v3.1.4 (Department of Physiology and Pharmacology, University of Strathclyde, UK; http://www.strath.ac.uk/Departments/PhysPharm/). All experiments were performed in voltage-clamp mode. Currents were filtered at a frequency of 5 kHz (−3 dB), using the 4-pole low-pass Bessel filter provided on the amplifier, and acquired at a sampling frequency of 1 kHz. The cell membrane potential was routinely held at −60 mV. Patch electrodes were pulled with a Sutter P87 (Novato, CA, USA) using a three stage horizontal pull and type GC120TF-10 borosilicate glass (Harvard Apparatus, Edenbridge, Kent, UK). Pipette resistances ranged from 2.0 to 3.5 MΩ. Series resistance was usually less than 5MΩ and voltage errors never exceeded 5 mV.
Application of solutions was achieved using a ValveBank 8II (Automate Scientific Inc., San Francisco, CA, USA). Cells were perfused using a gravity fed bath with a constant laminar flow of saline at a rate of 4-5 ml per min. The time taken for the baseline current to stabilise after changing saline concentrations (in the open-tip configuration) was used as an indicator of solution exchange, and showed that cells were completely submersed in test solution within 50-100 ms. After entering the whole-cell configuration, the membrane current was allowed to stabilise for at least 2 min before recordings were made.
For dose-response experiments, patch pipettes were filled with filtered (0.2 μm, Millipore) intracellular saline containing (mM), 140 CsCl, 1.0 MgCl2, 1.0 CaCl2, 10.0 EGTA and 10 HEPES; pH 7.2 with CsOH. Cells were continuously perfused with an extracellular solution containing 140 NaCl, 5.4 KCl, 1.0 MgCl2, 1.0 CaCl2 and 10 HEPES; pH 7.2 with NaOH. Solutions used to test the effects of external calcium were the same as above, but contained no added MgCl2, and either no added CaCl2 (referred to as 0 mM), 1 mM CaCl2, 3 mM CaCl2 or 10 mM CaCl2. All salines were prepared fresh each day.
Currents were analysed using the tools provided as part of Strathclyde Electrophysiology Software. Current desensitisation was fitted (5-95% of total current) to a single-exponential function. Owing to the slow rates of solution exchange (50-100 ms) when compared to the speed of activation, this parameter was not measured. Statistical analysis and curve fitting was performed using Prism v3.02 (GraphPad Software, San Diego, CA, USA, http://www.graphpad.com). Values are presented as mean ± SEM. Statistical analysis was performed using ANOVA in conjunction with a Dunnett’s post test. P values <0.05 are considered as statistically significant.
2.5. Radioligand binding
This was undertaken as previously described with minor modifications (Lummis et al., 1993). Briefly, transfected HEK 293 cell membranes were incubated in 10 mM HEPES buffer (pH 7.4) containing the 5-HT3 receptor antagonist [3H]granisetron for 1 h at 4 °C. Saturation binding (8 point) assays were performed using 0.10-40.0 nM [3H]granisetron. Non-specific binding was determined using 1 μM quipazine. Reactions were incubated for 1 h at 4 °C and radioactivity determined by scintillation counting (Beckman LS6000sc, Beckman Coulter Inc, CA, USA). Data were analyzed using Prism v3.02 by iterative curve fitting according to the equation: B = (Bmax· [L])/(K + [L]), where B is bound radioligand, Bmax is maximum binding at equilibrium, K is the equilibrium dissociation constant and [L] is the free concentration of radioligand. Values are presented as mean ± SEM. Statistical analysis was performed using ANOVA in conjunction with a Dunnett’s post test or Student’s t-test. P values <0.05 are considered as statistically significant.
2.6. Immunofluorescence
This was as described previously (Spier et al., 1999). Briefly, transfected cells were washed with three changes of Tris-buffered saline (TBS: 0.1 M Tris pH 7.4, 0.9% NaCl) and fixed using ice cold 4% paraformaldehyde in phosphate buffer (PB: 66 mM Na2HPO4, 38 mM NaH2PO4, pH 7.2). After two TBS washes, the cells were incubated overnight at 4 °C in pAb120; at 1:1600 in TBS. Biotinylated anti-rabbit IgG (Vector Laboratories, CA, USA) and fluorescein isothiocyanate (FITC) avidin D (Vector Laboratories, CA, USA) were used to detect bound antibody as stated in the manufacturer’s instructions. Coverslips were mounted in Vectashield mounting medium (Vector Laboratories, CA, USA). Immunofluorescence was observed using a confocal microscope.
3. Results
3.1. Wild type receptors
Under physiological conditions (1.0 mM CaCl2, 1.0 mM MgCl2), wild type 5-HT3 receptors responded to 5-HT with a rapidly activating and slowly desensitising inward current. Plotting current amplitude against a series of 5-HT concentrations yielded a log EC50 of −5.75 ± 0.02 (EC50;1.8 μM) and Hill slope of 2.31 ± 0.25 (n = 23), similar to previously published results (Hussy et al., 1994; Thompson and Lummis, 2003). Rates of desensitisation were well fitted by single exponential functions and increased with rising concentrations of 5-HT.
In the absence of external magnesium, raising the concentration of calcium (0 mM, 1 mM, 3 mM and 10 mM) had a number of concentration-dependent, reversible effects on the functional characteristics of the 5-HT3 receptor response (Fig. 2 and Table 1); it reduced the maximal current amplitude, shifted dose-response curves to the right and increased the rate of desensitisation. Hill co-efficients were unaltered.
Fig. 2.
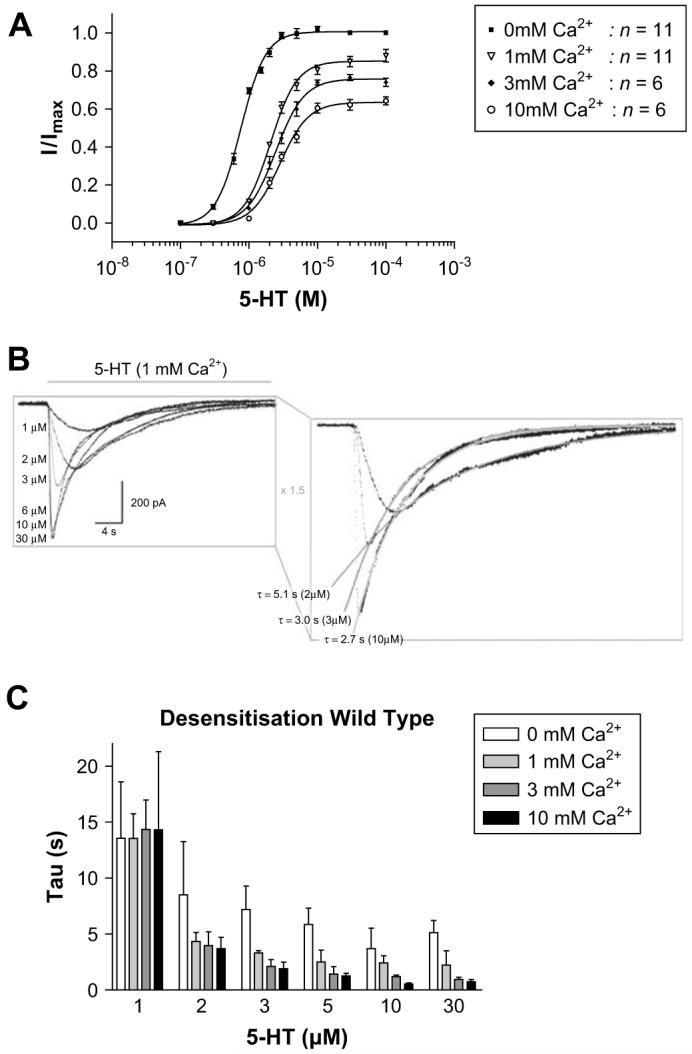
Wild type responses in the absence or presence of increasing concentrations of external calcium. Dose-response curves (A) displayed a calcium associated decrease in the peak current amplitude and the curves were shifted to higher 5-HT concentrations. (B) Example 5-HT3 receptor responses to varying concentrations of 5-HT and calcium. Current traces in response to varying concentrations of 5-HT (grey bar) in the presence of 1 mM calcium are shown (B, left), with exponential fits of desensitisation for the 2 μM, 3 μM and 10 μM 5-HT responses (B, right). The rate of desensitisation (C) increased as 5-HT concentrations were raised, and displayed additional increases in the presence of calcium. Values for desensitisation are mean ± SEM, n > 5.
Table 1.
Parameters derived from dose-response curves in the presence of varying external calcium concentrations
| Ext. Ca2+ (mM) | I/I max | log EC50 | EC50 (μM) | Hill slope | n |
|---|---|---|---|---|---|
| Wild type | |||||
| 0 | 1.01 ± 0.01 | −6.11 ± 0.02 | 0.8 | 2.39 ± 0.18 | 11 |
| 1 | 0.85 ± 0.01 | −5.68 ± 0.02 | 2.1 | 2.24 ± 0.19 | 11 |
| 3 | 0.76 ± 0.01 | −5.61 ± 0.02 | 2.5 | 2.08 ± 0.19 | 6 |
| 10 | 0.6 ± 0.02 | −5.55 ± 0.03 | 2.8 | 2.08 ± 0.26 | 6 |
| E213Q | |||||
| 0 | 0.99 ± 0.01 | −6.11 ± 0.02 | 0.8 | 2.45 ± 0.22 | 8 |
| 1 | 0.79 ± 0.01 | −5.56 ± 0.01 | 2.8 | 2.39 ± 0.16 | 10 |
| 3 | 0.59 ± 0.01 | −5.52 ± 0.02 | 3.0 | 2.42 ± 0.16 | 7 |
| 10 | 0.45 ± 0.02 | −5.10 ± 0.04 | 7.9 | 1.62 ± 0.19 | 6 |
| E215Q | |||||
| 0 | 1.00 ± 0.01 | −6.05 ± 0.01 | 0.9 | 2.98 ± 0.11 | 8 |
| 1 | 0.74 ± 0.01 | −5.82 ± 0.02 | 1.5 | 3.09 ± 0.39 | 7 |
| 3 | 0.71 ± 0.01 | −5.66 ± 0.01 | 2.2 | 2.54 ± 0.19 | 10 |
| 10 | 0.64 ± 0.02 | −5.48 ± 0.03 | 3.3 | 1.90 ± 0.24 | 7 |
| E277A/S297R | |||||
| 0 | 1.00 ± 0.01 | −5.77 ± 0.02 | 1.7 | 2.04 ± 0.16 | 4 |
| 1 | 1.00 ± 0.02 | −5.55 ± 0.04 | 2.8 | 1.64 ± 0.25 | 4 |
| 3 | 0.99 ± 0.03* | −5.72 ± 0.07 | 1.9 | 1.62 ± 0.30 | 6 |
| 10 | 0.96 ± 0.02* | −5.54 ± 0.04 | 2.9 | 1.35 ± 0.18 | 4 |
Data = mean ± SEM.
Sig. dif. (P < 0.05) to wild type at the same calcium concentration.
3.2. Identification of potential binding sites
Two adjacent and partially overlapping calcium binding sites were identified in the extracellular domain of the 5-HT3 receptor. Cations are typically coordinated by negatively charged amino acids, and a potential consensus calcium binding site was identified as a series of three glutamate residues at positions E213, E215 and E218 (Fig. 1A). A further potential site was identified by comparing sequence alignments and homology models of the 5-HT3 receptor (Thompson et al., 2005) with residues of a calcium bound AChBP crystal structure (PDB ID; 1i9b). In the AChBP structure, calcium was found to be coordinated by the side chains of D161 and D175, and the backbone of V176. These residues are homologous to D204, E218 and V219 in the 5-HT3 receptor (Fig. 1B).
3.3. E213Q currents
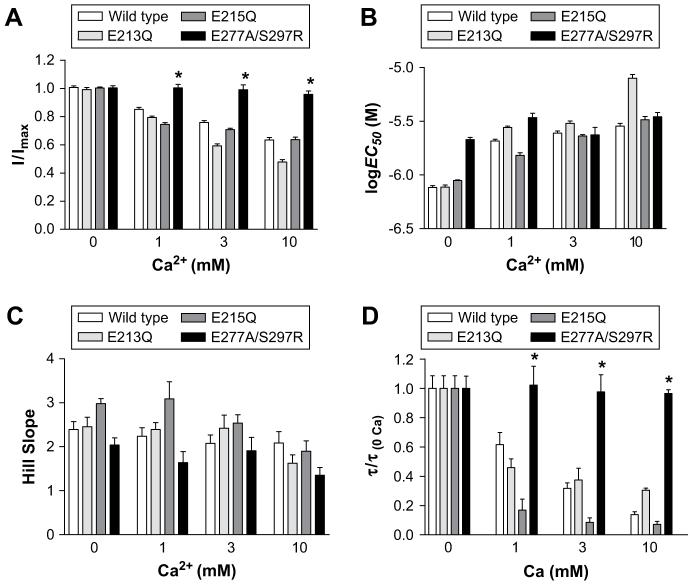
Dose-response curves yielded log EC50, Hill slope and peak current values that were not significantly altered when compared to wild type responses at the same calcium concentrations (Table 1, Figs. 3 and 6). E213Q mutants responded to 5-HT with rates of desensitisation that were ∼4-fold faster than wild type responses. To quantify calcium-dependent effects, values in the presence of varying calcium concentrations were normalised to values recorded at 0 mM and compared to wild type responses that had been normalised in the same way (Fig. 6D). This analysis showed that E213Q and wild type receptors displayed comparable relative increases in the rates of desensitisation in the presence of calcium.
Fig. 3.
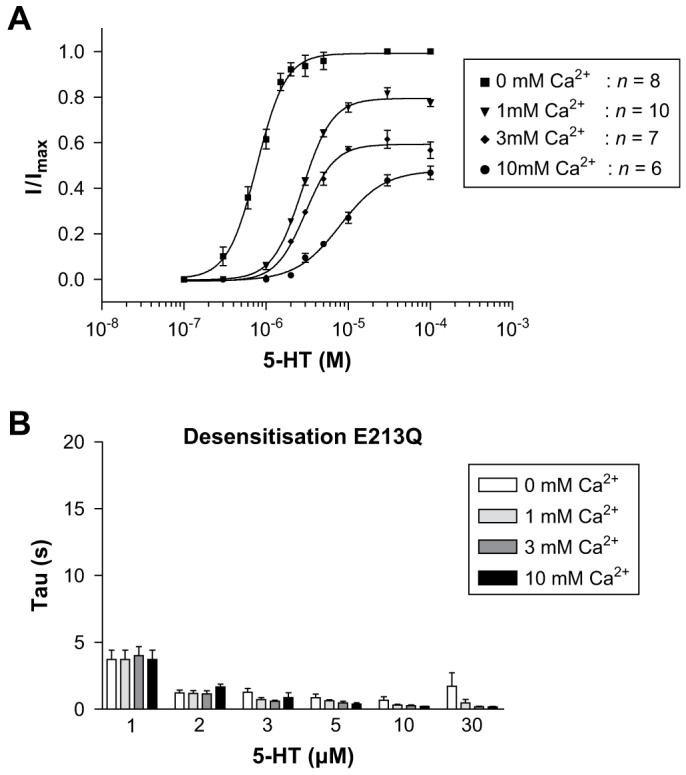
E213Q responses in the absence or presence of increasing concentrations of external calcium. Dose-response curves (A) displayed a calcium associated decrease in the peak current amplitude and the curves were shifted to higher 5-HT concentrations. The rate of desensitisation (B) increased as 5-HT concentrations were raised, and displayed additional increases in the presence of calcium. Values for desensitisation are mean ± SEM, n > 5.
Fig. 6.
A graphical comparison of peak current amplitude (A), pEC50 (B), Hill slopes and rates of desensitisation (D) in all of the mutants studied. For clarity, in D only the rates of desensitisation of the 10 μM 5-HT response are shown. All of the desensitisation rates in D have been normalised to the rate at 0 mM calcium, in order to allow for some of the increased baseline rates of desensitisation displayed by some mutants. Mean ± SEM values and sample size for A-C can be found in Table 1. Values for desensitisation are mean ± SEM, n > 5.
3.4. E215Q currents
Changes in the log EC50, Hill slope, peak current amplitude and rates of desensitisation were identical to wild type receptors across the range of calcium concentrations studied (Table 1, Figs. 4 and 6).
Fig. 4.
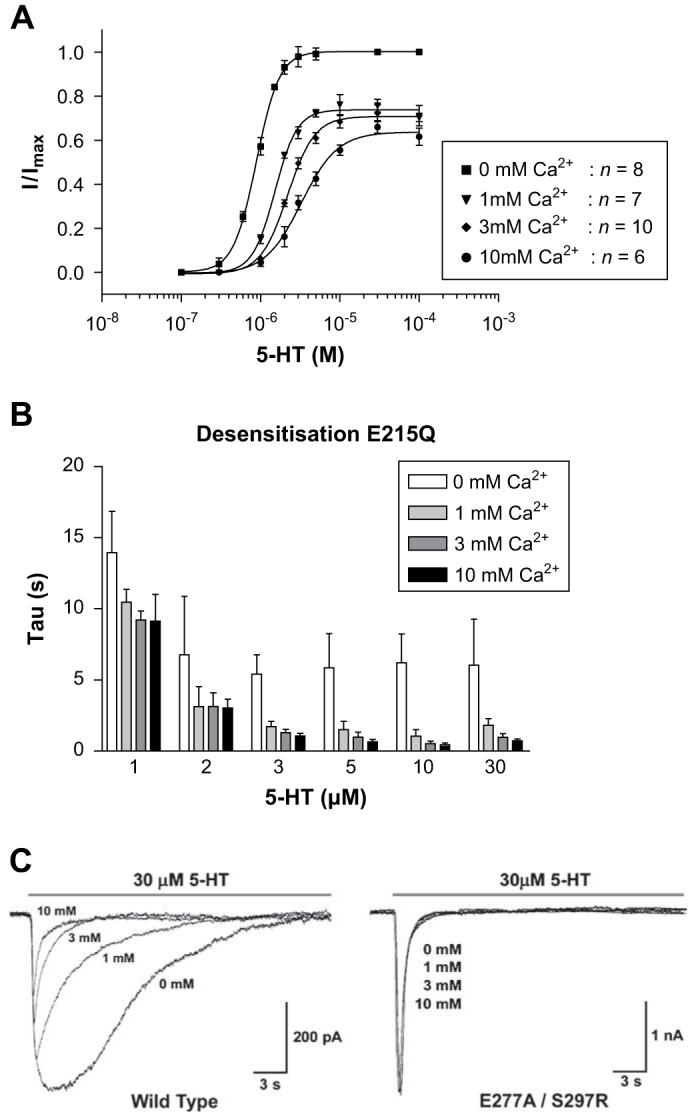
E215Q responses in the absence or presence of increasing concentrations of external calcium. Dose-response curves (A) displayed a calcium associated decrease in the peak current amplitude and the curves were shifted to higher 5-HT concentrations. The rate of desensitisation (B) increased as 5-HT concentrations were raised, and displayed additional increases in the presence of calcium. Values for desensitisation are mean ± SEM, n > 5. Calcium-dependent changes in the peak current amplitude and rates of desensitisation can be seen for wild type response (C, left), but were absent from the E277A/S297R mutant receptor (C, right).
3.5. Calcium impermeable mutant
We have previously found, using fluorometric calcium imaging, that combining an E277A substitution with S297R abolishes calcium permeability (Thompson and Lummis, 2003). Here we used this double mutant to find whether calcium modulation of the 5-HT3 receptor response was governed by regions other than the extracellular domain. When expressed in HEK 293 cells, the double mutant displayed responses that were similar to wild type receptors (Figs. 5 and 6). Examination of concentration-response curves at varying calcium concentrations showed that in contrast to the extracellular mutants, there were no significant changes in the log EC50, Hill slope or peak current amplitude with increasing concentrations of external calcium. E277A/S297R mutants responded to 5-HT with rates of desensitisation that were inherently faster than wild type responses, but when these rates were normalised to the values recorded at 0 mM calcium was found to have no effect (Figs. 4C and 6D). When E277A is expressed alone, we have previously shown that has a reduced level of calcium permeability (Thompson and Lummis, 2003). At E277A mutants, the modulatory effects of calcium were restored (data not shown).
Fig. 5.
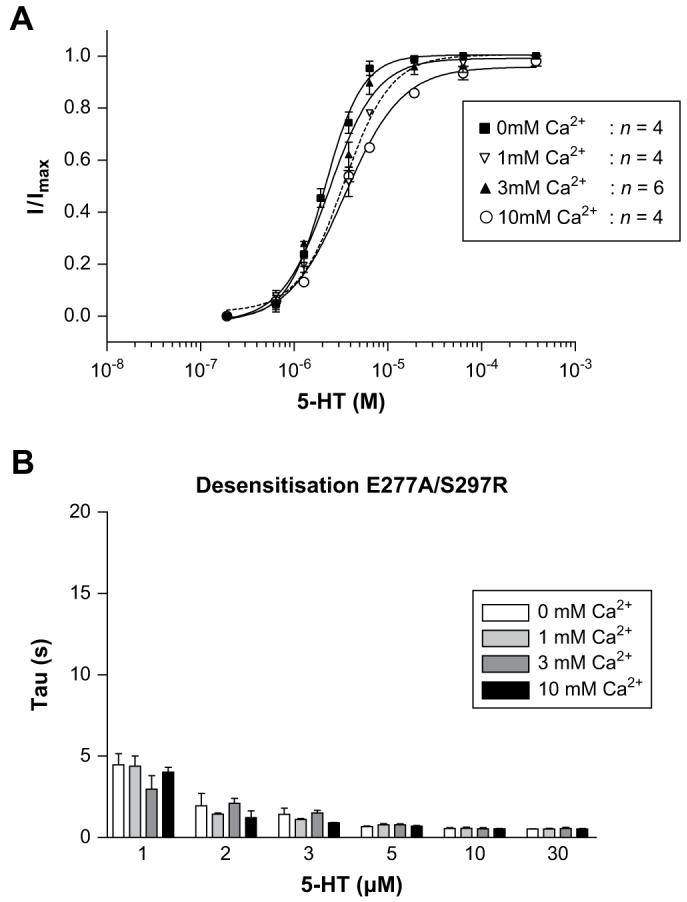
E277A/S297R responses in the absence or presence of increasing concentrations of external calcium. Dose-response curves (A) were not altered by the presence of increasing calcium concentrations. Unlike the other mutants studied here, there was no decrease in the peak current amplitude or shift in the dose-response curves with increasing calcium concentrations. Desensitisation (B) was unaltered by external calcium. Values for desensitisation are mean ± SEM, n > 5.
3.6. Non-functional mutants
Application of 1 mM 5-HT to patch-clamped HEK 293 cells transfected with D204N, E218Q or V219L mutant receptor cDNA was unable to elicit a response (n > 50 for each mutant).
3.7. [3H]Granisetron binding and immunofluorescence
Binding affinities (Kd) for wild type and mutant receptors were calculated using radiolabelled [3H]granisetron in the presence or absence of 10 mM calcium (Table 2). At 0 mM calcium, the Kd values of wild type, E213Q, E215Q, D204N, E277A/S297R and V219L mutants were similar. In the presence of 10 mM calcium, only wild type and E277A/S297R receptors displayed a small, but significant, increase in Kd. No radioligand binding was observed for E218Q mutants in either 0 mM or 10 mM calcium.
Table 2.
[3H]granisetron binding affinities of wild type and mutant 5-HT3 receptors
| Kd (nM) in 0 mM Ca2+ | Kd (nM) in 10 mM Ca2+ | |
|---|---|---|
| Wild type | 0.64 ± 0.08 (9) | 1.93 ± 0.03 (4)* |
| E213Q | 0.79 ± 0.21 (9) | 0.61 ± 0.17 (5) |
| E215Q | 0.41 ± 0.16 (7) | 0.44 ± 0.08 (6) |
| E218Q | NB (3) | NB (3) |
| D204N | 0.84 ± 0.17 (7) | 0.76 ± 0.09 (5) |
| E277A/S297R | 0.33 ± 0.04 (4) | 0.68 ± 0.04 (4)* |
| V219L | 0.82 ± 0.23 (6) | 1.14 ± 0.34 (3) |
Data = mean ± SEM, (n) NB = no binding.
Sig. dif. (P < 0.05) for the same mutant when compared at 0 and 10 mM external calcium.
Owing to the absence of radioligand binding for E218Q mutants, expression of this receptor was further examined using a 5-HT3 specific antibody (Spier et al., 1999). No immunofluorescence was observed in non-permeabilised cells, indicating that these receptors were unable to reach the cell surface (Fig. 7).
Fig. 7.
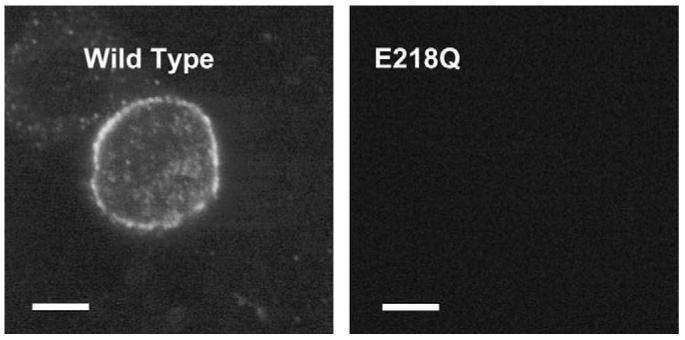
Confocal images of immunofluorescent 5-HT3 receptors labelled with pAb120 in non-permeabilised HEK 293 cells. Cells transfected with wild type cDNA displayed a conspicuous halo of fluorescence, indicating that receptors were expressed and had reached the cell surface. In contrast, E218Q mutant receptors were not detected. Scale bar = 25 μm.
4. Discussion
The aim of this study was to determine if residues that contribute to potential calcium binding residues in the extracellular domain of the 5-HT3 receptor are responsible for the modulatory effects of calcium. One potential calcium binding site consists of three charged glutamates (E213, E215 & E218). Neutralisation of the charge in E213Q mutants generates receptors with increased rates of desensitisation when compared to wild type receptors, although the calcium-mediated changes in the kinetics and current amplitude were similar to wild type. The functional properties of E215Q mutant receptors appeared identical to wild type receptors, but in contrast to wild type receptors, both E213Q and E215Q mutant receptors displayed no increase in [3H]granisetron binding affinity in the presence of calcium. E218Q mutants were not expressed. Three residues were also identified in a second potential binding site (D204, E218 & V219), one residue of which overlaps with the first binding site. Conserved substitutions at D204 and V219 revealed no change in [3H[granisetron binding affinity in the presence of calcium, but both these mutants were non-functional; this suggests that D204, E218 & V219 have an essential role in the structure, trafficking and/or function of the receptor. We also examined a calcium impermeant mutant receptor (E277A/S297R), which showed a calcium-dependent increase in Kd similar to wild type receptors, but displayed none of the calcium-dependent changes in peak current or rates of desensitisation. These data suggest that the effects on ligand binding are affected through sites in the extracellular domain, while calcium-dependent modulation of the 5-HT current response is mediated by calcium binding to a site either in the ion channel or within the intracellular region. This hypothesis is supported by a recent study which located residues at the extracellular end of the pore and in the intracellular region as important determinants of calcium permeability and conduction, although it was not reported whether these residues affected calcium-dependent changes in the peak current amplitude and desensitisation (Livesey et al., 2008).
External calcium is one of a number of ions which can modulate the activity of 5-HT3 (and many other) receptors, and it is believed to act through specific calcium binding sites (Peters et al., 1988; Ceresa and Limbird, 1994; Eddins et al., 2002b; Hu and Lovinger, 2005). However, accurately predicting the location of calcium binding sites is difficult as calcium may bind to a variety of protein structures, provided there are appropriately placed charged residues. For example, an in silico prediction of calcium binding sites in the α7 nACh receptor indicated five possible sites, only one of which was finally implicated in calcium-modulation of the response (Galzi et al., 1996). Based on the arrangement of residues along the linear sequence of this region, it was also suggested that they would form an EF hand. High resolution structures of AChBP and the nACh receptor have since shown that there is no EF hand, and that calcium is more likely to be coordinated by amino acid side-chains within loop F and the adjacent Cys-loop (Brejc et al., 2001). The sites within our own study are located in the same loop F/Cys-loop region as those in the α7 nACh receptor. Here we have shown that increasing the external calcium concentration from 0 mM to 10 mM causes a small, but significant increase in the Kd, for [3H]granisetron at wild type receptors. We observed similar calcium-dependent increases in Kd in the calcium impermeant E277A/S297R mutant receptor, but changes were absent from E213Q, E215Q, D204N and V219L mutant receptors. The change in ligand affinity may reflect calcium-dependent structural alterations in the F-loop, a region that is known to be important for ligand binding and which has been shown to coordinate calcium in AChBP (Niemeyer and Lummis, 2001; Thompson et al., 2006b; Brejc et al., 2001; Nishio et al., 1994). There have also been reports of competitive inhibition of α7 nACh receptors by monovalent cations, but in this study mutations in the adjacent β-strands also strongly affected calcium-mediated effects, indicating a further non-competitive mechanism (Akk and Auerbach, 1996; Le Novere et al., 2002; McLaughlin et al., 2006). Importantly, calcium-mediated changes at different Cys-loop receptors are varied, and the mechanism of inhibition at α7 nACh and 5-HT3 receptors are unlikely to be the same.
E213 and E215 are conserved in all homologues of 5-HT3A receptors and form a negatively charged region that could potentially assist in co-ordinating the binding of calcium. A sequence alignment shows that the residues responsible for coordinating calcium in AChBP (D161, D175 & V176) have similar counterparts in the 5-HT3 receptor (D204, E218 & V219) (Galzi et al., 1996; Brejc et al., 2001; Thompson and Lummis, 2006). Mutation of some of these residues (E213, E215, D204 and V219) resulted in loss of the calcium-mediated increase in Kd, suggesting a possible contribution of these residues to this increase, although it seems unlikely that the small effect observed in wild type receptors has a major physiological significance. However, the data reveal that E218Q mutants were non-functional, displayed no [3H]granisetron binding, and there was no cell surface immunolabelling, indicating that this residue is important for expression at the membrane. In addition, substitution of D204 or V219 produced non-functional receptors, although binding affinities for [3H]granisetron were similar to wild type receptors, suggesting that these residues do not affect the binding site, but play a role in receptor function, perhaps by affecting the structural integrity of a region that is involved in channel gating downstream of the binding site.
No calcium binding sites have yet been unequivocally identified in the 5-HT3 receptor, although it has been suggested that a site may exist within the pore or within the M3-M4 loop; there is a decrease in single channel conductance in the presence of calcium, and neutralisation of a conserved aspartate residue (adjacent to S297) that reduces calcium permeability, can eliminate calcium-mediated effects (Brown et al., 1998; Hu and Lovinger, 2005; Livesey et al., 2008). The reduction in the peak current and the non-surmountable shift in the dose-response curve that we observed would support this hypothesis, as these changes are consistent with a non-competitive mechanism. Interactions within the pore would be expected to be voltage-dependent, and although reports vary, there is evidence for this in 5-HT3 receptors expressed in Xenopus oocytes and in native cells (Eiselé et al., 1993; Maricq et al., 1991; McMahon and Kauer, 1997; Van Hooft and Wadman, 2003). Indeed, a mathematical model of the 5-HT3 receptor pore created by Van Hooft and Wadman, 2003 describes potential binding sites at channel residues 13′ and −4′. Binding in the ion channel has also been proposed for the α7 nACh receptor and it is notable that the M2 pore lining residues of the α7 nACh and 5-HT3 receptor are identical from the 13′ location down through to −4′ (Thompson and Lummis, 2006; Lyford et al., 2002; Eddins et al., 2002a).
4.1. Conclusions
We have mutated four charged amino acids and V219 in a region spanning residues 204-218 in the 5-HT3 receptor extracellular domain to determine their role in calcium-mediated effects on 5-HT3 receptor binding and function. Our results show that mutations to these residues can eliminate the calcium-dependent change in antagonist binding affinity observed in wild type receptors, and may indicate that these residues contribute to a calcium binding site, or introduce subtle structural changes that effect a more distal calcium binding site. Calcium-dependent modulation of the 5-HT current was unaffected by mutations at E213 and E215, while substituting E218, D204 and V219 prevented the formation of functional 5-HT3 receptors, suggesting a structural role for these residues. We speculate that this might be mediated by interactions with calcium, although there is currently no direct evidence for this in the 5-HT3 receptor. The modulatory effects of calcium upon peak current amplitude and kinetics could be abolished using a calcium impermeant mutant of the 5-HT3 receptor (E277A/S297R). These results suggest that residues within the extracellular domain may subtly influence calcium-dependent changes in ligand binding, but the major determinant of calcium-mediated modulation of the 5-HT3 receptor response is located within the channel or at an intracellular site.
Acknowledgments
This work was supported by a grant from the Wellcome Trust. S.C.R.L. is a Wellcome Trust Senior Research Fellow in Basic Biomedical Science.
Abbreviations
- HEK 293
human embryonic kidney cell line 293
- 5-HT
5-hydroxytryptamine (serotonin)
- 5-HT3-R
5-hydroxytryptamine receptor type 3
References
- Akk G, Auerbach A. Inorganic, monovalent cations compete with agonists for the transmitter binding site of nicotinic acetylcholine receptors. Biophysical Journal. 1996;70:2652–2658. doi: 10.1016/S0006-3495(96)79834-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Galzi JL, Devillers-Thiery A, Bertrand S, Changeux JP. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor. Proceedings of the National Academy of Science USA. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–726. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Brown AM, Hope AG, Lambert JJ, Peters JA. Ion permeation and conduction in a human recombinant 5-HT3 receptor subunit (h5-HT3A) Journal of Physiology. 1998;507:653–665. doi: 10.1111/j.1469-7793.1998.653bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceresa BP, Limbird LE. Mutation of an aspartate residue highly conserved among G-protein-coupled receptors results in nonreciprocal disruption of alpha 2-adrenergic receptor-G-protein interactions. A negative charge at amino acid residue 79 forecasts alpha 2A-adrenergic receptor sensitivity to allosteric modulation by monovalent cations and fully effective receptor/G-protein coupling. Journal of Biological Chemistry. 1994;269:29557–29564. [PubMed] [Google Scholar]
- Chen CA, Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988;6:632–638. [PubMed] [Google Scholar]
- Eddins D, Lyford LK, Lee JW, Desai SA, Rosenberg RL. Permeant but not impermeant divalent cations enhance activation of nondesensitizing alpha(7) nicotinic receptors. American Journal of Physiology - Cell Physiology. 2002a;282:C796–C804. doi: 10.1152/ajpcell.00453.2001. [DOI] [PubMed] [Google Scholar]
- Eddins D, Sproul AD, Lyford LK, McLaughlin JT, Rosenberg RL. Glutamate 172, essential for modulation of L247T alpha7 ACh receptors by Ca2+, lines the extracellular vestibule. American Journal of Physiology - Cell Physiology. 2002b;283:C1454–C1460. doi: 10.1152/ajpcell.00204.2002. [DOI] [PubMed] [Google Scholar]
- Eiselé J-L, Bertrand S, Galzi J-L, Devillers-Thiéry A, Changeux J-P, Bertrand D. Chimaeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature. 1993;366:479–483. doi: 10.1038/366479a0. [DOI] [PubMed] [Google Scholar]
- Galzi JL, Bertrand S, Corringer PJ, Changeux JP, Bertrand D. Identification of calcium binding sites that regulate potentiation of a neuronal nicotinic acetylcholine receptor. EMBO Journal. 1996;15:5824–5832. [PMC free article] [PubMed] [Google Scholar]
- Gill CH, Peters JA, Lambert JJ. An electrophysiological investigation of the properties of a murine recombinant 5-HT3 receptor stably expressed in HEK 293 cells. British Journal of Pharmacology. 1995;114:1211–1221. doi: 10.1111/j.1476-5381.1995.tb13335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XQ, Lovinger DM. Role of aspartate 298 in mouse 5-HT3A receptor gating and modulation by extracellular Ca2+ Journal of Physiology. 2005;568:381–396. doi: 10.1113/jphysiol.2005.092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard PC, Lummis SCR. Zn2+ enhancement of the recombinant 5-HT3 receptor is modulated by divalent cations. European Journal of Pharmacology. 2000;394:189–197. doi: 10.1016/s0014-2999(00)00143-6. [DOI] [PubMed] [Google Scholar]
- Hussy N, Lukas W, Jones KA. Functional properties of a cloned 5-hydroxytryptamine ionotropic receptor subunit: comparison with native mouse receptors. Journal of Physiology (Lond) 1994;481:311–323. doi: 10.1113/jphysiol.1994.sp020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Yakel JL. The 5-HT3 receptor channel. Annual Review of Physiology. 1995;57:447–468. doi: 10.1146/annurev.ph.57.030195.002311. [DOI] [PubMed] [Google Scholar]
- Jordan M, Schallhorn A, Wurm FM. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Research. 1996;24:596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley SP, Dunlop JI, Kirkness EF, Lambert JJ, Peters JA. A cytoplasmic region determines single-channel conductance in 5-HT3 receptors. Nature. 2003;424:321–324. doi: 10.1038/nature01788. [DOI] [PubMed] [Google Scholar]
- Kunkel TA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proceedings of the National Academy of Science USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Novere N, Grutter T, Changeux JP. Models of the extracellular domain of the nicotinic receptors and of agonist- and Ca2+-binding sites. Proceedings of the National Academy of Science USA. 2002;99:3210–3215. doi: 10.1073/pnas.042699699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey MR, Cooper MA, Deeb TZ, Carland JE, Kozuska J, Hales TG, Lambert JJ, Peters JA. Structural determinants of Ca2+permeability and conduction in the human 5-hydroxytryptamine type 3A receptor. Journal of Biological Chemistry. 2008;283:19301–19313. doi: 10.1074/jbc.M802406200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lummis SC, Sepulveda MI, Kilpatrick GJ, Baker J. Characterization of [3H]meta-chlorophenylbiguanide binding to 5-HT3 receptors in N1E-115 neuroblastoma cells. European Journal of Pharmacology. 1993;243:7–11. doi: 10.1016/0014-2999(93)90160-j. [DOI] [PubMed] [Google Scholar]
- Lyford LK, Lee JW, Rosenberg RL. Low-affinity Ca2+ and Ba2+ binding sites in the pore of alpha7 nicotinic acetylcholine receptors. Biochimica et Biophysica Acta. 2002;1559:69–78. doi: 10.1016/s0005-2736(01)00437-0. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- McLaughlin JT, Fu J, Sproul AD, Rosenberg RL. Role of the outer beta-sheet in divalent cation modulation of alpha7 nicotinic receptors. Molecular Pharmacology. 2006;70:16–22. doi: 10.1124/mol.106.023259. [DOI] [PubMed] [Google Scholar]
- McMahon LL, Kauer JA. Hippocampal interneurons are excited via serotonin-gated ion channels. Journal of Neurophysiology. 1997;78:2493–2502. doi: 10.1152/jn.1997.78.5.2493. [DOI] [PubMed] [Google Scholar]
- Niemeyer MI, Lummis SC. The role of the agonist binding site in Ca2+ inhibition of the recombinant 5-HT3A receptor. European Journal of Pharmacology. 2001;428:153–161. doi: 10.1016/s0014-2999(01)01251-1. [DOI] [PubMed] [Google Scholar]
- Nishio H, Negishi Y, Inoue A, Nakata Y. Differential effects of divalent cations on specific 3H-GR 65630 binding to 5-HT3 receptors in rat cortical membranes. Neurochemistry International. 1994;24:259–266. doi: 10.1016/0197-0186(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Peters JA, Hales TG, Lambert JJ. Divalent cations modulate 5-HT3 receptor-induced currents in N1E-115 neuroblastoma cells. European Journal of Pharmacology. 1988;151:491–495. doi: 10.1016/0014-2999(88)90550-x. [DOI] [PubMed] [Google Scholar]
- Quirk PL, Rao S, Roth BL, Siegel RE. Three putative N-glycosylation sites within the murine 5-HT3A receptor sequence affect plasma membrane targeting, ligand binding, and calcium influx in heterologous mammalian cells. Journal of Neuroscience Research. 2004;77:498–506. doi: 10.1002/jnr.20185. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A Laboratory Manual. Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Spier AD, Wotherspoon G, Nayak SV, Nichols RA, Priestley JV, Lummis SCR. Antibodies against the extracellular domain of the 5-HT3 receptor label both native and recombinant receptors. Molecular Brain Research. 1999;71:369. doi: 10.1016/s0169-328x(99)00055-8. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Lummis SC. A single ring of charged amino acids at one end of the pore can control ion selectivity in the 5-HT3 receptor. British Journal of Pharmacology. 2003;140:359–365. doi: 10.1038/sj.bjp.0705424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Lummis SCR. The relationship between structure and function in the 5-HT3 receptor: the transmembrane domain. In: Arias HR, editor. Biological and Biophysical Aspects of Ligand-Gated Ion Channel Receptor Superfamilies. Research Signpost; Kerala, India: 2006. pp. 155–170. [Google Scholar]
- Thompson AJ, Price KL, Reeves DC, Chan SL, Chau PL, Lummis SC. Locating an antagonist in the 5-HT3 receptor binding site using modeling and radioligand binding. Journal of Biological Chemistry. 2005;280:20476–20482. doi: 10.1074/jbc.M413610200. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Zhang L, Lummis SCR. The 5-HT3 Receptor. In: Roth BL, editor. The Serotonin Receptors: From Molecular Pharmacology to Human Therapeutics. Humana Press; New Jersey, USA: 2006a. pp. 439–457. [Google Scholar]
- Thompson AJ, Padgett CL, Lummis SC. Mutagenesis and molecular modeling reveal the importance of the 5-HT3 receptor F-loop. Journal of Biological Chemistry. 2006b;281:16576–16582. doi: 10.1074/jbc.M601265200. [DOI] [PubMed] [Google Scholar]
- Turchin A, Lawler JF., Jr. The primer generator: a program that facilitates the selection of oligonucleotides for site-directed mutagenesis. Biotechniques. 1999;26:672–676. doi: 10.2144/99264st02. [DOI] [PubMed] [Google Scholar]
- Van Hooft JA, Wadman WJ. Ca2+ ions block and permeate serotonin 5-HT3 receptor channels in rat hippocampal interneurons. Journal of Neurophysiology. 2003;89:1864–1869. doi: 10.1152/jn.00948.2002. [DOI] [PubMed] [Google Scholar]