Case
Ms. L, a 32-year-old G5 P2 A2 (gravida 5, para 2, abortions 2) woman, is admitted to hospital because of premature rupture of membranes at an estimated 34 weeks of gestation. She has had only intermittent prenatal care and no prenatal tests. This is an unplanned pregnancy, and she is not sure of the time of conception. Her 2 older children have been in foster care since birth, because child welfare authorities assessed her ability to parent as inadequate at the time; 1 child is in the process of adoption. Although child welfare authorities have documented a history of problem drinking, the woman denies any drinking during the current pregnancy. Her physical and neurologic examinations are unremarkable. Although she has sought routine prenatal care only periodically during her pregnancy, she describes developing a desire to parent this child in recent weeks. She denies any “recent” use of alcohol or cocaine, but will not be more specific. Informed consent for drug testing is obtained; no alcohol is detected in her blood, and a urine toxic screen is negative for cocaine, heroin, amphetamines and cannabinoids. Her hemoglobin level is 130 g/L, leukocyte count is 8.5 х 109/L, mean corpuscular volume is 82 fL, electrolytes and creatinine levels are normal, and gamma glutamyl transferase level is 75 (normally < 45) U/L. The woman's baby is delivered vaginally. His Apgar score is 8 at 1 minute and 10 at 5 minutes. His birth weight is 1.1 kg (below the third percentile for age) and head circumference 28.5 cm (fifth percentile for age). Findings from the physical and neurologic examinations of the baby are unremarkable, and he does not require assisted ventilation. Breastfeeding is started successfully.
What aspects of this case make you concerned that this child is at risk of fetal alcohol spectrum disorder (FASD)? What additional screens or laboratory tests might further indicate the possibility of FASD? What physical and neurodevelopmental deficits might present later in life if the child has FASD?
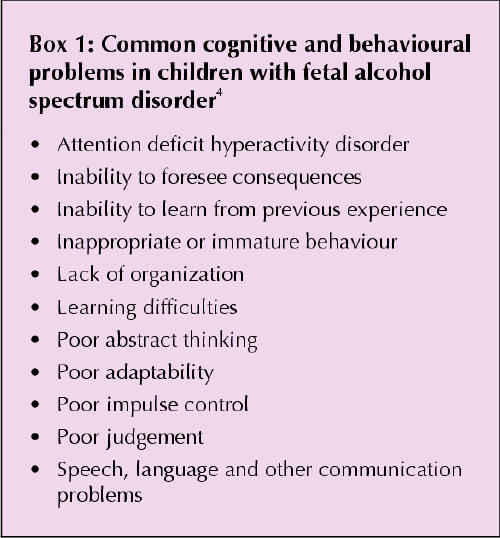
At least half of Canadian and American women drink socially1 and half of all pregnancies are unplanned;2 thus, an estimated quarter of all newborns (about 100 000 infants a year in Canada) are exposed to some alcohol during early gestation. Fetal alcohol syndrome (FAS) was originally described as intrauterine and postnatal growth retardation (below the third percentile); specific facial changes (short palpebral fissures [2 standard deviations below normal for age], smooth philtrum and thin vermilion border of the upper lip); and adverse brain effects (mainly mental retardation).3,4 However, during the late 1970s and 1980s, it became apparent that this classic triad of symptoms was relatively uncommon in the offspring of heavy drinkers (occurring in only 4%–5%).5 More often (in 30%–40% of children of heavy drinkers), the brain injury manifests as mild rather than severe cognitive dysfunction and a more subtle and complex pattern of neurobehavioural problems (Box 1) with or without physical features of classic FAS. The broader term “fetal alcohol spectrum disorder” (FASD) has been coined in recent years to encompass the wide range of adverse fetal effects of ethanol — from the classic FAS to its more partial presentations. Overall, it is estimated that FASD affects up to 9.1 of every 1000 babies born in the United States and Canada.6,7
Box 1.
Identifying pregnant women who are problem drinkers
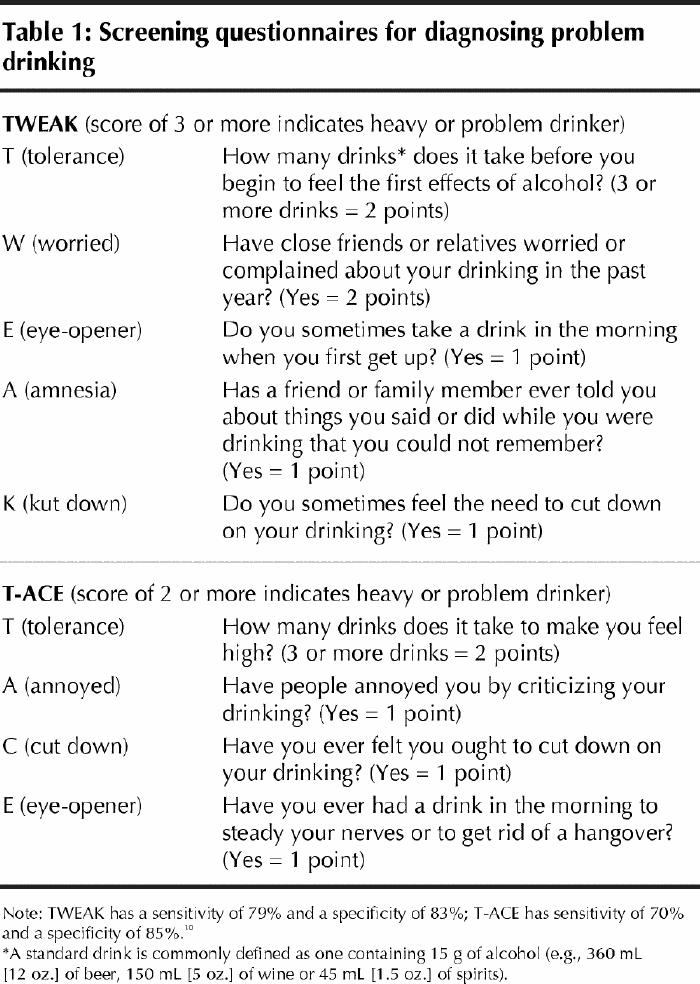
Although safe alcohol intake levels during pregnancy are difficult to define, questionnaires can identify women who are at greater risk of having a child with FASD.8 Two such questionnaires (TWEAK and T-ACE) can be used to screen all pregnant women to identify those at risk (Table 1).9 The CAGE questionnaire is less appropriate for screening pregnant women, because it is less sensitive than TWEAK and T-ACE.9,10 (Maternal and infant laboratory markers of possible antenatal alcohol use are discussed later.)
Table 1
Defining the spectrum of disabilities
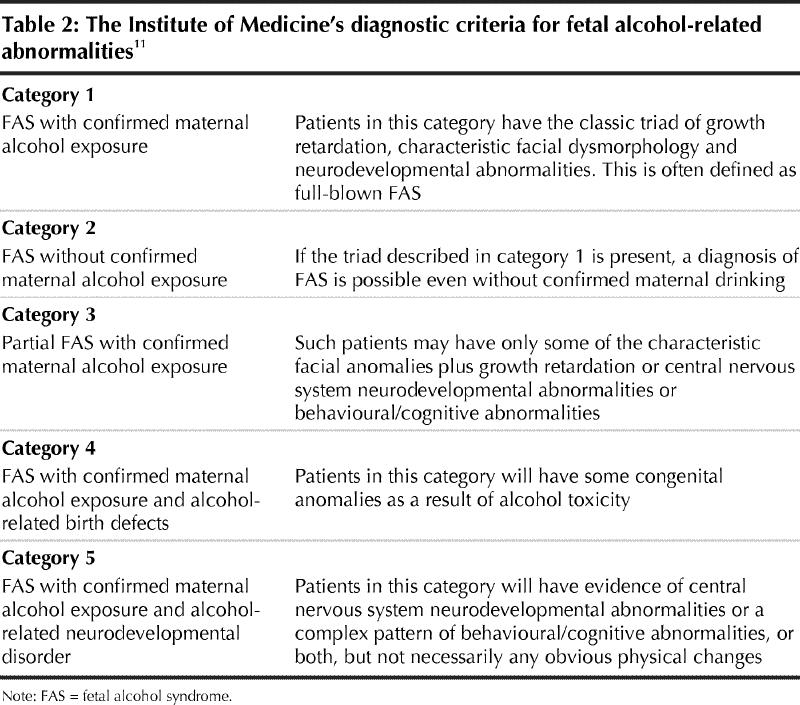
In 1996 the Institute of Medicine defined several categories of fetal alcohol pathology, from full-blown cases (i.e., history of maternal drinking, intrauterine and postnatal growth retardation, typical facial changes and brain dysfunction) to cases in which there are neurodevelopmental effects but no physical changes.11 The institute permits diagnosis of FAS only when there is evidence of maternal drinking, except when the pathognomonic facial changes are apparent, because other conditions do not elicit such changes. The institute's categories cover the range of so-called “primary disabilities” seen in children with FASD (Table 2).
Table 2
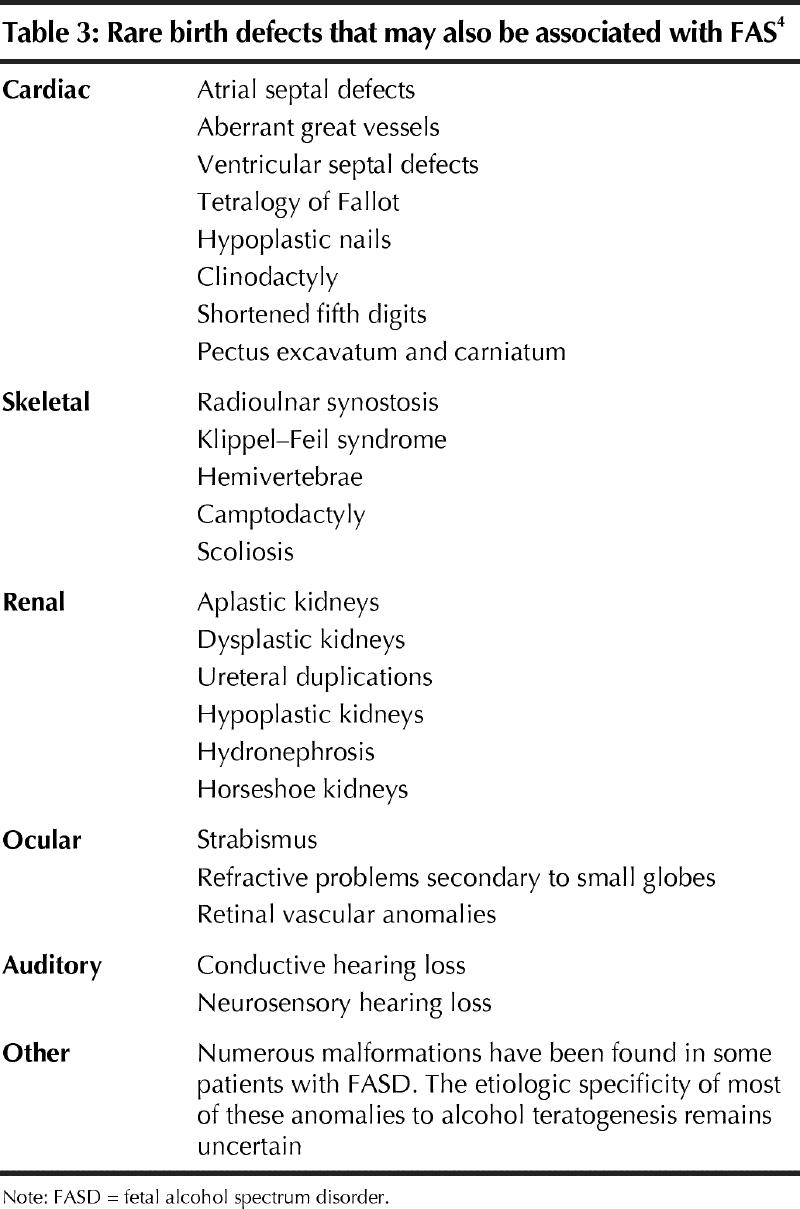
Facial signs of FAS are most evident between 8 months and 8 years of age; hence, their vague appearance in the newborn should not be misinterpreted. For the same reason, when examining an adolescent or adult, earlier childhood pictures may be useful to uncover facial features that may have disappeared. It is also important to evaluate photographs taken when the child is not smiling, because smiling leads to narrowing of the upper lip and thinning of the philtrum. Other physical malformations may also occur in FASD, although the frequency and occurrence of these malformations is poorly defined (Table 3).
Table 3
The complex neurobehavioural problems may manifest themselves insidiously. For example, attention deficit hyperactivity disorder (ADHD), diagnosed in about half of children with FASD, becomes apparent usually at or near school age. Frequently, these children exhibit impaired social adaptive ability and impaired executive functions, which reflect prefrontal cortex dysfunction. Secondary disabilities9 are those believed to occur as a result of primary disabilities and are revealed as mental health problems, school dropout and trouble with the law (Table 4).
Table 4

Coles and colleagues12 found that, among the factors protecting against severity of outcomes of FAS, the age of diagnosis is important. Early diagnosis leads to improved management of these children and, hence, decreases the burden of secondary disabilities. Although many children with extensive prenatal alcohol exposure appear normal at birth, they may later be identified with many subtle neurobehavioural deficits not apparent in their early years.
Although overall rates of FASD among heavy drinkers may be relatively low, it is of substantial clinical importance that a woman who has given birth to an affected child has a high risk of having subsequent children also affected if she continues to drink.13
Counselling of pregnant women who are light drinkers
Counselling of women who drank small amounts of alcohol before realizing they had conceived is a complex but important task, because an estimated 25% of all pregnant Canadians fall into this group. About 90% of Canadian women know that alcohol “is not good for the unborn baby” and many believe that even brief and mild exposure to alcohol poses fetal risk.14 Two recent meta-analyses failed to show adverse fetal effects after mild social drinking (up to several drinks a week) by nonalcoholic women.15,16 In contrast, recent studies suggest significant differences in aggressive and externalized behaviour in 6-year-old children with prenatal exposure to as little as 1 alcoholic beverage a week compared with an unexposed control group.17 It is clear, however, that substantial prenatal alcohol exposure — either heavy daily or weekend binge drinking — is the rule in children diagnosed with classic FAS.13
For physicians, it is a complex task to counsel women to abstain from any drinking during pregnancy while reassuring those who were inadvertently and mildly exposed that, although the threshold of ethanol embryopathy is unknown, there is currently no widely established evidence of fetal risk with such exposure. This “double message” is often poorly understood by the public. Nevertheless, it is unclear what, if any, threshold exists, and the best advice is against the use of alcohol during pregnancy. For women who appear disproportionately worried after having consumed only very small amounts of alcohol before realizing they were pregnant, it is important to remember that more than half the babies of heavy drinkers appear to be spared adverse effects. The reason why one child appears so dramatically affected and another, with similar exposure, appears unaffected is unknown, but it may be due to pharmacogenetic variability in ethanol metabolism, placental transfer or fetal handling of the molecule.13
Laboratory markers of maternal drinking
Several laboratory tests can help to either support or refute the historical information obtained from screening questionnaires. Elevated liver enzymes (specifically isolated gamma glutamyl transferase [GGT] elevations) and a mean corpuscular volume of less than 98 fL may indicate chronic alcoholism.
In 1998 Stoler and colleagues18 found that the most sensitive combination of laboratory test results predicting maternal alcohol dependence are an elevated GGT level, a mean corpuscular volume of less than 98 fL, a high carbohydrate-deficient transferrin level and a high whole-blood-associated acetaldehyde level.
Women who use other drugs (cocaine, heroin and barbiturates) while pregnant are at increased risk of also abusing alcohol; thus, testing women and infants for these drugs can also identify children at risk of FASD.19
Although it is often in the best interests of the child to identify mothers who have addiction problems, informed consent is still required before testing the woman or her child. If the patient does not understand the potential ramifications of a positive test result, then informed consent has not been obtained.20
Because of the short elimination time of many drugs of abuse, analysis of neonatal blood or urine is likely to miss most cases. The hair babies are born with develops during the last trimester, whereas meconium begins to develop in the second trimester of pregnancy. Studies have showed that neonatal hair and meconium are sensitive biologic markers of in utero exposure to cocaine and other drugs of abuse, including heroin, marijuana and barbiturates.4 In addition to documenting in utero exposure late in pregnancy, a positive neonatal hair test result indicates drug abuse long after pregnancy was diagnosed and, hence, is a marker of maternal addiction. Because there is evidence of health risks for infants cared for by addicted mothers, such information is crucial in ensuring the safety of the infant and in mobilizing medical and social assistance.
A novel meconium test developed at The Hospital for Sick Children, Toronto, makes it possible to detect maternal drinking in the second trimester of pregnancy (Fig. 1).21,22 Although its exact clinical role is still being determined, this test can detect FAEEs in a sample of neonatal meconium obtained within the first 2–3 days of life. It has a sensitivity of 100% and a specificity of 98% for identifying children of heavy alcohol drinkers.22
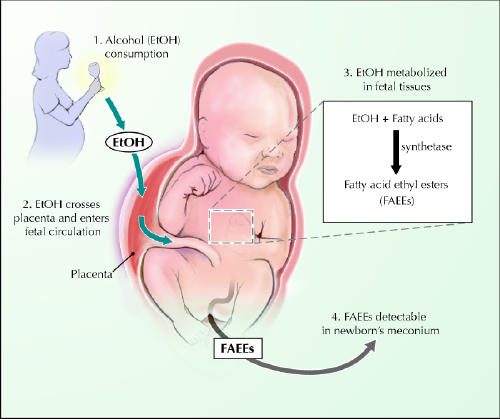
Fig. 1: Neonatal meconium assay for fatty acid ethyl esters (FAEEs). Most of the body's load of ethanol is eliminated after enzyme oxidation to acetaldehyde and water. However, ethanol is also esterified with fatty acids to FAEEs, which can be found in adult blood and tissue. It is now evident that FAEEs can be found in neonatal meconium and may reflect maternal drinking in pregnancy. Photo: Chesley Sheppard
The case revisited
There are several initial clues leading to the suspicion of Ms. L's problem drinking, including her previous history of drinking while pregnant. She has a positive TWEAK score (5 points). Her elevated GGT level and low-normal mean corpuscular volume may also be clues to a problem with chronic alcohol abuse. Although the results of her initial toxicology screen are negative, this is not surprising given the short elimination time of many of these substances in the body. Ms. L consents to additional testing of her baby after birth, including hair and meconium tests. Hair analysis reveals the metabolite of cocaine, benzoylecgonine, at 5 ng/g of hair (limit of detection 0.5 ng/g). The meconium test is positive for FAEEs at 40 nmol/g (a negative result is 2 nmol/g or less22) and for benzoylecgonine at 1.2 μg/g (detection limit is 50 ng/g). Analysis of the mother's hair is positive for cocaine and its metabolites (cotinine 30 ng/g and benzoylecgonine 42 ng/g).
Thus, according to the history and laboratory evidence, the baby meets many of the criteria for FASD: confirmed alcohol exposure and physical features of FASD, including intrauterine growth retardation, small head circumference and short palpebral fissure. FAS is diagnosed. The baby was also exposed in utero to cocaine, which is associated with potential teratogenic effects, including microencephaly.23
At delivery, it is too early to assess whether the baby has any neurodevelopmental effects of the prenatal alcohol exposure, unless these are heralded by gross structural brain anomalies (noted through diagnostic imaging) or severe neurologic impairment. He has an estimated 4%–5% risk of classic FAS (category 1 of the Institute of Medicine classification) and a 30%–40% chance of having an alcohol-related neurodevelopmental disorder (category 5 of the institute's classification). In addition to care by his primary care providers, this baby could benefit from close and frequent neurodevelopmental follow-up, optimally by a multidisciplinary team consisting of a developmental psychologist, a pediatrician and an occupational therapist (other professionals may include a speech and language pathologist and a physiotherapist). It is also important to address the mother's alcohol dependence by offering her care options, such as addiction treatment, mental health therapy and support.24 While being sensitive to cultural, spiritual, religious and emotional needs of the mother, it is important to ensure that she is offered effective contraception while she is struggling with her addiction program.
Conclusion
Thirty-five years after FAS was first described,25 the diagnosis of FASD continues to present major challenges, some of which are reflected in this practice review. There is often no history of maternal drinking, and when this is coupled with a lack of pathognomonic physical features or neurobehavioural information, many cases cannot be diagnosed with reasonable conviction. Moreover, among offspring of alcohol-dependent women, there is a well-defined clustering of neuropsychiatric morbidity, which may not necessarily reflect intrauterine effects of ethanol. Last, but not least, diagnostic services for FASD are scarce, and most physicians do not feel well informed or prepared to diagnose FASD.8 Providing physicians with tools to diagnose and manage FASD can have an impact on the most preventable form of neurodevelopmental deficit in Canadian children.
Acknowledgments
Gideon Koren is a senior scientist of the Canadian Institutes for Health Research (CIHR). Some of the work presented here was supported by grants from the CIHR, the Hospital for Sick Children's Translational Research Competition and Physicians Services Inc., Toronto, Ont.
Footnotes
This article has been peer reviewed.
Competing interests: None declared.
Correspondence to: Dr. Gideon Koren, Division of Clinical Pharmacology and Toxicology, The Hospital for Sick Children, 555 University Ave., Toronto ON M5G 1X8; fax 416 813-7562; gkoren@sickkids.ca
References
- 1.Floyd RL, Decoufle P, Hungerford DW. Alcohol use prior to pregnancy recognition. Am J Prev Med 1999;17(2):101-7. [DOI] [PubMed]
- 2.Martin JA, Park MM, Sutton PD: Births: preliminary data for 2001. Natl Vital Stat Rep 2002;50(10):1-20. [PubMed]
- 3.Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet 1973;2:999-1001. [DOI] [PubMed]
- 4.Koren G, Nulman I. The Motherisk guide to diagnosing fetal alcohol spectrum disorder. Toronto: The Hospital for Sick Children; 2002.
- 5.Alcohol use among women of childbearing age — United States, 1991–1999. MMWR Morb Mortal Wkly Rep 2002;51(13):273-6. [PubMed]
- 6.Williams RJ, Odaibo FS, McGee JM. Incidence of fetal alcohol syndrome in northeastern Manitoba. Can J Public Health 1999;90(3):192-4. [DOI] [PMC free article] [PubMed]
- 7.Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, et al. Incidence of fetal alcohol syndrome and prevalence of alcohol related neurodevelopmental disorder. Teratology 1997;56(5):317-26. [DOI] [PubMed]
- 8.Nevin AC, Parshuram C, Nulman I, Koren G, Einarson A. A survey of physicians' knowledge regarding awareness of maternal alcohol use and the diagnosis of FAS. BMC Fam Pract 2001;3(1):2. [DOI] [PMC free article] [PubMed]
- 9.Bradley KA, Boyd-Wickizer J, Powell SH, Burman ML. Alcohol screening questionnaires in women: a critical review. JAMA 1998;280(2):166-71. [DOI] [PubMed]
- 10.Chang G, Wilkins-Haug L, Berman S, Goetz MA, Behr H, Hiley A. Alcohol use and pregnancy: improving identification. Obstet Gynecol 1998;91(6):892-8. [DOI] [PubMed]
- 11.Committee to Study Fetal Alcohol Syndrome, Division of Biobehavioral Sciences and Mental Disorders, Institute of Medicine. Fetal alcohol syndrome: diagnosis, epidemiology, prevention, and treatment. Washington: National Academy Press; 1996.
- 12.Coles CD, Kable JA, Drews-Botsch C, Falek A. Early identification of risk for effects of prenatal alcohol exposure. J Stud Alcohol 2000;61(4):607-16. [DOI] [PubMed]
- 13.Abel EL. Fetal alcohol abuse syndrome. New York: Plenum Press; 1998.
- 14.Koren G, Koren T, Gladstone J. Mild maternal drinking and pregnancy outcome: preceived versus true risks. Clin Chim Acta 1996;246(1-2):155-62. [DOI] [PubMed]
- 15.Polygenis D, Wharton S, Malmberg C, Sherman N, Kennedy D, Koren G, et al. Moderate alcohol consumption during pregnancy and the incidence of fetal malformations: a meta-analysis. Neurotoxicol Teratol 1998;20(1):61-7. [DOI] [PubMed]
- 16.Makarechian N, Agro K, Devlin J, Trepanier E, Koren G, Einarson T. The association between moderate alcohol consumption during pregnancy and spontaneous abortion, stillbirth and premature birth: a meta-analysis. Can J Clin Pharmacol 1998;5:169-76.
- 17.Sood B, Delaney-Black V, Covington C, Nordstrom-Klee B, Ager J, Templin T, et al. Prenatal alcohol exposure and childhood behaviour at age 6 to 7 years: I. dose-response effect. Pediatrics 2001;108(2):E34. [DOI] [PubMed]
- 18.Stoler JM, Huntington KS, Peterson CM, Peterson KP, Daniel P, Aboagye KK, et al. The prenatal detection of significant alcohol exposure with maternal blood markers. J Pediatr 1998;133(3):346-52. [DOI] [PubMed]
- 19.Klein J, Chan D, Karaskov T, Koren G. Prevalence of prenatal alcohol and illicit substance exposure in neonates — assessment by meconium analysis [abstract]. Ther Drug Monit 2003;25(4):490.
- 20.Etchells E, Sharpe G, Walsh P, Williams JR, Singer PA. Bioethics for clinicians: 1. Consent. CMAJ 1996;155(2):177-80. [PMC free article] [PubMed]
- 21.Bearer CF, Lee S, Salvator AE, Minnes S, Swick A, Yamashita T, et al. Ethyl linoleate in meconium: a biomarker for prenatal ethanol exposure. Alcohol Clin Exp Res 1999;23(3):487-93. [PMC free article] [PubMed]
- 22.Chan D, Bar-Oz B, Pellerin B, Paciorek C, Klein J, Kapur B, et al. Population baseline of meconium fatty acid ethyl esters among infants of nondrinking women in Jerusalem and Toronto. Ther Drug Monit 2003;25(3):271-8. [DOI] [PubMed]
- 23.Addis A, Moretti ME, Ahmed Syed F, Einarson TR, Koren G. Fetal effects of cocaine; an updated meta-analysis. Reprod Toxicol 2001;15(4):341-69. [DOI] [PubMed]
- 24.Grant TM, Ernst CC, Streissguth AP. An intervention with high-risk mothers who abuse alcohol and drugs: the Seattle Advocacy Model. Am J Public Health 1996;86(12):1816-7. [PubMed]
- 25.Lemoine P. The history of alcoholic fetopathies. J FAS Int 2003;1:e2. [PubMed]


