Abstract
Unrelated developmental neurotoxicants nevertheless converge on common functional and behavioral outcomes. We used PC12 cells, a model of neuronal development, to explore similarities and differences for organophosphate pesticides (chlorpyrifos, diazinon), an organochlorine pesticide (dieldrin) and a metal (Ni2+), focusing on transcriptional profiles related to their differentiation into acetylcholine, dopamine and norepinephrine phenotypes. Agents were introduced at 30 μM for 24 or 72 hr, treatments devoid of cytotoxicity. Using microarrays, we examined the mRNAs encoding the proteins involved in neurotransmitter biosynthesis, storage, and degradation, along with the complete panoply of receptors for each transmitter. All three pesticides evoked concordant patterns of effects on genes involved in neural growth and neurite extension, with a distinctly different pattern for Ni2+. All four toxicants promoted differentiation into the dopamine phenotype at the expense of the acetylcholine phenotype, involving separable effects of each agent on the various gene families; however, there were major differences in the ability of each to promote or repress the norepinephrine phenotype. Chlorpyrifos and diazinon, although displaying many similarities in their transcriptional profiles, also showed major disparities in keeping with their known differences in synaptic and behavioral outcomes after neonatal exposures to these agents in vivo. Surprisingly, there were closer similarities among diazinon, dieldrin and Ni2+ than for each agent to chlorpyrifos. Our results illustrate how cell culture systems, combined with microarray technology, can screen for developmental neurotoxicants, serving as a model for alternative approaches to the detection and characterization of the impact of exogenous chemicals on brain development.
Keywords: Acetylcholine, Catecholamines, Chlorpyrifos, Diazinon, Dieldrin, Dopamine, Metal neurotoxicity, Microarrays, Neuronal differentiation, Neurotoxicity, Nickel, Norepinephrine, Organochlorine insecticides, Organophosphate insecticides, PC12 cells
Introduction
Rather than following a preordained fate, developing neurons display plasticity of the choice of neurotransmitter phenotypes, so that alterations of synaptic activity and expression of neurotrophic factors can influence the “wiring” of developing neuronal circuits [5,12,13,27]. Consequently, exposure to environmental contaminants that promote or interfere with synaptic activity or expression/function of neurotrophins can result in miswiring, leading to neurobehavioral anomalies. In that manner, otherwise unrelated agents might converge on common endpoints by augmenting or impairing neural activity, or by eliciting similar alterations at the level of trophic factors. The organophosphate pesticide, chlorpyrifos, provides a prime example of how these events take place. Chlorpyrifos exposure in the fetus or neonate differentially activates and suppresses acetylcholine (ACh) and monoamine systems [48,49,51], and also has profound effects on expression and function of several families of neurotrophic factors [10,11,39,62-64]; this agent evokes inappropriate choices of neurotransmitter phenotypes in the developing brain, producing mismatches between presynaptic innervation and postsynaptic target receptors [1,2,4,23,31,37,51,54,57,59-61,65].
The developmental neurotoxicity of the organophosphates depends on mechanisms unrelated to their shared property as cholinesterase inhibitors [16,20,43,48,49,51], and consequently, there are major differences in the outcomes from exposure to agents such as diazinon or parathion as compared to chlorpyrifos [32,44,47,52,53,55,57,58,60,62,63,66,69,70]. In several recent studies, we examined the similarities and differences in the effects on trophic factors among developmental neurotoxicants within the organophosphate class as well as other classes [62-64] and found major disparities in the effects of chlorpyrifos and diazinon that could account for dissimilar outcomes; but in addition, we found surprising concordance between the effects of diazinon and the organochlorine pesticide, dieldrin [57,64]. In the current study, we explore whether these underlying effects on trophic factors dictate the differences and similarities in developmental decision-making directed toward neurodifferentiation and neurotransmitter phenotype. We chose to study PC12 cells, a standard in vitro model for neuronal development [68] that has already been shown to mimic the mechanisms and outcomes underlying organophosphate-induced developmental neurotoxicity in rodent models of neonatal exposure [7,8,21,22,24,25,31,32,38,41,44,45,56,57,63,64,67,71]. With the introduction of nerve growth factor (NGF), PC12 cells differentiate to form neuritic projections and the phenotypic characteristics of ACh and catecholamine (CA) neurons, the latter primarily involving dopamine, and to a lesser extent, norepinephrine [26,67,68]. Besides the organophosphates and dieldrin, we also evaluated the effects of a metal, Ni2+, for contrast with the pesticides, since we previously found that chlorpyrifos, diazinon, dieldrin and Ni2+ all promote the dopamine phenotype at the expense of the ACh phenotype, but likely through different underlying mechanisms [31,57]. Here, we examined the transcriptional profiles of the entire family of genes related to these phenotypes to reveal the underlying events and to identify the mechanisms that drive the similarities and differences among developmental neurotoxicants.
Aside from their importance in testing our hypothesis, all four agents studied here appear on the registry of Superfund Chemicals [74] and thus represent significant environmental concerns. For diazinon, exposures of inner-city women during pregnancy are comparable to those seen with chlorpyrifos [76]. Organochlorines such as dieldrin produce fetal neural damage [75], in part through their interaction with GABAA receptors [14] but also through other mechanisms such as oxidative stress [34,35]. Because PC12 cells do not express GABAA receptors [28,72], any similarities in effects between dieldrin and other agents will perforce reflect these additional mechanisms which are more likely to represent convergent targets for otherwise unrelated chemicals. Nickel compounds readily cross the placenta and accumulate in fetal tissues, including the brain, at concentrations (up to 2 μg/g) comparable to that of lead [15], greatly exceeding maternal levels [30]. Although there is little information about the developmental neurotoxicity of Ni2+, it shares similar properties with lead and cadmium for blockade of calcium channels [9], specifically involving events in neurodifferentiation [42]. We recently showed similarities between the effects of Ni2+ on neurotransmitter choice in PC12 cells and those elicited by the organophosphates in the same model [57].
Materials and Methods
Cell cultures
Because of the clonal instability of the PC12 cell line [26], the experiments were performed on cells that had undergone fewer than five passages. As described previously [46,67], PC12 cells (American Type Culture Collection, 1721-CRL, obtained from the Duke Comprehensive Cancer Center, Durham, NC) were seeded onto poly-D-lysine-coated plates in RPMI-1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% inactivated horse serum (Sigma Chemical Co., St. Louis, MO), 5% inactivated fetal bovine serum (Sigma), and 50 μg/ml penicillin streptomycin (Invitrogen). Incubations were carried out with 7.5% CO2 at 37°C, standard conditions for PC12 cells. To initiate neurodifferentiation [31,57,68] twenty-four hours after seeding, the medium was changed to include 50 ng/ml of 2.5 S murine NGF (Invitrogen). Along with the NGF, we added 30 μM of each of the test agents: chlorpyrifos (Chem Service, West Chester, PA), diazinon (Chem Service), dieldrin (Chem Service) or NiCl2 (Sigma). The concentration was chosen from earlier studies that demonstrated adverse effects on differentiation of PC12 cells without outright cytotoxicity [32,44,57,63]. Because of the limited water solubility of the three insecticides, these agents were dissolved in dimethylsulfoxide (final concentration 0.1%), which was also added to the control cultures and to cultures containing NiCl2; this concentration of dimethylsulfoxide has no effect on PC12 cell growth or differentiation [44,46,67]. Cultures were examined 24 and 72 hr after commencing exposure, with 5-8 independent cultures evaluated for each treatment at each time point. We used two time points so as to be able to evaluate changes in gene expression regardless of whether the mRNA for a given gene has a rapid turnover (and hence can rise rapidly) or a slower turnover that would require a longer period to show corresponding increases or decreases. For chlorpyrifos, we evaluated the effects both on undifferentiated cells and during NGF-induced differentiation, whereas for the other agents, we studied only the effects during differentiation.
Microarray determinations
Our earlier studies detailed all the techniques for mRNA isolation, preparation of cDNA, conversion to cRNA incorporating cyanine-3 (reference RNA) or cyanine-5 (sample RNA), verification of RNA purity and quality, hybridization to the microarrays, washing and scanning [60,62,63]. These all involve commercial kits and standardized procedures, and since the current studies were done identically, the techniques will not be described here. The mRNA used for the reference standard was created by pooling aliquots from each of the samples in the study so as to ensure measurable levels of all genes expressed over the background. Similarly, array normalizations and error detection were carried out by standard procedures described previously [60,62,63]. We used Agilent Whole Rat Genome Arrays (Agilent Technologies, Palo Alto, CA), type G4131A for the studies of chlorpyrifos in undifferentiated and differentiating cells, and type G4131F for the studies of diazinon, dieldrin and nickel in differentiating cells. The two chips contain exactly the same sequences but the latter has a lower detection threshold; however, all the genes reported here passed the quality control filters with both arrays.
For many of the genes, the arrays contain multiple probes and/or replicates of the same probe in different locations on the chip, and these were used to verify the reliability of values and the validity of the measures on the chip. In these cases, to avoid artificially inflating the number of positive findings, we limited each gene to a single set of values, selecting those obtained for the probe showing the smallest intragroup variance. The other values for that gene were used only to corroborate direction and magnitude of change. We also validated the readings on the arrays through the use of duplicate arrays for selected samples [60,62].
Statistical procedures
Because of the requirement to normalize the data across arrays and within each gene, the absolute values for a given gene are meaningless, so only the relative differences between treatments can be compared. Accordingly, results are presented as means and standard errors of the percentage change from control values to allow for visual comparison of the effects across families of genes. However, statistical comparisons were based on the actual ratios (log-transformed, since the data are in the form of ratios) rather than the percent change.
Our design involved multiple planned comparisons of four agents at two time points, as well as the effects on one agent (chlorpyrifos) in undifferentiated vs. differentiating states. It was therefore important to consider the false positive rate and to protect against the increased probability of type 1 errors engendered by repeated testing of the same data base. Accordingly, before looking at effects on individual genes, we performed a global ANOVA incorporating all the variables in a single comparison: treatment, time, and all genes. Lower-order ANOVAs on subdivisions of the data set were then carried out as permitted by the interactions of treatment with the other variables. Finally, differences for individual treatments for a specified gene at a single time point were evaluated with Fisher's Protected Least Significant Difference. However, for a given gene where there was no interaction of treatment with other variables (time, differentiation state), only the main treatment effect was reported without subtesting of effects at a single time point. Treatment effects were considered significant at p < 0.05 (two-tailed, since we were interested in both increases and decreases in gene expression). In addition to these parametric tests of the direction and magnitude of changes in gene expression, we evaluated the incidence of significant differences as compared to the predicted false positive rate, using Fisher's Exact Test, applying a one-tailed criterion of p < 0.05, since only an increase above the false positive rate would be predicted; at the criterion of p < 0.05, one gene out of every 20 tested can be expected to show a difference at random. Finding a significant decrease in the incidence of detected differences relative to the false positive rate would be biologically implausible and statistically meaningless. Finally, concordance of patterns of effects between different agents was evaluated by linear regression analysis.
Results
Because the comparison between effects on undifferentiated and differentiating cells were conducted with only one agent (chlorpyrifos), we performed two sets of global statistical tests. For the evaluations of chlorpyrifos, ANOVA incorporating all factors (treatment, differentiation state, time, gene) identified interactions of treatment × time (p < 0.02), treatment × gene (p < 0.0001), treatment × state × gene (p < 0.0001), treatment × time × gene (p < 0.05) and treatment × state × time × gene (p < 0.0001). For the entire set of 57 genes, we found 37 showing significant differences, as compared to a predicted false positive rate of <3 genes (p < 10-11). The significant relationships held up for the two major subdivisions as well. For ACh-related genes, there were 17 showing significant differences out of a total of 26 (p < 0.00001), and for CA-related genes, there were 18 out of 27 (p < 10-6). For the study of diazinon, dieldrin and Ni2+ conducted in differentiating cells, global ANOVA (factors of treatment, gene, time) identified a main effect of treatment (p < 0.0001) and interactions of treatment × time (p < 0.05), treatment × gene (p < 0.0001) and treatment × time × gene (p < 0.0001). Out of 57 total genes, we found significant differences for 43 (p < 10-14 vs. the predicted false positive rate); for ACh-related genes, 19 showed significant differences out of 26 (p < 10-6), and for CA-related genes, there were differences for 20 out of 27 (p < 10-7). In light of the interactions of treatment with the other variables, we divided the data into the separate treatments for presentation, with genes grouped by functional relationships to growth processes or neurotransmitter phenotypes.
Growth-related genes
We evaluated four genes directly related to neuronal growth and to the development of neuritic projections that accompanies differentiation: growth-associated protein 43 (gap43) and the light (nfl), medium (nef3) and heavy (nefh) neurofilament polypeptides. Exposure of either undifferentiated or differentiating cells to chlorpyrifos evoked upregulation of both nfl and nef3, while leaving gap43 and nefh unaffected (Figure 1A). There were distinct similarities to the effects of diazinon, which also increased nef3 (Figure 1B) and dieldrin, which enhanced both nfl and nef3 (Figure 1C), although the latter also evoked a small but significant reduction in nefh expression. In contrast, exposure to Ni2+ suppressed both gap43 and nef3 (Figure 1D), effects that were not seen with any of the other agents.
Figure 1.
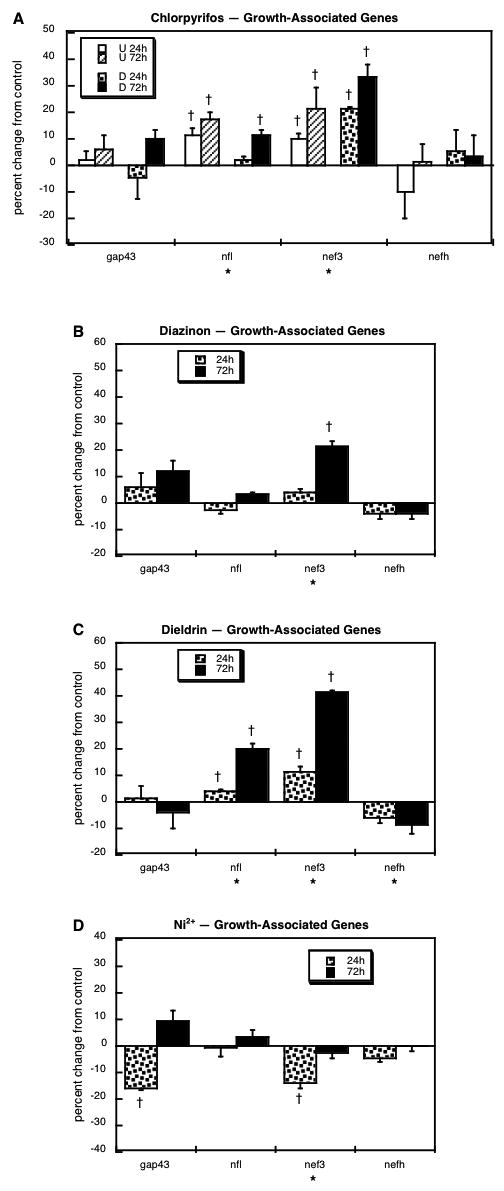
Effects of 30 μM chlorpyrifos (A), diazinon (B), dieldrin (C) or Ni2+ (D) exposure on expression of growth-associated genes. For chlorpyrifos, results are shown for both undifferentiated cells (U 24h, U 72h) and cells undergoing NGF-induced differentiation (D 24h, D 72h), whereas results for the other agents involve only differentiating cells. Asterisks shown below each gene denote a significant main treatment effect. Daggers denote genes for which treatment interacted with the other variables (differentiation state, time) and show the individual values for which treatment effects were present.
ACh-related genes
For genes involved in ACh synthesis, storage and degradation, we evaluated choline acetyltransferase (chat), the high-affinity presynaptic choline transporter (slc5a7), the low-affinity choline transporter that also transports creatine (slc6a8), the vesicular ACh transporter (slc18a3), and three cholinesterases, acetylcholinesterase (ache), the glycolipid-anchored form of acetylcholinesterase (hache) and butyrylcholinesterase (bche). Chlorpyrifos exposure strongly suppressed expression of chat and sl5a7, regardless of whether cells were in the undifferentiated or differentiating state (Figure 2A). There were small, but significant increments in slc6a8, slc18a3 and ache, whereas bche showed robust downregulation limited to undifferentiated cells. Like chlorpyrifos, diazinon evoked significant suppression of chat and smaller increases in slc6a8 and slc18a3 (Figure 2B); however, this organophosphate did not cause significant changes in any of the cholinesterase genes. Unlike the organophosphates, dieldrin failed to alter chat but did evoke upregulation of slc6a8 and slc18a3 (Figure 2C). On the other hand, the response to Ni2+ was completely unique, with strong downregulation of slc18a3 and a small, significant decrease in ache (Figure 2D).
Figure 2.
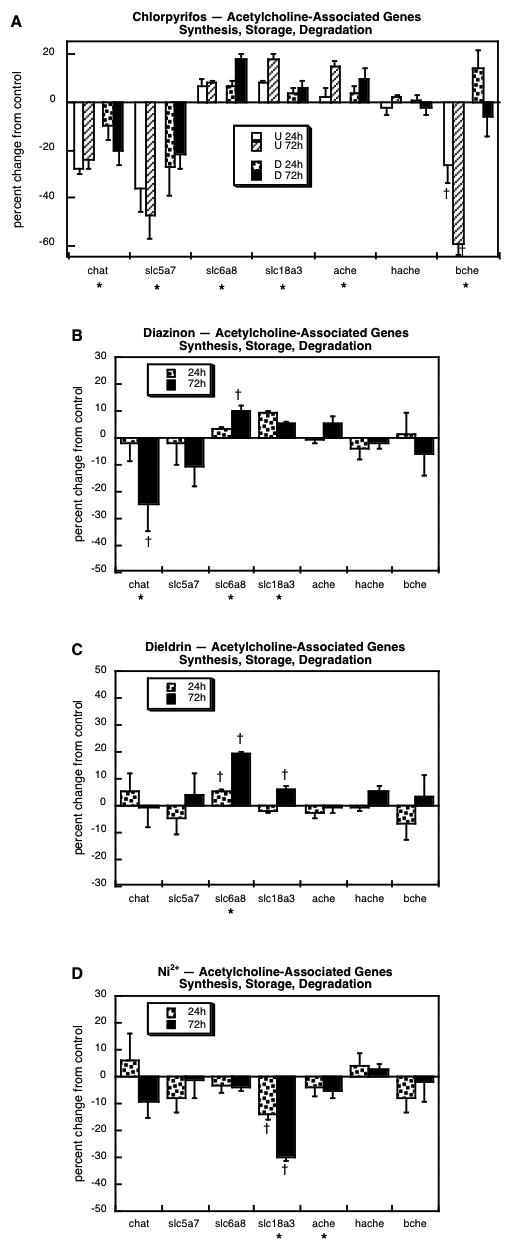
Effects of 30 μM chlorpyrifos (A), diazinon (B), dieldrin (C) or Ni2+ (D) exposure on expression of genes involved in the synthesis, storage and degradation of ACh. For chlorpyrifos, results are shown for both undifferentiated cells (U 24h, U 72h) and cells undergoing NGF-induced differentiation (D 24h, D 72h), whereas results for the other agents involve only differentiating cells. Asterisks shown below each gene denote a significant main treatment effect. Daggers denote genes for which treatment interacted with the other variables (differentiation state, time) and show the individual values for which treatment effects were present.
For muscarinic ACh receptor genes, we obtained measurements for subtypes 1 through 5: chrm1, chrm2, chrm3, chrm4 and chrm5. Chlorpyrifos exposure affected expression of four out of the five subtypes (Figure 3A). In undifferentiated cells, there was strong suppression of chrm1, but the same subtype was upregulated when chlorpyrifos exposure occurred during differentiation. Regardless of differentiation state, chlorpyrifos evoked upregulation of chrm2 expression and downregulation of chrm3. For chrm5, chlorpyrifos likewise evoked major reductions but only when exposure occurred during differentiation. The effects of diazinon on muscarinic ACh receptor gene expression in differentiating cells showed both similarities to, and differences from those of chlorpyrifos (Figure 3B). Diazinon evoked downregulation of both chrm1 and chrm2 but failed to affect chrm5. Further, diazinon evoked a small, but significant increase in chrm4 that was not obtained with chlorpyrifos. Even though it belongs to an unrelated chemical class, dieldrin exposure actually elicited some changes that were similar to those of diazinon, notably decreases in chrm1 and chrm2 (Figure 3C); however, dieldrin downregulated chrm4 and strongly upregulated chrm5, effects in the opposite direction from those obtained with diazinon (chrm4) or chlorpyrifos (chrm5). For this set of genes, Ni2+ exposure elicited some of the very same changes as seen with diazinon or dieldrin (Figure 3D): marked suppression of both chrm1 and chrm2, as well as downregulation of chrm4.
Figure 3.
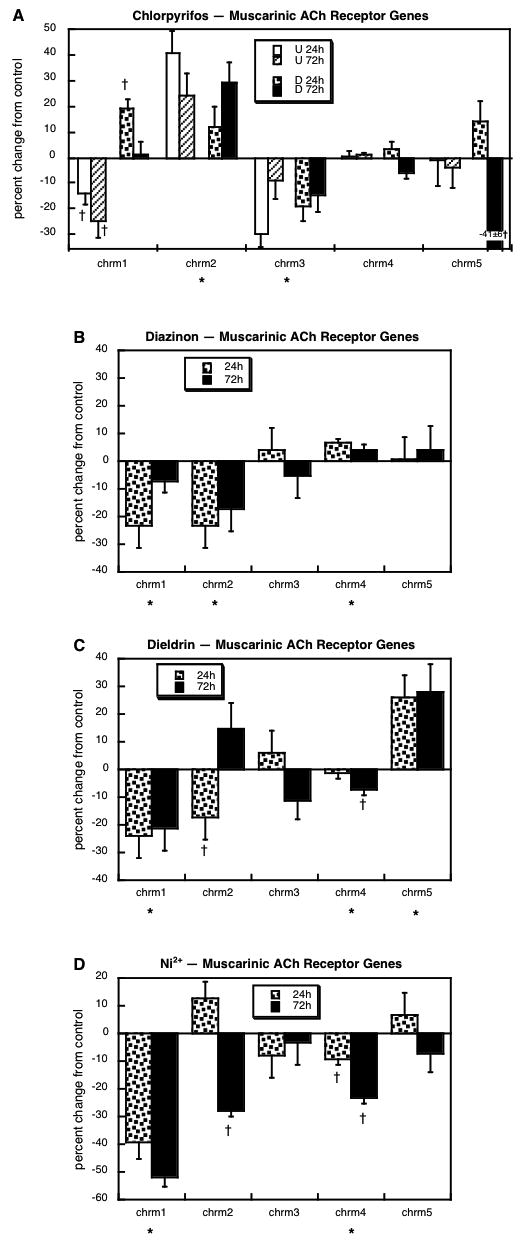
Effects of 30 μM chlorpyrifos (A), diazinon (B), dieldrin (C) or Ni2+ (D) exposure on expression of genes encoding the muscarinic ACh receptors. For chlorpyrifos, results are shown for both undifferentiated cells (U 24h, U 72h) and cells undergoing NGF-induced differentiation (D 24h, D 72h), whereas results for the other agents involve only differentiating cells. Asterisks shown below each gene denote a significant main treatment effect. Daggers denote genes for which treatment interacted with the other variables (differentiation state, time) and show the individual values for which treatment effects were present.
Results were obtained for eight of the nicotinic ACh receptor α-subunits: chrna2, chrna3, chrna4, chrna5, chrna6, chrna7, chrna9 and chrna10. Chlorpyrifos evoked major changes in gene expression that were highly dependent on differentiation state and restricted to specific subtypes (Figure 4A); some of these effects were substantially larger than those seen with other agents, so the reader should note that the scale in the graph for chlorpyrifos encompasses twice the range as for the others. In general, changes were much larger in undifferentiated cells, where chlorpyrifos downregulated chrna2 and upregulated chrna4 and chrna10 (Figure 4A); chrna9 showed a highly time-dependent change, with a large increase after 24h of exposure and an equally large decrease after 72h. In differentiating cells, chlorpyrifos evoked a small downregulation of chrna5, a large increase in chrna10, and a time-dependent change (decrease, then increase) in chrna9. The pattern obtained with exposure of differentiating cells to diazinon was completely different (Figure 4B). Three of the subtypes unaffected by chlorpyrifos showed significant decrements with diazinon (chrna2, chrna3, chrna7) and one other (chrna4) showed a time-dependent change (increased at 24h, decreased at 72h). Unlike chlorpyrifos, diazinon failed to evoke any change in expression of either chrna9 or chrna10, but did share a similar suppression of chrna5. The response to dieldrin exposure was quite similar to that of diazinon (Figure 4C): decreased expression of chrna2, chrna3 and chrna5 and a time-dependent change in chrna4, (initial increase disappearing by 72h). However, dieldrin evoked a small, significant upregulation of chrna7, the opposite effect to that seen with diazinon. Exposure to Ni2+ also produced decrements in chrna2 and chrna3 expression akin to those obtained with diazinon or dieldrin, and elicited decreases in two other subtypes (chrna4, chrna7) that were similarly affected by diazinon but not dieldrin, (Figure 4D). Uniquely, Ni2+ also produced a decrease in chrna10.
Figure 4.
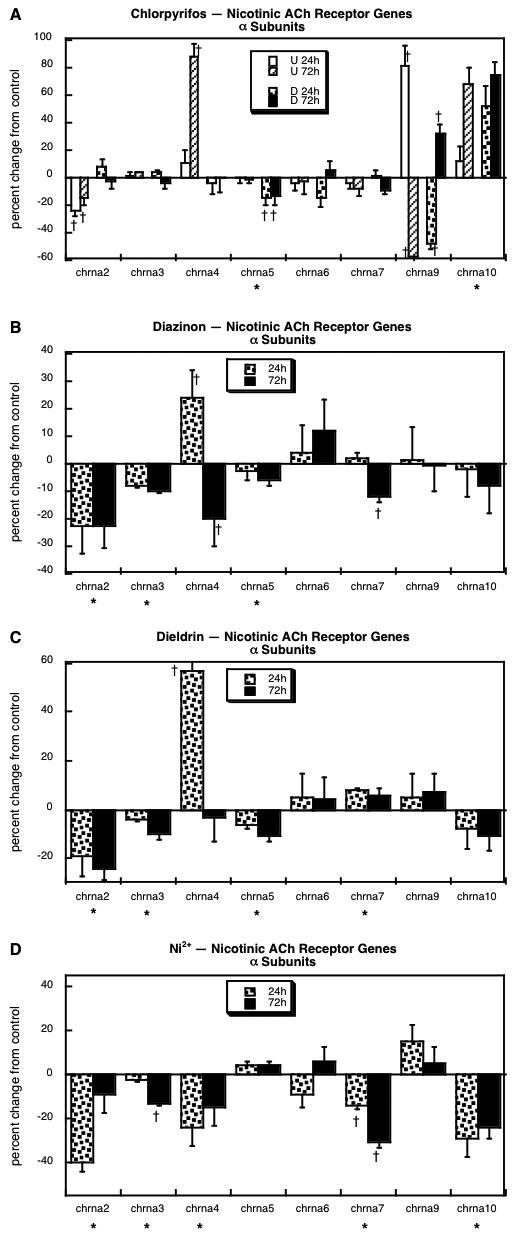
Effects of 30 μM chlorpyrifos (A), diazinon (B), dieldrin (C) or Ni2+ (D) exposure on genes encoding the nicotinic ACh receptor α subunits. For chlorpyrifos, results are shown for both undifferentiated cells (U 24h, U 72h) and cells undergoing NGF-induced differentiation (D 24h, D 72h), whereas results for the other agents involve only differentiating cells. Asterisks shown below each gene denote a significant main treatment effect. Daggers denote genes for which treatment interacted with the other variables (differentiation state, time) and show the individual values for which treatment effects were present. Note the different scale for (A), which covers a two-fold larger range of changes than the other panels.
Of the remaining nicotinic ACh receptor components, we obtained measurements for three β-subunits (chrnb2, chrnb3, chrnb4) as well as the δ, ε and γ subunits (chrnd, chrne, chrng, respectively). Chlorpyrifos exposure (Figure 5A) produced robust upregulation of chrnb3 and had effects on chrne that depended highly on whether cells were undifferentiated (decreased expression) or undergoing differentiation (increased expression at 24h). The effects of diazinon were dissimilar, with significant decrements in chrnb4 and chrng, and a time-dependent increase (24h) in chrnd (Figure 5B); like chlorpyrifos, diazinon evoked an initial rise in chrne expression, but the effect was much smaller and then showed a significant decrease after 72h of exposure. Exposure of differentiating cells to dieldrin evoked two changes that were similar to those of diazinon, decreases in chrnb4 and chrng, but it failed to affect chrne (Figure 5C). The effect of dieldrin on chrnd bore some resemblance to that of diazinon, namely a drop between 24h and 72h; however, for diazinon, the value at 24h was significantly elevated, so the subsequent decrease reduced the 72h value nonsignificantly, whereas there was no initial increase with dieldrin and a significant decrement at 72h. Exposure to Ni2+ also evoked a deficit in chrnb4 and chrng expression similar to those seen with diazinon or dieldrin (Figure 5D); Ni2+ significantly reduced chrnb3, an effect that was not significant for the other two agents, but it should be noted that the nonsignificant effects were in the same direction and were not themselves statistically distinguishable from the significant decrement obtained with Ni2+. However, the reduction in chrnb3 was clearly different from the effect of chlorpyrifos, which instead elicited a significant increase.
Figure 5.
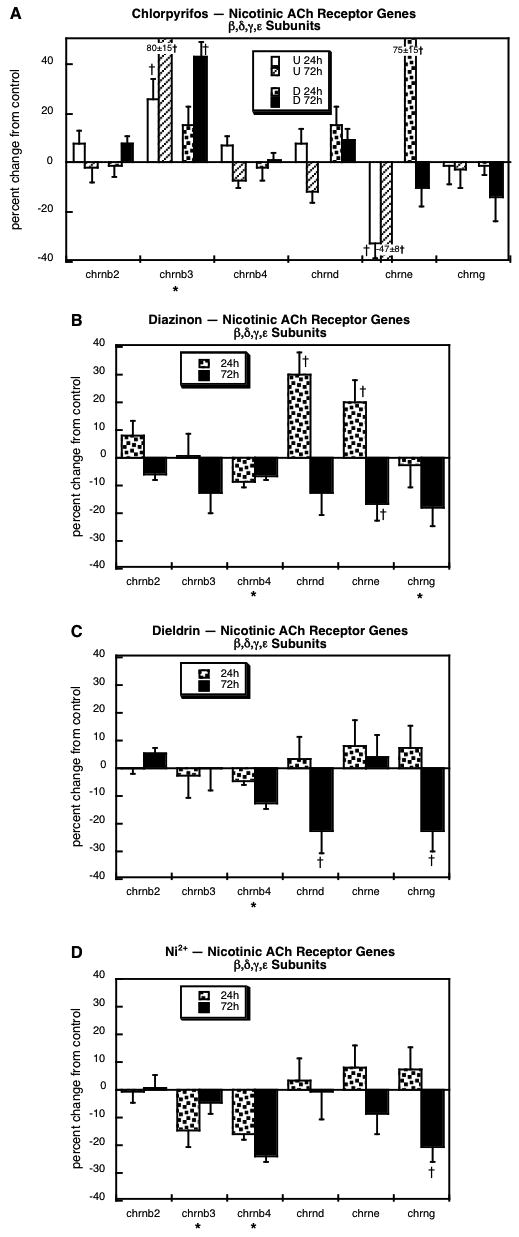
Effects of 30 μM chlorpyrifos (A), diazinon (B), dieldrin (C) or Ni2+ (D) exposure on genes encoding the nicotinic ACh receptor β, δ, ε and γ subunits. For chlorpyrifos, results are shown for both undifferentiated cells (U 24h, U 72h) and cells undergoing NGF-induced differentiation (D 24h, D 72h), whereas results for the other agents involve only differentiating cells. Asterisks shown below each gene denote a significant main treatment effect. Daggers denote genes for which treatment interacted with the other variables (differentiation state, time) and show the individual values for which treatment effects were present.
Catecholamine-related genes
We evaluated 11 genes related to CA synthesis and storage: tyrosine hydroxylase (th), dopamine β-hydroxylase (dbh), the presynaptic high-affinity norepinephrine transporter (slc6a2), the presynaptic high-affinity dopamine transporter (slc6a3), the vesicular monoamine transporter (slc18a1, slc18a2), chromogranins A and B (chga, chgb), monoamine oxidase A and B (maoa, maob) and catechol-O-methyltransferase (comt). Chlorpyrifos exposure evoked significant upregulation of both of the genes directly involved in CA biosynthesis (th, dbh) as well as the presynaptic norepinephrine transporter (slc6a2), whereas for the dopamine transporter (slc6a3), the effects were highly dependent on differentiation state and time (Figure 6A); chlorpyrifos evoked initial upregulation of slc6a3 in differentiating cells, followed by suppression at 72h. For the genes encoding the vesicular monoamine transporter, chlorpyrifos evoked a small reduction in slc18a1 in differentiating cells, a strong suppression of slc18a2 in undifferentiated cells, and a robust initial upregulation of the latter during differentiation. There were small increases in the chromogranins (significant for chga, nonsignificant for chgb because of slightly higher variability) and comt. With diazinon, there was a similar upregulation of th, but dbh expression was reduced instead of being increased (Figure 6B). The same dichotomy was evident in the effects of diazinon on the presynaptic transporters: diazinon failed to upregulate slc6a2 but markedly increased slc6a3. Also unlike chlorpyrifos, diazinon reduced expression of both of the vesicular transporter genes (slc18a1, slc18a2), as well as chgb, maob and comt. The pattern obtained with dieldrin was similar to that of diazinon in many respects: upregulation of th and slc6a3, and decreases in dbh, slc18a1 and chgb (Figure 6C). However, dieldrin evoked a reduction in slc6a2 and an increase in comt instead of a decrease, and failed to alter maob. Like the other three agents, Ni2+ evoked a significant increase in th expression but dbh was neither up- nor downregulated (Figure 6D). This agent also elicited significant elevations in slc18a1 and both of the chromogranins (chga, chgb), as well as reductions in slc6a2 and comt.
Figure 6.
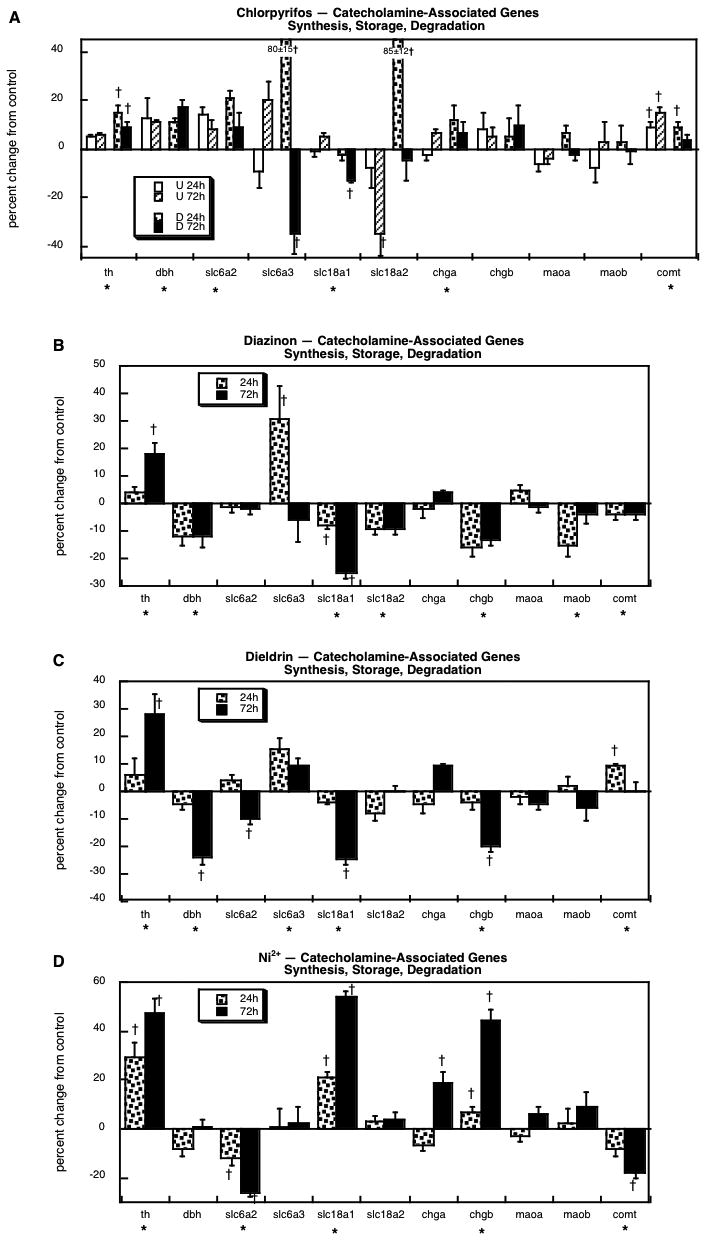
Effects of 30 μM chlorpyrifos (A), diazinon (B), dieldrin (C) or Ni2+ (D) exposure on genes involved in the synthesis, storage and degradation of catecholamines. For chlorpyrifos, results are shown for both undifferentiated cells (U 24h, U 72h) and cells undergoing NGF-induced differentiation (D 24h, D 72h), whereas results for the other agents involve only differentiating cells. Asterisks shown below each gene denote a significant main treatment effect. Daggers denote genes for which treatment interacted with the other variables (differentiation state, time) and show the individual values for which treatment effects were present.
Next, we evaluated the genes encoding five of the dopamine receptor subtypes: drd1a, drd2, drd3, drd4 and drd5. Chlorpyrifos exposure evoked upregulation of drd1a expression in both undifferentiated and differentiating cells, with a stronger effect in the former (Figure 7A). Similarly, the drd3 subtype showed small, but significant increases in response to chlorpyrifos in undifferentiated PC12 cells but not when cells were differentiating. In contrast, exposure of differentiating cells to any of the other agents produced dissimilar responses: diazinon's effect was limited to transient downregulation of drd2 (Figure 7B), whereas dieldrin upregulated drd3 (Figure 7C) and Ni2+ suppressed drd4 and drd5 (Figure 7D).
Figure 7.
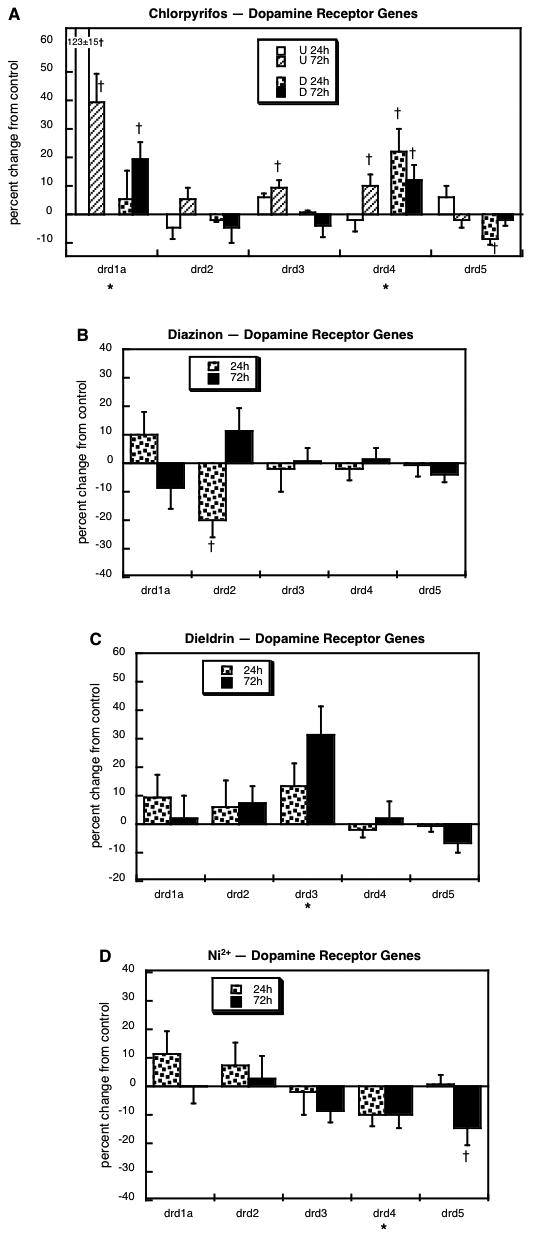
Effects of 30 μM chlorpyrifos (A), diazinon (B), dieldrin (C) or Ni2+ (D) exposure on genes encoding the dopamine receptors. For chlorpyrifos, results are shown for both undifferentiated cells (U 24h, U 72h) and cells undergoing NGF-induced differentiation (D 24h, D 72h), whereas results for the other agents involve only differentiating cells. Asterisks shown below each gene denote a significant main treatment effect. Daggers denote genes for which treatment interacted with the other variables (differentiation state, time) and show the individual values for which treatment effects were present.
We assessed multiple subforms of the α- and β-adrenergic receptors, along with two of the β-receptor kinases that modulate the coupling of these receptors to G-proteins: adra1a, adra1b, adra2a, adra2b, adra2c, adrb1, adrb2, adrb3, adrbk1 and adrbk2. The effects of chlorpyrifos were highly dependent on differentiation state (Figure 8A). In undifferentiated cells, chlorpyrifos evoked strong upregulation of adra1a, adra1d and adrb2, with lesser effects on adra2a (increase), adrb3 (decrease) and adrbk2 (decrease). However, in differentiating cells, the same agent decreased the expression of adra1a and adra1d, and induced adrb2, an effect not seen in the undifferentiated state. For three of the genes, adra2a, adrb3 and adrbk2, chlorpyrifos evoked the same changes regardless of whether the cells were undifferentiated or differentiating. With dieldrin exposure, the changes were far less notable, limited to a transient decrease in adrb3 expression (Figure 8B), one of the genes that was also decreased by chlorpyrifos. Dieldrin showed yet another pattern, with a small but significant decrease in adra2b, a transient increase in adrb1, a decrement in adrb2, and a minor rise in adrbk1 (Figure 8C). With Ni2+ exposure, we saw much more widespread changes: a transient decrease in adra1b, elevations in adra2a and adra2c, strong suppression of adrb2, and a small rise in adrbk1 (Figure 8D).
Figure 8.
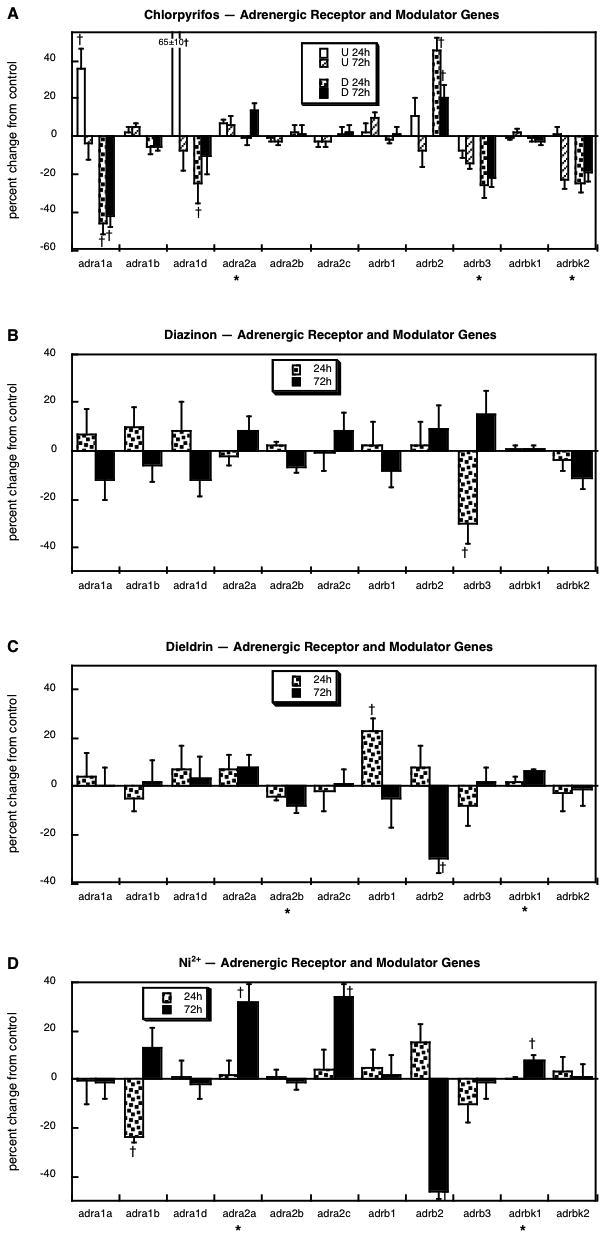
Effects of 30 μM chlorpyrifos (A), diazinon (B), dieldrin (C) or Ni2+ (D) exposure on genes encoding the adrenergic receptors and their modulators. For chlorpyrifos, results are shown for both undifferentiated cells (U 24h, U 72h) and cells undergoing NGF-induced differentiation (D 24h, D 72h), whereas results for the other agents involve only differentiating cells. Asterisks shown below each gene denote a significant main treatment effect. Daggers denote genes for which treatment interacted with the other variables (differentiation state, time) and show the individual values for which treatment effects were present.
Discussion
Our results indicate a number of key findings about the underlying mechanisms and interrelationships among diverse developmental neurotoxicants. First, all four agents affected differentiation into neuronal phenotypes in a manner distinct from their effect on general cell differentiation and neuritic outgrowth. Second, although they each elicited different transcriptional patterns, the effects were all consistent with a switch from the ACh to one or both of the CA phenotypes, the same conclusion reached from comparisons of their effects on enzymatic activity of tyrosine hydroxylase and choline acetyltransferase [31,57]. Third, and perhaps most interestingly, the transcriptional profiles readily explain many of the observed differences in outcomes from exposure to chlorpyrifos vs. diazinon in vivo [32,44,47,52,53,55,57,58,60,62,63,66,69,70], and at the same time predict that neonatal exposure to dieldrin will have effects quite similar to those of diazinon, even though the two compounds are otherwise unrelated. For the latter conclusion, correlation analysis provides a convenient way of summarizing these types of relationships (Table 1). Using the values for all of the genes and time points, we identified a small, but significant correlation between the effects of chlorpyrifos and diazinon, and essentially no relationship between chlorpyrifos and the other two agents. In contrast, the best correlation was between the effects of diazinon and dieldrin; the worst correlations, not surprisingly, were for any of the agents in relation to Ni2+. In discussing each of the sets of data, we will further utilize correlation analysis to emphasize similarities and differences involving separate families of genes.
Table 1. Correlations Among Different Neurotoxicants.
| Chlorpyrifos vs. Diazinon | Chlorpyrifos vs. Dieldrin | Chlorpyrifos vs. Ni2+ | Diazinon vs. Dieldrin | Diazinon vs. Ni2+ | Dieldrin vs. Ni2+ | |
|---|---|---|---|---|---|---|
| All genes | r = 0.20 p < 0.04 |
r = 0.003 NS |
r = -0.09 NS |
r = 0.54 p < 0.0001 |
r = 0.14 NS |
r = 0.16 NS |
| Growth genes | r = 0.70 p < 0.05 |
r = 0.81 p < 0.02 |
r = 0.23 NS |
r = 0.71 p < 0.05 |
r = 0.15 NS |
r = 0.16 NS |
| ACh and catecholamine phenotype* | r = 0.47 p < 0.05 |
r = 0.07 NS |
r = -0.04 NS |
r = 0.69 p < 0.002 |
r = -0.06 NS |
r = 0.05 NS |
| ACh receptors | r = 0.04 NS |
r = -0.09 NS |
r = -0.23 NS |
r = 0.60 p < 0.0001 |
r = 0.48 p < 0.003 |
r = 0.21 NS |
| CA receptors | r = 0.15 NS |
r = -0.04 NS |
r = 0.04 NS |
r = 0.11 NS |
r = 0.05 NS |
r = 0.47 P < 0.009 |
chat, slc5a7, slc18a3, th, dbh, slc6a2, slc6a3, slc18a1, slc18a2
Abbreviation: NS, not significant
For chlorpyrifos, there was relatively little relationship (r = 0.08, not significant) between responses in the undifferentiated state vs. cells undergoing differentiation when considering all of the genes we evaluated. This is consistent with previous observations of a peak of vulnerability to chlorpyrifos during the initial stages of differentiation [31,44,45,57,67], which corresponds to the critical window for targeting of brain development in vivo [3,4,40,48,49,51]. On the other hand, there was a highly consistent effect for undifferentiated vs. differentiating cells on the values for the genes that specifically define the emergence of the ACh phenotype, chat, sl5a7 and slc18a3: r = 0.93, p < 0.008. This relationship represented primarily a suppression of the ability to synthesize ACh, since there were overall reductions in the genes for chat, the enzyme required for ACh biosynthesis, and for sl5a7, the high-affinity presynaptic choline transporter that represents the rate-limiting factor in ACh biosynthesis [18,36]. The other ACh-related genes, although representative of the emergence of neuronal characteristics, are not selective for the ACh phenotype, since the low-affinity choline/creatine cotransporter, the cholinesterases and cholinergic receptors are all associated with ACh target cells as well as ACh neurons themselves. In contrast to the positive results for the ACh phenotypic genes, we did not observe a significant overall correlation for effects of chlorpyrifos in the undifferentiated state vs. differentiating cells for the corresponding set of genes related to CA synthesis and storage (th, dbh, slc6a2, alc6a3, slc18a1, slc18a2). That is not to say that there were not significant effects, but only that there is no overall coordination of the effects among the entire set of phenotype-specific genes. Chlorpyrifos did cause a parallel upregulation of four of the genes defining the CA phenotype in both undifferentiated and differentiating cells (th, dbh, slc6a2, comt), but those relationships did not hold for the remaining key genes (slc6a3, slc18a1, slc18a2). As for ACh, we evaluated many other CA-related genes that are not expressed solely in CA neurons: monoamine oxidase is a ubiquitous mitochondrial protein; the chromogranins, although directly involved in CA storage, are found in many other secretory granules and are actually prohormones [77]; and the dopamine and adrenergic receptors, like the ACh receptors, are also expressed on the target cells for CA neurons. Our findings for the key genes for ACh and CA phenotypes thus provide important clues as to one of the important outcomes of chlorpyrifos exposure in vivo: a net switch from the ACh to the CA phenotype that will, perforce, produce miswiring of neuronal circuits.
During neurodifferentiation, chlorpyrifos exposure disrupts the patterns of neuritic outgrowth, promoting dendrite formation at the expense of axonogenesis [29,78]. Here, we found upregulation of the genes commonly associated with the lower molecular weight neurofilament proteins that concentrate in the shorter projections (nfl, nef3), with essentially no change in the heavy neurofilament protein (nefh) or in gap43, which is associated with growth in general. Diazinon, too, is known to reduce axonogenesis both in vitro [6] and in vivo [55]. Here, we found a significant correlation between the effects of chlorpyrifos and diazinon on growth-related genes (Table 1), but the effects of diazinon were distinctly less, with no upregulation of nfl and a smaller increase in nef3. We therefore predict that diazinon will ultimately prove to have less of a promotional effect on dendrite formation than chlorpyrifos. By the same measures, dieldrin should have even greater effects; the correlation between dieldrin and chlorpyrifos was not only higher than that for diazinon, but also represented greater net effects of dieldrin on nfl and nef3, combined with a significant reduction in nefh, the protein most associated with axonal projections. In contrast, the growth-associated effects of Ni2+ did not correlate with those of any other agent; Ni2+ reduced the expression of both gap43 and nef3, indicating the likelihood of impaired general growth as well as reduced neurite formation.
In our earlier work delineating the effects of these four agents on neurodifferentiation, we found that all of them elicited a switch away from the ACh and toward the dopamine phenotype, as defined by the corresponding activities of neurotransmitter-specific enzymes [31,55]. Here, we observed significant correlations between the actions of chlorpyrifos and diazinon on expression of the genes defining these two phenotypes, and even more so between diazinon and dieldrin, but not for any other combinations (Table 1). Diazinon, like chlorpyrifos, suppressed chat expression while enhancing th, effects that clearly underlie the corresponding and parallel changes seen at the enzyme level for both agents [31,55]; both agents also evoked robust but transient stimulation of the gene encoding the presynaptic dopamine transporter, slc6a3. These are all consistent with promotion of the dopamine phenotype at the expense of the ACh phenotype. However, when we examined the genes selective for the norepinephrine phenotype, we found substantial differences between the two organophosphates. Chlorpyrifos increased expression of dbh, which is responsible for the conversion of dopamine to norepinephrine, as well as slc6a2, the presynaptic norepinephrine transporter, whereas diazinon reduced dbh and had no effect on slc6a2. We therefore anticipate that the effects of diazinon will diverge from those of chlorpyrifos specifically as they relate to the impact on noradrenergic neurons. Dieldrin had actions on the emergence of neurotransmitter phenotypes quite similar to those of diazinon, as indicated by the stronger correlation between these two agents (Table 1). Dieldrin, like diazinon, elevated th and slc6a3 expression but failed to reduce chat. Thus, although dieldrin, like the organophosphates, promotes a switch to the dopamine phenotype at the expense of the ACh phenotype, it does so solely by promoting the dopamine-related genes, rather than by a combination of enhanced dopaminergic character and suppressed ACh character. Also like diazinon, dieldrin suppressed dbh expression, and in addition, reduced expression of the norepinephrine transporter (slc6a2), so we would again anticipate that the CA-promotional effect would be limited to the dopamine subtype, with opposite effects for differentiation into norepinephrine neurons. Ni2+ enhanced th expression without affecting chat, but also showed some unique features, with strong induction of one of the vesicular monoamine transporter genes (slc18a1) and suppression of the vesicular ACh transporter gene (slc128a3). Ni2+ also significantly reduced expression of the norepinephrine transporter (slc6a2), so that taken together, this agent again appears to enhance the dopamine phenotype but not the norepinephrine phenotype, while suppressing different aspects of the ACh phenotype from the other agents. Thus, we have four different agents that ultimately all promote a switch in neurotransmitter “choice,” but by a variety of originating mechanisms, each represented by transcriptional events that show some similarities, but also major differences.
Although the neurotransmitter degradative enzymes and receptors can not be used to define whether cells are differentiating into specific neurotransmitter phenotypes, they all nevertheless represent important aspects of neuronal development that definitively influence cellular responses to neurotransmitter input. For degradative enzymes, although there were differences among the four neurotoxicants, there were only minor changes overall. However, genes encoding the receptors showed robust effects that were substantially dissimilar among the various agents. For muscarinic ACh receptors, chlorpyrifos stood out as unique, enhancing expression of chrm1 and chrm2 while suppressing chrm3 and chrm5; the other three agents all suppressed chrm1 and chrm2, while differing in their effects on the other muscarinic subtypes. We saw the same dichotomy for nicotinic ACh receptor α, β, γ, δ and ε subunits, with diazinon and dieldrin showing quite similar patterns, Ni2+ showing lesser similarities to diazinon and dieldrin, but chlorpyrifos displaying a pattern unique from those of the other agents (Table 1). The relationships were least strong for CA receptors, where the only significant correlation was between dieldrin and Ni2+ (Table 1); this means that each agent basically produces a unique pattern of changes in expression of the genes encoding dopamine and norepinephrine receptors. The main conclusion of the receptor evaluations, then, is that exposure of differentiating neuronotypic cells to each of the four agents alters the expression of these key neuronal features, effects that can be expected to contribute to disruption of synaptic function over and above changes in the neurotransmitter phenotype; and again, we would predict critical differences in the outcomes of exposure to agents of the same class (chlorpyrifos, diazinon) and surprising similarities among agents of different classes (diazinon, dieldrin, Ni2+).
Our results thus provide the mechanistic underpinnings that explain how exposure to two different organophosphates, chlorpyrifos and diazinon, given at the same time in brain development, at comparable doses that produce similar systemic effects, nevertheless can elicit substantially divergent outcomes in terms of neurochemistry, synaptic function and behavior [32,44,47,52,53,55,57,58,60,62,63,66,69,70]. The dissimilarities clearly reside in mechanisms other than their shared property as cholinesterase inhibitors, and as shown here, are likely to involve direct effects on developing neurons during the critical stage in which cells begin to differentiate into specific neurotransmitter phenotypes. Equally important, our results explain how diverse agents can nevertheless converge on a common set of outcomes, albeit through disparate originating mechanisms or through differential effects on members of key gene families that produce the same net outcome. We found surprising similarities in the effects of diazinon, dieldrin and Ni2+, agents whose developmental neurotoxicant actions are far less studied than are those of chlorpyrifos; the present results thus provide guidelines for the types of outcomes that are the best candidates for future evaluations of each of these toxicants with in vivo animal models. Finally, our results illustrate how cell culture systems combined with microarray technology, can provide screening techniques for developmental neurotoxicants, serving as a model for further design of alternative approaches to the detection and characterization of the impact of exogenous chemicals on brain development [17,19,33,50,73].
Acknowledgments
Research was supported by NIH ES10356. The authors state that they have no competing financial interests but have provided expert witness testimony on behalf of government agencies, corporations and/or individuals.
Abbreviations
- ACh
acetylcholine
- ANOVA
analysis of variance
- CA
catecholamine
- NGF
nerve growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ Health Perspect. 2005;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ Health Perspect. 2005;113:1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldridge JE, Seidler FJ, Meyer A, Thillai I, Slotkin TA. Serotonergic systems targeted by developmental exposure to chlorpyrifos: effects during different critical periods. Environ Health Perspect. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asmus SE, Parsons S, Landis SC. Developmental changes in the transmitter properties of sympathetic neurons that innervate the periosteum. J Neurosci. 2000;20:1495–1504. doi: 10.1523/JNEUROSCI.20-04-01495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Axelrad JC, Howard CV, McLean WG. The effects of acute pesticide exposure on neuroblastoma cells chronically exposed to diazinon. Toxicology. 2003;185:67–78. doi: 10.1016/s0300-483x(02)00592-9. [DOI] [PubMed] [Google Scholar]
- 7.Bagchi D, Bagchi M, Hassoun EA, Stohs SJ. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology. 1995;104:129–140. doi: 10.1016/0300-483x(95)03156-a. [DOI] [PubMed] [Google Scholar]
- 8.Bagchi D, Bhattacharya G, Stohs SJ. In vitro and in vivo induction of heat shock (stress) protein (Hsp) gene expression by selected pesticides. Toxicology. 1996;112:57–68. doi: 10.1016/0300-483x(96)03350-1. [DOI] [PubMed] [Google Scholar]
- 9.Benters J, Schafer T, Beyersmann D, Hechtenberg S. Agonist-stimulated calcium transients in PC12 cells are affected differentially by cadmium and nickel. Cell Calcium. 1996;20:441–446. doi: 10.1016/s0143-4160(96)90007-x. [DOI] [PubMed] [Google Scholar]
- 10.Betancourt AM, Burgess SC, Carr RL. Effect of developmental exposure to chlorpyrifos on the expression of neurotrophin growth factors and cell-specific markers in neonatal rat brain. Toxicol Sci. 2006;92:500–506. doi: 10.1093/toxsci/kfl004. [DOI] [PubMed] [Google Scholar]
- 11.Betancourt AM, Filipov NM, Carr RL. Alteration of neurotrophins in the hippocampus and cerebral cortex of young rats exposed to chlorpyrifos and methyl parathion. Toxicol Sci. 2007;100:445–455. doi: 10.1093/toxsci/kfm248. [DOI] [PubMed] [Google Scholar]
- 12.Black IB, Adler JE, Dreyfus CF, Jonakait GM, Katz DM, LaGamma EF, Markey KM. Neurotransmitter plasticity at the molecular level. Science. 1984;225:1266–1270. doi: 10.1126/science.6147894. [DOI] [PubMed] [Google Scholar]
- 13.Borodinsky LN, Spitzer NC. Activity-dependent neurotransmitter-receptor matching at the neuromuscular junction. Proc Natl Acad Sci. 2007;104:335–340. doi: 10.1073/pnas.0607450104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brannen KC, Devaud LL, Liu JP, Lauder JM. Prenatal exposure to neurotoxicants dieldrin or lindane alters tert-butylbicyclophosphorothionate binding to GABA(A) receptors in fetal rat brainstem. Dev Neurosci. 1998;20:34–41. doi: 10.1159/000017296. [DOI] [PubMed] [Google Scholar]
- 15.Casey CE, Robinson MF. Copper, manganese, zinc, nickel, cadmium and lead in human foetal tissues. Br J Nutrition. 1978;39:639–646. doi: 10.1079/bjn19780079. [DOI] [PubMed] [Google Scholar]
- 16.Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- 17.Coecke S, Goldberg AM, Allen S, Buzanska L, Calamandrei G, Crofton K, Hareng L, Hartung T, Knaut H, Honegger P, Jacobs M, Lein P, Li A, Mundy W, Owen D, Schneider S, Silbergeld E, Reum T, Trnovec T, Monnet-Tschudi F, Bal-Price A. Workshop report: incorporating in vitro alternative methods for developmental neurotoxicity into international hazard and risk assessment strategies. Environ Health Perspect. 2007;115:924–931. doi: 10.1289/ehp.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. 7th. Oxford University Press; New York: 1996. [Google Scholar]
- 19.Costa LG. Neurotoxicity testing: a discussion of in vitro alternatives. Environ Health Perspect. 1998;106 2:505–510. doi: 10.1289/ehp.98106505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Crumpton TL, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos in vivo and in vitro: effects on nuclear transcription factor involved in cell replication and differentiation. Brain Res. 2000;857:87–98. doi: 10.1016/s0006-8993(99)02357-4. [DOI] [PubMed] [Google Scholar]
- 22.Crumpton TL, Seidler FJ, Slotkin TA. Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos? Dev Brain Res. 2000;121:189–195. doi: 10.1016/s0165-3806(00)00045-6. [DOI] [PubMed] [Google Scholar]
- 23.Dam K, Garcia SJ, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure alters synaptic development and neuronal activity in cholinergic and catecholaminergic pathways. Dev Brain Res. 1999;116:9–20. doi: 10.1016/s0165-3806(99)00067-x. [DOI] [PubMed] [Google Scholar]
- 24.Das KP, Barone S. Neuronal differentiation in PC12 cells is inhibited by chlorpyrifos and its metabolites: is acetylcholinesterase inhibition the site of action? Toxicol Appl Pharmacol. 1999;160:217–230. doi: 10.1006/taap.1999.8767. [DOI] [PubMed] [Google Scholar]
- 25.Flaskos J, McLean WG, Hargreaves AJ. The toxicity of organophosphate compounds towards cultured PC12 cells. Toxicol Lett. 1994;70:71–76. doi: 10.1016/0378-4274(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 26.Fujita K, Lazarovici P, Guroff G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ Health Perspect. 1989;80:127–142. doi: 10.1289/ehp.8980127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez-Lira G, Lamas M, Romo-Parra H, Gutierrez R. Programmed and induced phenotype of the hippocampal granule cells. J Neurosci. 2005;25:6939–6946. doi: 10.1523/JNEUROSCI.1674-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hales TG, Tyndale RF. Few cell lines with GABAA mRNAs have functional receptors. J Neurosci. 1994;14:5429–5436. doi: 10.1523/JNEUROSCI.14-09-05429.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howard AS, Bucelli R, Jett DA, Bruun D, Yang DR. Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicol Appl Pharmacol. 2005;207:112–124. doi: 10.1016/j.taap.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Jacobsen N, Alfheim I, Jonsen J. Nickel and strontium distribution in some mouse tissues. Passage through placenta and mammary glands. Res Comm Chem Pathol Pharmacol. 1978;20:571–584. [PubMed] [Google Scholar]
- 31.Jameson RR, Seidler FJ, Qiao D, Slotkin TA. Chlorpyrifos affects phenotypic outcomes in a model of mammalian neurodevelopment: critical stages targeting differentiation in PC12 cells. Environ Health Perspect. 2006;114:667–672. doi: 10.1289/ehp.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jameson RR, Seidler FJ, Slotkin TA. Nonenzymatic functions of acetylcholinesterase splice variants in the developmental neurotoxicity of organophosphates: chlorpyrifos, chlorpyrifos oxon and diazinon. Environ Health Perspect. 2007;115:65–70. doi: 10.1289/ehp.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimmel GL. In vitro testing in developmental toxicity risk assessment. Teratology. 1998;58:25–26. doi: 10.1002/(SICI)1096-9926(199808)58:2<25::AID-TERA1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radical Biol Med. 2001;31:1473–1485. doi: 10.1016/s0891-5849(01)00726-2. [DOI] [PubMed] [Google Scholar]
- 35.Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase Cδ in dopaminergic cells: relevance to oxidative stress and dopaminergic degeneration. Neuroscience. 2003;119:945–964. doi: 10.1016/s0306-4522(03)00226-4. [DOI] [PubMed] [Google Scholar]
- 36.Klemm N, Kuhar MJ. Post-mortem changes in high affinity choline uptake. J Neurochem. 1979;32:1487–1494. doi: 10.1111/j.1471-4159.1979.tb11089.x. [DOI] [PubMed] [Google Scholar]
- 37.Levin ED, Addy N, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Dev Brain Res. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 38.Li WW, Casida JE. Organophosphorus neuropathy target esterase inhibitors selectively block outgrowth of neurite-like and cell processes in cultured cells. Toxicol Lett. 1998;98:139–146. doi: 10.1016/s0378-4274(98)00116-7. [DOI] [PubMed] [Google Scholar]
- 39.Mauro RE, Zhang L. Unique insights into the actions of CNS agents: lessons from studies of chlorpyrifos and other common pesticides. CNS Agents Med Chem. 2007;7:183–199. [Google Scholar]
- 40.Meyer A, Seidler FJ, Aldridge JE, Tate CA, Cousins MM, Slotkin TA. Critical periods for chlorpyrifos-induced developmental neurotoxicity: alterations in adenylyl cyclase signaling in adult rat brain regions after gestational or neonatal exposure. Environ Health Perspect. 2004;112:295–301. doi: 10.1289/ehp.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagata K, Huang CS, Song JH, Narahashi T. Direct actions of anticholinesterases on the neuronal nicotinic acetylcholine receptor channels. Brain Res. 1997;769:211–218. doi: 10.1016/s0006-8993(97)00707-5. [DOI] [PubMed] [Google Scholar]
- 42.Nikodijevic B, Guroff G. Nerve growth factor-stimulated calcium uptake into PC12 cells: uniqueness of the channel and evidence for phosphorylation. J Neurosci Res. 1992;31:591–599. doi: 10.1002/jnr.490310402. [DOI] [PubMed] [Google Scholar]
- 43.Pope CN. Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Health. 1999;2:161–181. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- 44.Qiao D, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos modeled in vitro: comparative effects of metabolites and other cholinesterase inhibitors on DNA synthesis in PC12 and C6 cells. Environ Health Perspect. 2001;109:909–913. doi: 10.1289/ehp.01109909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiao D, Seidler FJ, Slotkin TA. Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol Appl Pharmacol. 2005;206:17–26. doi: 10.1016/j.taap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Qiao D, Seidler FJ, Violin JD, Slotkin TA. Nicotine is a developmental neurotoxicant and neuroprotectant: stage-selective inhibition of DNA synthesis coincident with shielding from effects of chlorpyrifos. Dev Brain Res. 2003;147:183–190. doi: 10.1016/s0165-3806(03)00222-0. [DOI] [PubMed] [Google Scholar]
- 47.Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, Levin ED. Developmental diazinon neurotoxicity in rats: later effects on emotional response. Brain Res Bull. 2008;75:166–172. doi: 10.1016/j.brainresbull.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ Health Perspect. 1999;107 1:71–80. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Slotkin TA. Guidelines for developmental neurotoxicity and their impact on organophosphate pesticides: a personal view from an academic perspective. NeuroToxicology. 2004;25:631–640. doi: 10.1016/S0161-813X(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 51.Slotkin TA. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC, editor. Toxicity of Organophosphate and Carbamate Pesticides. Elsevier Academic Press; San Diego: 2005. pp. 293–314. [Google Scholar]
- 52.Slotkin TA, Bodwell BE, Levin ED, Seidler FJ. Neonatal exposure to low doses of diazinon: long-term effects on neural cell development and acetylcholine systems. Environ Health Perspect. 2008;116:340–348. doi: 10.1289/ehp.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slotkin TA, Bodwell BE, Ryde IT, Levin ED, Seidler FJ. Exposure of neonatal rats to parathion elicits sex-selective impairment of acetylcholine systems in brain regions during adolescence and adulthood. Environ Health Perspect. 2008 doi: 10.1289/ehp.11451. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slotkin TA, Cousins MM, Tate CA, Seidler FJ. Persistent cholinergic presynaptic deficits after neonatal chlorpyrifos exposure. Brain Res. 2001;902:229–243. doi: 10.1016/s0006-8993(01)02387-3. [DOI] [PubMed] [Google Scholar]
- 55.Slotkin TA, Levin ED, Seidler FJ. Comparative developmental neurotoxicity of organophosphate insecticides: effects on brain development are separable from systemic toxicity. Environ Health Perspect. 2006;114:746–751. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slotkin TA, MacKillop EA, Ryde IT, Seidler FJ. Ameliorating the developmental neurotoxicity of chlorpyrifos: a mechanisms-based approach in PC12 cells. Environ Health Perspect. 2007;115:1306–1313. doi: 10.1289/ehp.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slotkin TA, MacKillop EA, Ryde IT, Tate CA, Seidler FJ. Screening for developmental neurotoxicity using PC12 cells: comparisons of organophosphates with a carbamate, an organochlorine and divalent nickel. Environ Health Perspect. 2007;115:93–101. doi: 10.1289/ehp.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slotkin TA, Ryde IT, Levin ED, Seidler FJ. Developmental neurotoxicity of low-dose diazinon exposure of neonatal rats: effects on serotonin systems in adolescence and adulthood. Brain Res Bull. 2008;75:640–647. doi: 10.1016/j.brainresbull.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slotkin TA, Seidler FJ. The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Dev Brain Res. 2005;158:115–119. doi: 10.1016/j.devbrainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 60.Slotkin TA, Seidler FJ. Comparative developmental neurotoxicity of organophosphates in vivo: transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res Bull. 2007;72:232–274. doi: 10.1016/j.brainresbull.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slotkin TA, Seidler FJ. Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: critical periods for regional and sex-selective effects. Reprod Toxicol. 2007;23:421–427. doi: 10.1016/j.reprotox.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 62.Slotkin TA, Seidler FJ, Fumagalli F. Exposure to organophosphates reduces the expression of neurotrophic factors in neonatal rat brain regions: similarities and differences in the effects of chlorpyrifos and diazinon on the fibroblast growth factor superfamily. Environ Health Perspect. 2007;115:909–916. doi: 10.1289/ehp.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slotkin TA, Seidler FJ, Fumagalli F. Targeting of neurotrophic factors, their receptors, and signaling pathways in the developmental neurotoxicity of organophosphates in vivo and in vitro. Brain Res Bull. 2008;76:424–438. doi: 10.1016/j.brainresbull.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Slotkin TA, Seidler FJ, Fumagalli F. Unrelated developmental neurotoxicants elicit similar transcriptional profiles for effects on neurotrophic factors and their receptors in an in vitro model. Neurotoxicol Teratol. 2008 doi: 10.1016/j.ntt.2008.11.006. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Functional alterations in CNS catecholamine systems in adolescence and adulthood after neonatal chlorpyrifos exposure. Dev Brain Res. 2002;133:163–173. doi: 10.1016/s0165-3806(02)00284-5. [DOI] [PubMed] [Google Scholar]
- 66.Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ Health Perspect. 2006;114:1542–1546. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol Appl Pharmacol. 1998;151:182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- 68.Teng KK, Greene LA. Cultured PC12 cells: a model for neuronal function and differentiation. In: Celis JE, editor. Cell Biology: A Laboratory Handbook. Academic Press; San Diego: 1994. pp. 218–224. [Google Scholar]
- 69.Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol Teratol. 2008;30:38–45. doi: 10.1016/j.ntt.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, Agoos S, Kallepalli A, Rastogi A, Braddy D, Wells C, Perraut C, Seidler FJ, Slotkin TA, Levin ED. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Res Bull. 2008 doi: 10.1016/j.brainresbull.2008.08.019. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tuler SM, Hazen AA, Bowen JM. Release and metabolism of dopamine in a clonal line of pheochromocytoma (PC12) cells exposed to fenthion. Fund Appl Toxicol. 1989;13:484–492. doi: 10.1016/0272-0590(89)90284-4. [DOI] [PubMed] [Google Scholar]
- 72.Tyndale RF, Hales TG, Olsen RW, Tobin AJ. Distinctive patterns of GABAA receptor subunit mRNAs in 13 cell lines. J Neurosci. 1994;14:5417–5428. doi: 10.1523/JNEUROSCI.14-09-05417.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.U.S. Environmental Protection Agency. Opportunities to Improve Data Quality and Children's Health Through the Food Quality Protection Act. [7 July 2006];Report no 2006-P-00009. 2006 Available: http://www.epa.gov/oig/reports/2006/20060110-2006-P-00009.pdf.
- 74.U.S. National Library of Medicine. Superfund Chemicals in TOXMAP. [9 August 2006];2006 Available: http://toxmap.nlm.nih.gov/toxmap/main/sfChemicals.jsp.
- 75.Uzoukwu M, Sleight SD. Dieldrin toxicosis: fetotoxicosis, tissue concentrations, and microscopic and ultrastructural changes in guinea pigs. Am J Vet Res. 1972;33:579–583. [PubMed] [Google Scholar]
- 76.Whyatt RM, Camann DE, Kinney PL, Reyes A, Ramirez J, Dietrich J, Diaz D, Holmes D, Perera FP. Residential pesticide use during pregnancy among a cohort of urban minority women. Environ Health Perspect. 2002;110:507–514. doi: 10.1289/ehp.02110507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winkler H, Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience. 1992;49:497–528. doi: 10.1016/0306-4522(92)90222-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang D, Howard A, Bruun D, Ajua-Alemanj M, Pickart C, Lein PJ. Chlorpyrifos and chlorpyrifos-oxon inhibit axonal growth by interfering with the morphogenic activity of acetylcholinesterase. Toxicol Appl Pharmacol. 2008;228:32–41. doi: 10.1016/j.taap.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


