Summary
The critical role of the ubiquitin-26S proteasome system in regulation of protein homeostasis in eukaryotes is well established. In contrast, the impact of the ubiquitin-independent proteolytic activity of proteasomes is poorly understood. Through biochemical analysis of mammalian lysates, we find that the 20S proteasome, latent in peptide hydrolysis, specifically cleaves more than 20% of all cellular proteins. Thirty intrinsic proteasome substrates (IPSes) were identified and in vitro studies of their processing revealed that cleavage occurs at disordered regions, generating stable products encompassing structured domains. The mechanism of IPS recognition is remarkably conserved in the eukaryotic kingdom, as mammalian and yeast 20S proteasomes exhibit the same target specificity. Further, 26S proteasomes specifically recognize and cleave IPSes at similar sites, independent of ubiquitination, suggesting that disordered regions likely constitute the universal structural signal for IPS proteolysis by proteasomes. Finally, we show that proteasomes contribute to physiological regulation of IPS levels in living cells and the inactivation of ubiquitin-activating enzyme E1 does not prevent IPS degradation. Collectively, these findings suggest a significant contribution of the ubiquitin-independent proteasome degradation pathway to the regulation of protein homeostasis in eukaryotes.
Keywords: 20S proteasome, 26S proteasome, protein degradation, endoproteolytic cleavage, ubiquitin-independent proteasome degradation
Introduction
Proteasomes are responsible for the majority of non-lysosomal protein degradation in eukaryotic cells.1,2 The catalytic sites of these degradation machines are concealed within the internal cavity of the 20S proteasome, thereby preventing unwarranted proteolysis of cellular proteins. The 20S core particle consists of 28 subunits arranged in a cylindrical form of four rings.3 The two inner rings are assembled from seven distinct β-subunits, three of which are catalytically active in each ring. The two outer rings consist of seven homologous α-subunits and a protein substrate can get access to the internal catalytic sites of the 20S core through a narrow axial pore of the α-ring. The 20S proteasome is usually latent because the N-termini of α-subunits project towards the axial pore of the ring and form a gate that blocks the entrance of substrates into the internal proteolytic chamber. Further, the 20S proteasome can neither recognize ubiquitin modifications nor actively unfold protein substrates. Different regulatory complexes (PA700/19S cap or PA28/11S regulator) attach to one or both of the endplates of the barrel-shaped 20S core particle to form proteasome species with distinct proteolytic properties4,5,6; this docking rearranges the blocking residues of α-subunits and thereby opens the gate, allowing for substrate entry. The 19S regulatory particle together with the 20S catalytic core forms the 26S proteasome holoenzyme. The 19S cap recognizes ubiquitinated proteins, unfolds these degradation substrates in an ATP-dependent manner and threads them into the internal proteolytic chamber of the 20S proteasome, wherein the polypeptide is cleaved after amino acids flanking the scissile peptide bonds by any of the three catalytic subunits. The ubiquitin-26S proteasome system (UPS) has been intensively characterized and is currently considered to be the major degradation pathway of cellular proteins.
Several studies demonstrated that both the 20S proteasome and the 26S proteasome degrade oxidized and chemically unfolded proteins, as well as specific natively disordered proteins, in a ubiquitin-independent manner.7–9 Endoproteolytic cleavage at polypeptide bonds in two natively disordered proteins was also demonstrated.10 We have previously shown that the native 20S proteasome specifically cleaves two structured cellular proteins, the eIF3a subunit of eIF3 and the eIF4G subunit of eIF4F, at internal sites leading to the generation of stable cleavage products.11 Since then, an increasing number of studies identified other structured proteins that are processed to a various extent by 20S and/or 26S proteasomes independent of ubiquitination.12–16 These findings suggest that cleavage of specific cellular proteins by proteasomes could potentially be a widespread mechanism in eukaryotes. To address this question we performed biochemical analysis of both mammalian and yeast lysates, in search of cellular proteins that are processed by the 20S proteasome. This survey revealed an unexpectedly high proportion (over 20%) of cellular proteins targeted by this degradation pathway and a remarkable conservation of the mechanism of substrate recognition and processing by eukaryotic 20S proteasomes. Thirty mammalian Intrinsic Proteasome Substrates (IPSes) were studied further and the distinct nature of their processing revealed that cleavage occurred at sites within extended disordered regions. Further, purified 26S proteasomes specifically recognized and cleaved IPSes at similar sites in vitro, implicating that an extended disordered region could be the key structural determinant in targeting cellular proteins to ubiquitin-independent processing by proteasomes. Since this proteolysis spares structured domains, the Intrinsic Proteasome Degradation Pathway (IPDP) could generate cleavage products that have a function distinct from that of the intact protein. The cleavage rate of different IPSes by 20S and 26S proteasomes differed, suggesting that proteasome species can have distinct roles in regulation of different IPSes in the cell. Finally, we show that proteasomes contribute to differential regulation of IPS levels in cell cultures and that the inhibition of ubiquitination does not prevent IPS degradation, in contrast to proteins targeted by the UPS. Collectively, our findings suggest that the ubiquitin-independent IPDP is a widespread mechanism that contributes to the regulation of protein turnover in eukaryotes.
Results
20S proteasome specifically cleaves more than twenty percent of cellular proteins
The discovery of the non-lysosomal protein degradation pathway, the proteasome, and the ubiquitin-proteasome system were all achieved via the biochemical analysis of fractionated rabbit reticulocyte lysate.1,2 To evaluate the magnitude of the ubiquitin-independent proteolytic activity of proteasomes, we used this classical system for large-scale biochemical analysis, in which we sought to identify native cellular proteins that are specific substrates of highly purified native 20S proteasomes.
First, we verified that purification yielded 20S proteasomes with a closed substrate entry pore. The gate formed by the N-termini of α-subunits constrains the access of small peptides into the internal chamber, accounting for low peptidase activity of the 20S proteasome. Treatment with 0.02% SDS or specific hydrophobic peptides, such as suc-FLF-mna, opens the gate, thereby stimulating the access and hydrolysis of model peptide substrates.17,18 We found that either of the treatments stimulated more than six fold both the chymotrypsin-like and caspase-like activities of catalytic cores in hydrolyzing Suc-LLVY-amc and Ac-nLPnLD-amc, respectively (Supplementary Fig. 1), consistent with the closed gate conformation of purified 20S proteasomes.
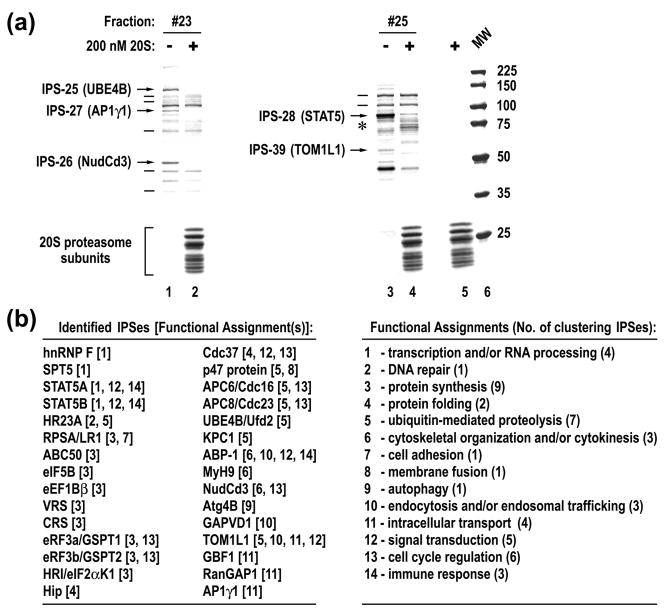
Next, we fractionated RRL by a variety of biochemical techniques. Seventy two different fractions, typically containing five to twenty five distinct protein species, were incubated in the absence or presence of 20S proteasomes and then visualized on Blue R stained SDS-PAGE. Visual analysis of protein species revealed either a complete disappearance or an evident reduction in intensity of specific bands following incubation with 20S proteasomes (Fig. 1a, arrows). Importantly, the integrity of other protein species in the same reactions was unaffected, even for those present in small amounts (Fig. 1a, lines). The appearance of new protein species, likely corresponding to proteolytic products of specific substrates of the 20S proteasome, was also noticed in many reactions (Fig. 1a, lane 4, asterisk). The cleavage of protein targets was suppressed by the addition of the specific proteasome inhibitors MG132 and epoxomicin (Supplementary Fig. 2). Clearly, the native 20S proteasome specifically recognizes and cleaves certain proteins, referred to as IPSes. Analysis of seventy two reactions revealed 258 IPSes out of a total of 1167 detectable protein species, affording a statistically sound assessment that ~22% of cellular proteins are specific substrates of the catalytic cores.
Fig. 1.
20S proteasome specifically cleaves multiple cellular proteins. (a) An example of analysis of cellular proteins for the presence of IPSes. Gel filtration elution fractions were incubated for 3 hours with or without 20S proteasomes and stained with Blue R, following SDS-PAGE. Protein species that were cleaved or remained intact are marked with arrows or lines to the left of the reactions. The position of new protein species that appeared after incubation with 20S proteasomes is marked with an asterisk. The last lane is a MW marker. (b) Summary of identified IPSes and their known functional engagements.
Thirty IPS species were randomly selected, purified to different extents, excised from gel matrices, and identified by mass spectrometry. The identity of most IPSes, and complexes they associated with, was further verified by different methods (see Supplementary Table 1). Of note, all identified IPSes lacked ubiquitin modification, as judged by their mobility as well as the absence of any peptides corresponding to ubiquitin throughout LC-MS/MS analysis. Fig. 1b is a summary of the identified proteins, along with their known functional engagements. The identified IPSes function in a variety of cellular processes, ranging from signal transduction to cellular organization. Of interest, a significant proportion of IPSes are involved in regulation of protein synthesis and degradation, suggesting that the IPDP may largely control protein turnover by modulating these fundamental processes.
20S proteasome cleaves IPSes at different rates with generation of distinct cleavage products
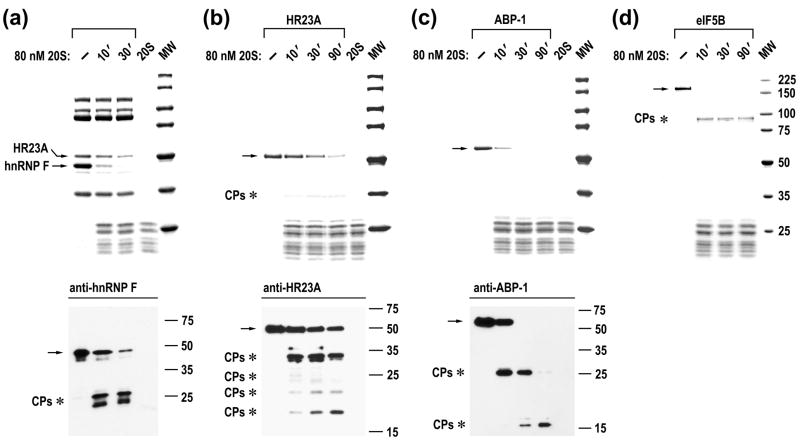
To address the mechanism of IPS processing by 20S proteasomes, we first followed the course of cleavage for all thirty IPSes, fourteen of which were highly purified. IPS cleavage was monitored by Blue R staining and immunoblot analysis, using antibodies specific to seventeen proteins. In vitro analysis revealed that 20S proteasomes cleave twenty two out of the thirty IPSes into discrete proteolytic products (Fig. 2; also see Supplementary Table 1). Cleavage of certain IPSes generated large products that were stable upon further incubation with 20S proteasomes (e.g., eIF5B, Fig. 2d), whereas processing of other proteins included generation of intermediate products that were further cleaved into smaller species (e.g., ABP-1, Fig. 2c). Differences in the cleavage rate of individual IPSes were apparent upon examination of fractions containing several IPSes (e.g., 20S proteasomes cleave hnRNP F faster than HR23A protein, Fig. 2a). When similar amounts of highly purified IPSes were examined, a tenfold range in the cleavage rate was observed (Fig. 2b to d; compare cleavage rate of HR23A (the slowest) with that of ABP-1 and eIF5B). Thus, IPSes have distinct intrinsic half-reaction times when exposed to 20S proteasomes, likely due to differences in structural organization.
Fig. 2.
Analysis of IPS cleavage by 20S proteasome. Time courses of cleavage of partly purified HR23A and hnRNP F (a) and highly purified proteins (b–d). Reactions were assembled as indicated above the panels. Upper panels, Blue R stained SDS-PAGE; bottom panels, immunoblot analysis. The positions of intact IPSes (arrows) and cleavage products (CPs, asterisks) are marked to the left of the panels.
20S proteasomes cleave IPSes at disordered regions sparing structured domains
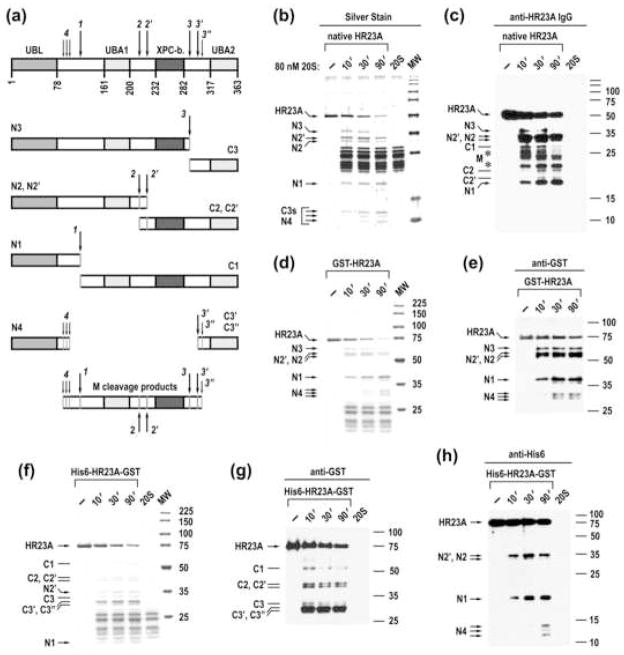
Cleavage of IPSes by 20S proteasomes generated distinct proteolytic products, suggesting that specific sites or protein regions were recognized. To determine whether cleavage sites are related to protein domain organization, we used HR23A and p47 proteins as models because their structural organization is characterized in molecular detail. HR23A contains four structured domains, connected by flexible linker regions (schemed in Fig. 3a).19 Silver staining and immunoblot analysis of the time course of cleavage of native HR23A protein by 20S proteasomes revealed products ranging in size from 10 to 38 kDa, with larger products progressively processed into smaller species over the incubation course, suggesting that HR23A is cleaved at several sites (Fig. 3b and c). Recombinant GST-HR23A and His6-HR23A-GST were cleaved at a rate similar to that of native HR23A, with cleavage products readily observed on Blue R stained SDS-PAGE and by immunoblot analysis, using antibodies specific to terminal tags (Fig. 3d to h). Based upon the mobility of “tagged” cleavage products and the time-course of their generation, the primary sites of cleavage were mapped to the three flexible linker regions of HR23A (Fig. 3a). Thus, the 20S proteasome cleaves HR23A in disordered regions, resulting in the generation of products that contain a variable number of structured domains. This mode of cleavage appears to be a general property of the catalytic core. Using a similar approach, we have shown that the native 20S proteasome cleaves p47 protein, which contains three structured domains that are connected by two extended flexible regions,20 at two disordered regions, generating products that encompass structured domains (Supplementary Fig. 3). Additionally, mass spectrometry analysis of GAPVD1 cleavage products revealed cleavage events at internal sites that preserved both the N- and C-terminal structured domains (Supplementary Fig. 4). Of interest, the cleavage sites were mapped to internal regions for all three proteins, suggesting that the endoproteolytic proteolysis is a widespread mechanism.
Fig. 3.
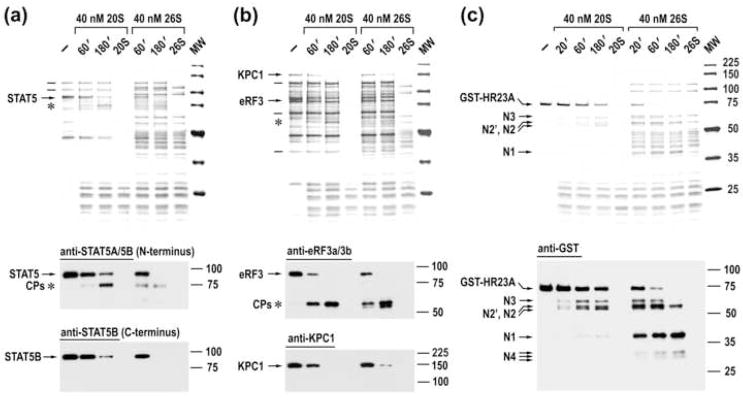
20S proteasome cleaves HR23A at internal disordered regions while sparing structured domains. (a) The model of HR23A cleavage. The upper lane schematically represents the structure of HR23A, the empty boxes denote flexible regions and the filled boxes correspond to the ubiquitin-like (UBL), ubiquitin associated (UBA), and XPC protein-binding (XPC-b) domains. Positions of cleavage sites (arrows with numbers) are tentative, based upon the mapping of cleavage products with specific antibodies and the mobility of the products. (b, c) Time course of cleavage of native rabbit HR23A protein. Reactions were assembled as indicated and analyzed by silver staining and western blotting with anti-serum to human HR23A protein. The assignment of cleavage products is based upon their mobility and time course of their generation. Cleavage products that likely comprise the middle portion of HR23A are marked with asterisks. (d, e) Time course of cleavage of GST-human HR23A recombinant protein. (f–h) Time course of cleavage of His6-human HR23A-GST. Reactions were stained with Blue R or probed with antibodies specific to terminal tags as indicated above the panels. The positions of C-terminal (Cx) and N-terminal (Nx) cleavage products are marked with lines and arrows, respectively, to the left of the panels.
Evolutionarily distant 20S proteasomes specifically cleave cellular IPSes
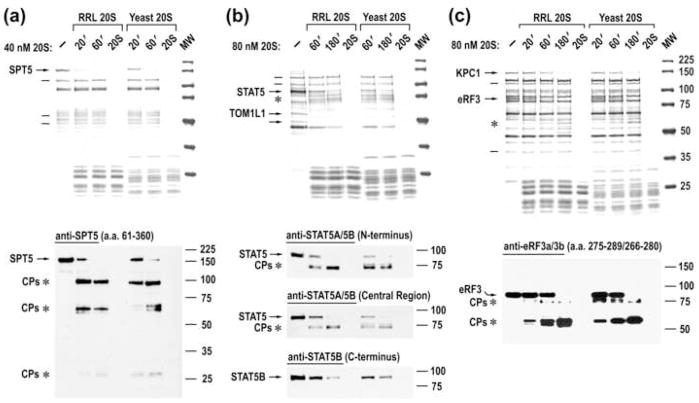
In order to determine whether the mechanism of selective protein cleavage by 20S proteasomes is evolutionarily conserved in eukaryotes, we fractionated yeast lysates and then examined them for the presence of IPSes, using the same approach described for RRL. Similar to the results obtained in the mammalian cell system, the yeast 20S proteasome also specifically cleaved a significant proportion of yeast proteins (data not shown). We then tested yeast 20S proteasomes for specific selection and cleavage of mammalian IPSes. Remarkably, all of the mammalian IPSes contained in individual fractions were specifically cleaved by yeast 20S proteasomes, whereas other cellular proteins that were not processed by mammalian catalytic cores remained intact (Fig. 4, upper panels). Further, yeast 20S proteasomes cleaved mammalian IPSes with generation of cleavage products similar to those produced by mammalian catalytic cores, suggesting cleavage at similar sites (Fig. 4, bottom panels). Moreover, IPSes with no known homologues in yeast, such as STAT5, were recognized by yeast 20S proteasomes and cleaved at appropriate sites (Fig. 4b). The reciprocal experiments, when partly purified fractions containing yeast proteins were incubated with yeast or mammalian catalytic cores, revealed yeast IPSes that were specifically cleaved by either of the 20S proteasome species (Supplementary Fig. 5). Collectively, these results demonstrate that the mechanism of IPS selection and cleavage by 20S proteasomes are conserved throughout the eukaryotic kingdom and suggest that the IPDP is an evolutionarily ancient and universal proteolytic pathway.
Fig. 4.
Mammalian and yeast 20S proteasomes specifically recognize and cleave the same mammalian IPSes. (a–c) Blue R staining of SDS-PAGE of time courses of cleavage of partly purified rabbit IPSes with rabbit or yeast 20S proteasomes (upper panels) and immunoblot analysis of these reactions (bottom panels). The positions of IPSes (arrows), their cleavage products (asterisks), and some of the stable cellular proteins (lines) are marked to the left of the panels.
26S proteasomes specifically cleave cellular IPSes
To investigate how the docking of a regulatory complex(es) onto the catalytic core would affect IPS recognition and cleavage, 26S proteasomes were purified from RRL and largely separated from free catalytic cores by gel filtration. Purified proteasomes contained mainly 19S caps and a peak elution fraction comprised singly and doubly capped species at approximate ratio of 8:3, respectively (Supplementary Fig. 6) 26S proteasomes were about fivefold more active in hydrolysis of model peptide substrates than 20S proteasomes (Supplementary Fig. 6e), as expected from their open gate conformation.
Next, we examined whether 26S proteasomes specifically recognize and cleave IPSes within a mixture of native cellular proteins, similar to 20S proteasomes. Sixteen fractions, containing partly purified IPSes, were incubated with 26S proteasomes or 20S proteasomes and the integrity of protein species was visualized on Blue R stained SDS-PAGE. As subunits of the 26S proteasome mask many cellular proteins, only species with mobility distinct from that of proteasomal subunits were surveyed. This analysis revealed that all discernable IPSes contained in individual fractions were cleaved by 26S proteasomes, whereas cellular proteins that were not processed by 20S proteasomes also remained intact upon incubation with 26S proteasomes (Fig. 5a and b, upper panels; also see Supplementary Fig. 7a).
Fig. 5.
26S and 20S proteasomes specifically cleave the same IPSes. Blue R staining of SDS-PAGE of time courses of cleavage of partly purified native IPSes (a, b) and recombinant GST-HR23A (c) with 20S and 26S proteasomes (upper panels) and immunoblot analysis of these reactions (bottom panels).
Further, immunoblot analysis of time courses of cleavage of twelve native IPSes, along with recombinant HR23A and p47 proteins, revealed that 26S proteasomes cleave IPSes with generation of cleavage products similar to those produced by free catalytic cores, suggesting cleavage at the same, presumably disordered regions (Fig. 5, bottom panels; Supplementary Figs. 7a and 9b along with Supplementary Table 1). Cleavage products of some IPSes were more labile upon further incubation with 26S proteasomes (e.g., STAT5 cleavage products, Fig. 5a), consistent with a model where the rest of the protein is unfolded and digested sequentially, following the initial cleavage at a disordered region.21 Cleavage products of other IPSes were equally stable following incubation with either 20S or 26S proteasomes (e.g., eRF3, Fig. 5b; SPT5, Supplementary Fig. 7a). Likely, these cleavage products consist of tightly folded structures that hinder processive proteasomal degradation, which has been shown for certain protein domains.22
26S proteasomes cleaved a number of IPSes at a rate 2–3 fold faster than free catalytic cores (e.g., STAT5, Fig. 5a), while the cleavage rate of other IPSes was similar or somewhat slower than that by 20S proteasomes (e.g., eRF3 and KPC1, Fig. 5b). Of note, the presence of the 19S cap significantly stimulated the cleavage of HR23A (Fig. 5c), likely due to specific binding of the protein to the 26S proteasome.23
Finally, cleavage of several IPSes was studied using a fraction of 26S proteasomes that contained singly and doubly capped species at approximate ratio of 2:5, respectively (Supplementary Fig. 8a). No appreciable differences in IPS processing were observed between the two pools of 26S proteasomes, enriched in singly or doubly capped species (Supplementary Figs. 7b, 8b, and 9).
Proteasomes contribute to physiological regulation of IPS levels in living cells largely independent of ubiquitination
Proteasomes were implicated in degradation of only two out of the thirty IPSes identified: HR23A is specifically degraded during S phase of cell cycle 24 and the phosphorylated form of STAT5A is specifically degraded in the nucleus;25 in both cases, degradation was mediated by ubiquitination of substrates. To ascertain the biological relevance of our in vitro findings, we investigated the role of proteasomes and ubiquitination in regulation of different IPSes, identified in this study and previously,11 in the living cell. First, we evaluated the expression levels of multiple IPSes during continuous growth of two cell lines, LoVo and Huh-7, and the contribution of proteasomes to IPS regulation. Although similar results were obtained in both cell cultures, data is shown for LoVo cells as they were efficiently arrested by contact inhibition and prolonged treatment with proteasome inhibitors did not induce a marked cytotoxic effect.
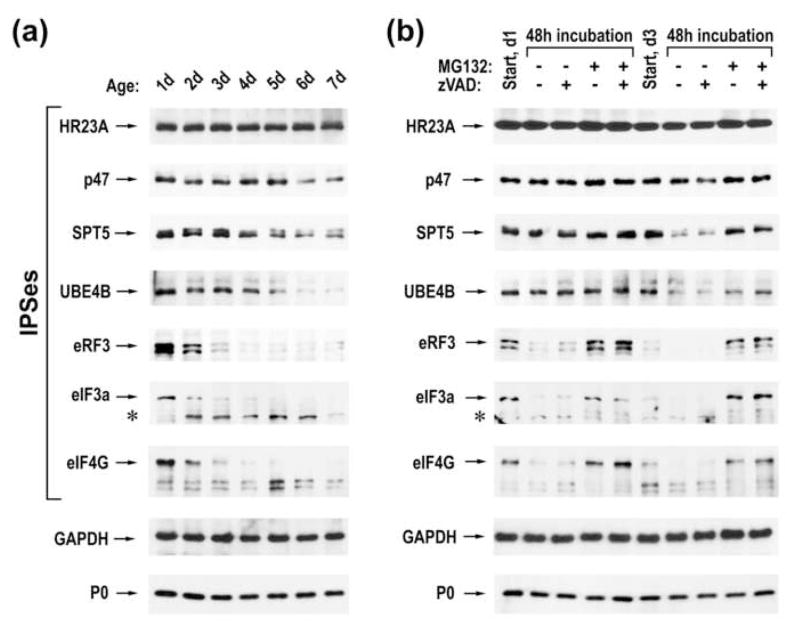
We found that the abundance of different IPSes changed unequally during seven day growth of LoVo cells (Fig. 6a). For example, little changes in the level of HR23A were detected. Levels of p47, SPT5, and UBE4B did not change upon the transition from the proliferative (day 1) to resting (day 3) state, but were differentially reduced thereafter. In contrast, a rapid decline in the amounts of IPSes involved in protein biosynthesis (eRF3, eIF3a, and eIF4G) was observed between days one to three (during the transition of cells from proliferative to resting state) and low levels of these factors were maintained after entering quiescence. This dramatic reduction was likely due to the cleavage of translation factors by proteasomes, as an accumulation of eIF3a cleavage products was observed concomitant with a decrease in the intact factor.
Fig. 6.
Proteasomes differentially regulate levels of different IPSes. (a) Immunoblot analysis of LoVo lysates harvested at different days, using antibodies specific to different IPSes as well as to GAPDH and ribosomal protein P0. (b) Immunoblot analysis of LoVo lysates harvested at different days following treatment with 5 μM MG132 and 50 μM z-VAD-FMK as indicated above the panel. The positions of intact proteins and their cleavage products (asterisk) are indicated to the left of the panel.
To demonstrate that proteasomes were responsible for the decline in IPS levels during cell growth, one or three day old LoVo cells were incubated in the presence or absence of the proteasome inhibitor MG132, caspase inhibitor z-VAD-FMK, or their combination. Cellular lysates were harvested before and after 48 hours of treatment and then subjected to immunoblot analysis (Fig. 6b). A modest accumulation of HR23A was observed when either one or three day old cells were treated with MG132, indicating that proteasomes continuously degrade the protein at a rather slow rate. A minor accumulation of SPT5 and UBE4B was observed when one day old cells were treated with MG132, suggesting that these IPSes were not targeted for proteasomal destruction during cellular transition from dividing to resting state. The reduction of SPT5 and UBE4B levels between days three to five was prevented by treatment with MG132, implicating proteasomes in their accelerated decay in quiescent cells. High levels of eRF3, eIF3a, and eIF4G were restored by proteasome inhibition, beginning on day one of culture. Thus, it appears that proteasomes are responsible for the rapid cleavage of these IPSes during the transition of cells from proliferative to resting state. Further, treatment of three day old cells with MG132 resulted in the accumulation of intact translation factors to levels comparable to those characteristic of one day old cells, suggesting that IPSes involved in translational control are continuously synthesized, even after entering quiescence, but then rapidly cleaved by proteasomes. Of note, the accumulation of IPSes following the inhibition of proteasomes is reversible. Thus, the surplus of eIF3a and eIF4G was rapidly diminished following the removal of MG132, with low protein levels (characteristic of quiescent cells) observed at 24 hours after the relief from the proteasome inhibition (Supplementary Fig. 10). Similarly, proteasomes degraded the surplus of UBE4B, albeit at a slower rate, adjusting the protein quantity to low levels at 36 hours after the MG132 removal. These results demonstrate that proteasomes regulate physiological levels of different IPSes via degradation.
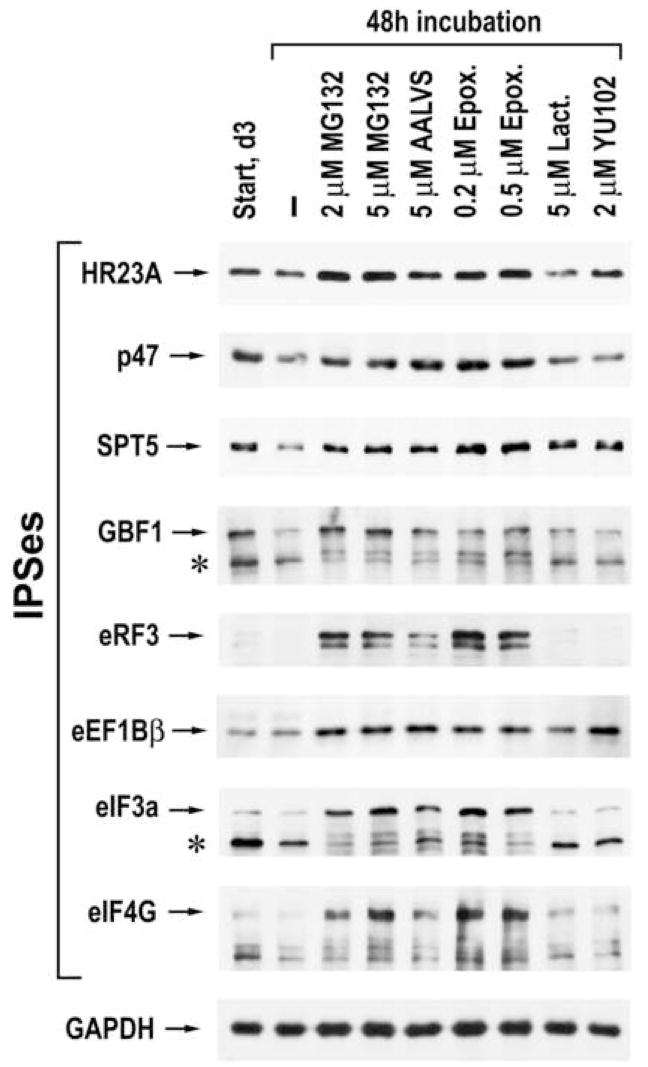
To alleviate any concerns that treatment of cells with MG132 could also inhibit the activity of other resident cellular proteases, three day old LoVo cells were treated with four additional proteasome inhibitors that differ in their potency and specificity of inactivation of the three distinct proteolytic centers of the proteasome. Immunoblot analysis of these cellular lysates revealed that the cleavage of all IPSes tested was prevented by MG132 and two highly specific proteasome inhibitors, epoxomicin and Ada-(Ahx)3-(Leu)3-vinyl sulphone (AALVS) (Fig. 7). This result validates the involvement of proteasomes in the regulation of IPS levels in the cell and suggests little contribution from other cellular proteases. Two other proteasome inhibitors, YU102 and lactacystin, were only effective in the protection of certain IPSes (e.g., SPT5, HR23A, eEF1Bβ). These variations in the efficacy of protection are likely attributable to differences between IPSes in the composition of scissile peptide bonds that are preferentially cleaved by one of the three distinct catalytic sites of the proteasome and differences in the specific inactivation of these proteolytic centers between the inhibitors.
Fig. 7.
The effect of different proteasome inhibitors on IPS levels. LoVo cells were treated as indicated above the panels and the lysates were analyzed by western blotting, using antibodies specific to the indicated proteins.
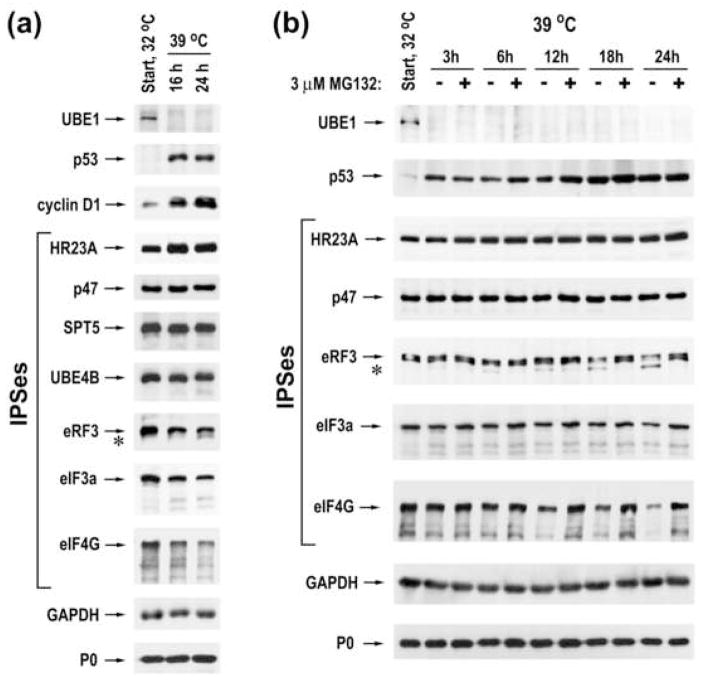
Finally, we evaluated the importance of protein ubiquitination in IPS degradation. The mouse ts85 cell line contains a temperature-sensitive ubiquitin-activating enzyme E1 (UBE1) and as a consequence, protein degradation via the ubiquitin-dependent pathway is drastically reduced at the restrictive temperature.26,27 UBE1 was rapidly degraded at the restrictive temperature (Fig. 8a and b, top rows), as expected. The disruption of ubiquitination resulted in a dramatic accumulation of tumor suppressor p53 and cyclin D1, whose levels are controlled by the UPS (Fig. 8a), consistent with previous reports.28,29 A moderate increase of HR23A was also observed, indicating that the ubiquitin-dependent proteolytic pathway also contributes to regulation of its levels. However, accumulation of other IPSes was not detected after blocking protein ubiquitination (Fig. 8a). Moreover, levels of translation factors eRF3, eIF3a, and eIF4G decreased after growth at the restrictive temperature. Additionally, a faster migrating species of eRF3 accumulated (Fig. 8, asterisk), likely corresponding to proteasomal cleavage products. Treatment of cells with MG132 prevented both the reduction in levels of translation factors as well as the appearance of eRF3 cleavage products (Fig. 8b). Collectively, these results suggest that many cellular IPSes can be degraded by proteasomes independent of ubiquitination.
Fig. 8.
Proteasomes degrade many IPSes in a ubiquitin-independent manner. (a) ts85 cells were grown one day at the permissive temperature (32°C) and harvested immediately (start) or after 16 and 24 hours of further growth at the restrictive temperature (39°C). Cellular lysates were analyzed by western blotting, using antibodies specific to the indicated proteins. (b) Immunoblot analysis of ts85 cells grown at 32°C and harvested immediately and at different time points after further growth at 39°C in the absence or in the presence of 3 μM MG132.
Discussion
The ubiquitin-26S proteasome system (UPS) is essential for proper cellular function and is considered to be the major mechanism of proteasomal degradation of cellular proteins. The system relies upon ATP-dependent enzymatic steps and multiple enzymes that ensure timely ubiquitination of specific protein substrates via diverse regulatory mechanisms. Accumulating data now shows that certain cellular proteins are degraded by proteasomes either completely ubiquitin-independent or by both pathways.8,9,15,30,31 Our findings highlight the importance of the ubiquitin-independent mechanism of protein degradation by proteasomes, as it may regulate the stability and function of more than 20% of cellular proteins. Unlike the UPS, the intrinsic proteasome degradation pathway (IPDP) does not require a complex system of substrate-modifying enzymes and can be executed by both the 26S and the 20S proteasomes. “Free” 20S particles are highly abundant proteasome species in mammalian cells32,33 and in contrast to 26S proteasomes, which are heavily engaged in degradation of ubiquitylated proteins, these species can function solely in the IPDP and markedly contribute to regulation of IPSes.
One critical question is what structural motifs afford targeting of a cellular protein to proteasomes. IPSes are specifically cleaved by evolutionarily distant 20S proteasomes as well as by 26S proteasomes, whereas many other cellular proteins are not, suggesting the existence of a universal structural signature shared by all IPSes. Inspection of structured domains and amino acid sequences revealed only one rather broad structural feature shared by ~70% of IPSes, α-helix rich regions (data not shown). However, the actual folds of such regions are not shared by any considerable number of protein substrates. This study and previous reports10,11,16 suggest that 20S proteasomes cleave IPSes at disordered regions, outside of structured domains. The presence of an accessible disordered region that can thread through the substrate channel and reach the internal proteolytic centers is apparently a prerequisite for IPS cleavage by the catalytic core, since 20S proteasome cannot unfold protein substrates. Such regions likely constitute a critical structural determinant for IPS cleavage by the 26S proteasome as well. This view is consistent with recent reports that nonubiquitylated tightly folded proteins are refractory to proteasomal proteolysis even in the presence of ATP34 and extended disordered regions greatly facilitate protein degradation by 26S proteasomes.21,35 Conceivably, all cellular IPSes contain susceptible disordered regions while proteins that are resistant to the IPDP lack such regions.
IPSes were cleaved by 20S proteasomes in the closed gate conformation, suggesting that certain interactions between the catalytic core and an IPS promote opening of the gate and translocation of specific disordered amino acid sequences into the internal catalytic chamber. Several studies demonstrated that 20S proteasome effectively degrades oxidized proteins in vitro and appears to play a major role in degradation of these damaged proteins in cell cultures.7,9,36 Davies and colleagues proposed that partial unfolding and exposure of internal hydrophobic patches of amino acids are key to selective recognition and degradation of oxidized proteins by the 20S proteasome.7,37 In this model, the hydrophobic patches bind to the α-subunits of the 20S proteasome, thereby opening the substrate channel. It is quite likely that cellular IPSes, in their native conformation, contain specific surface hydrophobic patches that confer both binding to the catalytic core and gate opening. Consistent with this notion is our observation that the addition of hydrophobic peptides impeded IPS cleavage, probably due to the competition for binding sites on the surface of the 20S proteasome (unpublished data). Conceivably, the actual composition of surface hydrophobic patch(es) and extended disordered region(s) could largely determine the efficiency of IPS recognition and processing by 20S proteasomes. Indeed, we observed significant variations in the cleavage rate of different IPSes, suggesting that proteins might have evolved structurally to control their degradation by catalytic cores. Further, docking of the 19S cap exerts differential effects on IPS cleavage. Some IPSes were cleaved two- to threefold faster by the 26S proteasome, likely due to the open gate conformation of this species and accelerated translocation of a disordered region into the catalytic chamber. Other IPSes were processed by 20S and 26S proteasomes at similar rates, suggesting that certain interactions between a protein substrate and the endplate of the catalytic core augment specific binding to the 20S particle, thereby compensating for the impeded entrance of disordered region to the catalytic sites. The cleavage rate of HR23A was dramatically stimulated by docking of the 19S cap, likely due to specific tethering of the protein to the 26S proteasome, mediated by interactions between UBL domain of HR23A and Rpn10 subunit of the 19S cap.23 Similarly, eIF3a subunit of eIF3 was cleaved by 26S proteasomes significantly faster than by catalytic cores (data not shown), likely because of additional interactions between the 19S cap and other subunits of eIF3.38,39
The UPS typically degrades protein substrates processively, whereas the IPDP generates products encompassing structured domains. An important question is whether IPS cleavage leads to the production of new protein species with functional properties distinct from those of the intact protein. Recent reports demonstrated that 20S proteasome cleaves translation initiation factors eIF3 and eIF4F to produce species with distinct functional capabilities11 and processes NF-κB1 precursor p105 into a functional p50 product.13 Limited proteolysis of certain ubiquitylated proteins by the 26S proteasome, yielding biologically active fragments, was also reported.22,40 While the functional consequences of IPS cleavage were not examined in this study, some implications of their cleavage could be proposed based upon the analysis of cleavage products and reports published by other investigators. For example, proteasomes cleave STAT5 proteins to produce a 75 kDa-long species that lacks the carboxy-terminal transactivation domain, as evidenced by immunoblot analysis using antibodies specific to different regions of the proteins (Figs. 4b and 5a). Naturally occurring C-terminally truncated forms of STAT5A and STAT5B have been observed in different cell types, where they are generated by the action of cellular proteases whose identity has not been established yet.41 These truncated STAT5 proteins are activated in response to cytokine signaling by tyrosine phosphorylation, bind DNA, and prevent transcription activation of STAT5-targeted genes, thereby exerting a dominant negative activity. Proteolytic processing of STAT5 is critical for developmental regulation of myeloid cells and its disturbance has been implicated in a number of human disorders. Future investigations should address how the IPDP contributes to the regulation of STAT5 biology. Collectively, these observations suggest that the IPDP can regulate cellular processes not only via destruction of IPSes but perhaps also by the generation of specific proteolytic species with distinct functions.
The IPDP appears to contribute to differential regulation of IPS levels during cell growth, highlighting one important aspect of this pathway in cellular physiology. We observed a dramatic decrease in levels of critical translation factors during cellular transition from dividing state to quiescence, suggesting that their cleavage by proteasomes can contribute to a well-known phenomenon of translational attenuation in resting/quiescent cells.42 A broader question is how the IPDP regulates levels of different IPSes at distinct stages of cell life. One potential mechanism may involve regulation via association of the catalytic core with different regulatory complexes. In support of this idea, in vitro studies showed that the 20S proteasome is more active in degradation of the cell cycle regulator p21cip1 than the 26S proteasome, containing 19S caps.43 On the other hand, association of the catalytic core with the REGγ complex significantly stimulated p21 degradation in vitro and in cell cultures, due to specific interactions between the REGγ lid and the target protein.43,44 This study also shows differences in the activity of 20S and 26S proteasomes in cleavage of specific IPSes. In addition, certain cellular proteins can assist the targeting of a specific substrate to catalytic cores. For example, MDM2 was shown to facilitate the delivery of Rb protein to the 20S proteasome, thereby stimulating the ubiquitin-independent degradation of Rb in vitro and in cell cultures.14 Further, association of IPSes with specific proteins may hamper their recognition by the IPDP. Mathes and colleagues demonstrated that free IκBα is rapidly degraded by proteasomes independent of ubiquitination and NF-κB binding precludes this degradation pathway.45 In contrast to free protein, NF-κB-bound IκBα is degraded via the UPS, highlighting distinct roles of the IPDP and the UPS in regulation of protein homeostasis. Finally, the intracellular colocalization of proteasome species with particular IPSes likely contributes to the differential cleavage of cellular IPSes. The abundance, composition, and intracellular localization of proteasomes (as well as specific regulatory complexes and protein adaptors) vary markedly throughout cell cycle progression, differentiation, development, ageing, environmental cues and pathology.46,47 Further expansion of this field will afford a better understanding of the role of the IPDP in the physiology of cells and organisms.
Materials and methods
Plasmids
Plasmids pQE-30-p47,48 pGex-4T-2-hHR23A,24 and pET28-eIF4GI(697–1076)49 have been previously described. The entire ORF of hHR32A was PCR-amplified from pcDNA3-V5-hHR23A50 with addition of a coding sequence for His6 tag to the N terminus, digested with BspHI and NotI, and ligated into the NcoI/NotI-digested pET28- GST vector to generate pET28-His6-HR23A-GST vector. pET28-GST was constructed by PCR amplification of the GST ORF from pGEX-2TK (GE Healthcare), using primers containing HindIII-NotI and XhoI restriction sites, digested with HindIII and XhoI, and ligated into HindIII/XhoI-digested pET28a (Novagen). The eIF3a coding region, spanning amino acids 495 to 891, was PCR amplified from pUC19-eIF3a,51 digested with NdeI and NotI, and ligated into NdeI/NotI-digested pET28a to generate pET28-His6-eIF3a(495–891) vector.
Protein purification
1L rabbit reticulocyte lysate (RRL) (Green Hectares) was fractionated into ribosomal salt wash (RSW) and postribosomal fractions (PRF) as described.11 5 μM MG132 and Protease Inhibitor Cocktail “Complete”, at the concentration recommended by the manufacturer (Roche), were added to RRLs immediately after thawing the lysates and during suspension of the polysomes. All chromatography purification steps were carried out at 4°C with buffer A containing 20 mM Tris-HCl (pH 7.5), 1 mM DTT, 0.1 mM EDTA, 10% glycerol, and KCl at the stated concentration (i.e., A0 contains no salt, A100 contains 100 mM KCl, etc.). RSW and PRF were fractionated by ammonium sulfate cuts, dialyzed against buffer A100, and further fractionated by consecutive step-elution from DEAE cellulose column (DE52; Whatman) and phosphocellulose column (P11; Whatman). Elution fractions were dialyzed against buffer A100, applied to an FPLC monoQ HR 5/5 column (GE Healthcare), and 1 ml fractions were collected across a 30 ml 100–500 mM KCl gradient, by use of an AKTA FPLC system (GE Healthcare). Individual or combinations of two adjacent monoQ elution fractions were concentrated to 200 μl using Vivaspin 10,000 MWCO HY (VivaScience), applied to an FPLC gel filtration column (GE Healthcare) equilibrated with buffer A200, and 0.5 ml fractions were collected following elution with A200. Additional purification steps included KCl gradient elution from 1 ml HiTRAP Blue and Phenyl FF columns (GE Healthcare) as indicated (see Supplementary Table 1). The final purification step included a second separation on monoQ column wherefrom ~300 μl peak fractions were collected, divided into aliquots, frozen and stored at −80°C until used.
Recombinant GST-HR23A, His6-HR23A-GST, His6-p47, eIF4GI(697–1076), and His6-eIF3a(495–891) were expressed in Eschericia coli BL21(DE3) (Novagen) and purified by chromatography using GST-Bind Resin (Novagen) and/or Ni-NTA agarose (Qiagen) according to the manufacturer’s protocols. HR23A and p47 proteins were then applied to the FPLC monoQ HR 5/5 column and fractions collected across a 100–500 mM KCl gradient. p47 protein was concentrated and applied on Superdex 200 10/30 GL column (GE Healthcare) wherefrom the peak was collected between 15 and 15.5 ml.
Proteasome purification
Rabbit 20S proteasomes were purified from RRL PRF by the previously described protocol.11 The yeast 20S proteasomes were purified from 10 liters of Saccharomyces cerevisiae, strain BJ2168 (a gift from D. Bishop, University of Chicago), grown on YPD to the stationary phase. After centrifugation, the cell pellet was resuspended in a fourfold volume of Buffer Y containing 20 mM Tris-HCl (pH 7.5), 100 mM KCl, 5 mM MgCl2, and 2mM DTT. Cells were lysed with a French press and the extract clarified as described by Glickman et al.52 The extracts were fractionated into the RSW and PRF and 20S proteasomes were purified from PRF using the same protocol as for purification of rabbit catalytic cores. 20S proteasomes were concentrated using Vivaspin 30,000 MWCO HY (VivaScience), divided into aliquots, frozen and stored at −80°C until used.
26S proteasomes were purified from 600 ml RRL PRF. All purification steps were carried out at 4°C with buffer E containing 20 mM Tris-HCl (pH 7.5), 2 mM MgCl2, 1 mM DTT, 10% glycerol, 1 mM ATP, and KCl at the stated concentration. PRF were spun for 4.5 h at 200,000 × g. The resulting proteasome-containing pellets were dissolved in buffer E100 and loaded onto DEAE cellulose column. After washing with buffer E100, proteasomes were eluted with buffer E250, diluted with buffer E0 to contain 100 mM KCl, and loaded onto P11 phosphocellulose column. The flowthrough fraction was then applied to the FPLC monoQ HR 5/5 column and 1 ml fractions were collected across a 30 ml 100–500 mM KCl gradient. Proteasome-containing fractions (eluted between 330 and 400 mM KCl) were concentrated to 300 μl using Vivaspin 30,000 MWCO HY (VivaScience), applied to an FPLC Superose 6 10/600 GL gel filtration column (GE Healthcare) equilibrated with buffer E150, and 0.7 ml fractions were collected following elution with the same buffer. 26S proteasome-containing elution fractions #26 and #29 (enriched in doubly capped and singly capped species, respectively) were divided into aliquots, frozen and stored at −80°C until used.
The integrity of purified proteasomes was verified by electron microscopy visualization of uranyl acetate stained particles put onto freshly glow-discharged and carbon-coated copper grids as described previously.11
Proteolytic assays
To measure peptidase activity, 5 μM Suc-LLVY-amc or 50 μM Ac-nLPnLD-amc (Biomol International) were incubated with 10 nM 20S proteasomes for one hour at 37°C in buffer C (20 mM Tris-HCl (pH 7.5), 2 mM DTT) supplemented or not with 40 μM suc-FLF-mna or 0.02% SDS. Similar conditions were used to measure peptidase activity of 26S proteasomes except for reactions were assembled in buffer D (20 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 0.04 mM ATP) supplemented or not with 1 mM ATP. The reactions were stopped by the addition of 1% SDS and fluorescence of released AMC was measured (excitation, 380 nm; emission, 460 nm).
To study IPS processing, reactions were assembled in buffer B (20 mM Tris-HCl (pH 7.5), 100 mM KCl, 2 mM MgCl2, 0.25 mM spermidine, 2 mM DTT) with the indicated amounts of proteasomes and incubated at 37°C. Buffer B was supplemented with 3–4% glycerol to maintain the native state of proteasomes. 26S proteasome-containing reactions included 1mM ATP. 20 to 40 μl aliquots were transferred from the reactions to Laemmli sample buffer at indicated time points and immediately boiled for 5 minutes. The proteins were resolved by SDS-PAGE and stained with Blue R or silver (GE Healthcare) or, when indicated, transferred to PVDF membranes for immunoblot analysis. For detection of IPSes, 6 μl aliquots from gel-filtration elution fractions were incubated with or without 200 nM 20S proteasome for three hours in 20 μl reactions. The analyzed fractions did not contain any endogenous proteolytic activity, as their incubation alone had no effect on the integrity of any protein species. Pre-incubation of 20S proteasomes with 25 μM MG132 for 5 min at 37°C prevented IPS cleavage, further verifying the specificity of cleavage reactions.
Protein sequencing
Purified IPSes were subjected to 200 mm-long 9% SDS-PAGE and stained with 0.5% Coomassie blue G-250. The bands were cut from the gel matrices and sent for sequencing by LC-MS/MS to the Proteomics Core Laboratory of the University of Chicago.
Cell culture and treatments
Human colorectal adenocarcinoma cell line LoVo was obtained from American Type Culture Collection and maintained as recommended. Human liver carcinoma cell line Huh-7 was maintained as described previously.11 For all experiments, cells were seeded in 25 cm2 Falcon tissue culture flasks at amounts that form 60–70% confluent monolayers the next day (day 1) and 100% confluent monolayers by day 3. The growth media was replaced with fresh media containing 5% FBS at day one and every third day, in order to avoid serum and amino acid starvation. At the beginning of treatments, the replacement media was supplemented with 0.05% DMSO (control cells) or the following proteasome inhibitors purchased from Biomol International: MG132, epoxomicin, Ada-(Ahx)3-(Leu)3-vinyl sulphone, lactacystin, and YU102. In addition, cells were treated with 50 μM z-VAD-FMK (Sigma). The cell monolayers were harvested and boiled at indicated time points in total lysis buffer (50 mM Tris pH 6.8, 1% SDS, 350 μM β-mercaptoethanol, 10% glycerol, and 0.1% bromophenol blue).
The ts85 mouse mammary carcinoma cell line, kindly provided by D. Finley (Harvard Medical School), was maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Gibco) at permissive temperature (32°C) in 25 cm2 Falcon tissue culture flasks. Cell cultures were harvested before and after incubation at restrictive temperature (39°C) in the absence or presence of MG132 in total lysis buffer.
Immunoblot analysis and antibodies
Proteins were transferred onto Immobilin-PSQ (Millipore) in 10mM CAPS/10% Methanol (pH 11.0) and detected according to standard procedures, using antibodies specific for proteins of interest. Polyclonal chicken antibodies specific to eIF4G, eIF3a, and p47 were generated by “Alpha Diagnostic International” (Texas) following the immunization of chickens with recombinant proteins His6-p47, His6-eIF3a(495–891), and eIF4GI(697–1076) and purification of IgG antibodies by protein-coupled affinity chromatography. Anti-HR23A was kindly provided by P. M. Howley (Harvard Medical School); anti-hnRNP F by C. Milcarek (University of Pittsburgh). The following commercial antibodies were used: anti-HIP55/ABP-1 (ab2836-100, Abcam), anti-HRI (sc-30143, Santa Cruz), anti-APC8 (sc-20988, Santa Cruz), anti-APC6/Cdc16 (sc-6393, Santa Cruz), anti-eRF3 (ab23889, Abcam), anti-GBF1 (#612116, BD Transduction Laboratories), anti-eEF1Bβ/eEF1δ (ab13962-100, Abcam), anti-UBE4B (#611966, BD Transduction Laboratories), anti-STAT5A/5B against central region (#9310, Cell Signaling), anti-STAT5A/5B against the N-terminus (sc-836, Santa Cruz), anti-STAT5B against the C-terminus (sc-1656, Santa Cruz), anti-LR1/RPSA (ab711, Abcam), anti-SPT5 (sc-28678, Santa Cruz), anti-MyH9/IIA (M8064, Sigma), anti-KPC1/RNF123 (H00063891-M01, ABNOVA (Taiwan) Corporation), anti-His6 (27-4710-01, GE Healthcare), anti-GST (27-4577-01, GE Healthcare), anti-UBE1 (sc-53555, Santa Cruz), anti-p53 (sc-6243, Santa Cruz), anti-cyclin D1 (sc-753, Santa Cruz), anti-GAPDH (sc-25778, Santa Cruz), anti-ribosomal P0 (R2031-25, US Biological).
Supplementary Material
Acknowledgments
We are grateful to our colleagues for providing the plasmids, antibodies, and cells used in this study. We thank G. Khramtsova and A. Puschell for technical assistance. The project described was supported by Grant Number R01AI064489 from the National Institute of Allergy and Infectious Diseases to EVP. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. JMB was supported by T32 AI065382, Biodefense Training in Host-Pathogen Interactions at The University of Chicago.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ciechanover A. Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Hematology. 2006;2006:1–12. doi: 10.1182/asheducation-2006.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg AL. Functions of the proteasome: from protein degradation and immune surveillance to cancer therapy. Biochem Soc Trans. 2007;35:12–17. doi: 10.1042/BST0350012. [DOI] [PubMed] [Google Scholar]
- 3.Wolf DH, Hilt W. The proteasome: a proteolytic nanomachine of cell regulation and waste disposal. Biochim Biophys Acta. 2004;1695:19–31. doi: 10.1016/j.bbamcr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Bajorek M, Glickman MH. Keepers at the final gates: regulatory complexes and gating of the proteasome channel. Cell Mol Life Sci. 2004;61:1579–1588. doi: 10.1007/s00018-004-4131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeMartino GN, Gillette TG. Proteasomes: machines for all reasons. Cell. 2007;129:659–662. doi: 10.1016/j.cell.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Reichsteiner M, Hill CP. Mobilizing the proteolytic machine: cell biological roles of proteasome activators and inhibitors. Trends Cell Biol. 2005;15:27–33. doi: 10.1016/j.tcb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Grune T, Merker K, Sandig G, Davies KJ. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem Biophys Res Commun. 2003;305:709–718. doi: 10.1016/s0006-291x(03)00809-x. [DOI] [PubMed] [Google Scholar]
- 8.Hoyt MA, Coffino P. Ubiquitin-free routes into the proteasome. Cell Mol Life Sci. 2004;61:1596–1600. doi: 10.1007/s00018-004-4133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orlowski M, Wilk S. Ubiquitin-independent proteolytic functions of the proteasome. Arch Biochem Biophys. 2003;415:1–5. doi: 10.1016/s0003-9861(03)00197-8. [DOI] [PubMed] [Google Scholar]
- 10.Liu CW, Corboy MJ, DeMartino GN, Thomas PJ. Endoproteolytic activity of the proteasome. Science. 2003;299:408–411. doi: 10.1126/science.1079293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baugh JM, Pilipenko EV. 20S proteasome differentially alters translation of different mRNAs via the cleavage of eIF4F and eIF3. Mol Cell. 2004;16:575–586. doi: 10.1016/j.molcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Lonard DM, Jung SY, Malovannaya A, Feng Q, Qin J, Tsai SY, Tsai MJ, O’Malley BW. The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGgamma proteasome. Cell. 2006;124:381–392. doi: 10.1016/j.cell.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 13.Moorthy AK, Savinova OV, Ho JQ, Wang VY, Vu D, Ghosh G. The 20S proteasome processes NF-κB1 p105 into p50 in a translation-independent manner. EMBO J. 2006;25:1945–1956. doi: 10.1038/sj.emboj.7601081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sdek P, Ying H, Chang DL, Qiu W, Zheng H, Touitou R, Allday MJ, Xiao ZX. MDM2 promotes proteasome-dependent ubiquitin-independent degradation of retinoblastoma protein. Mol Cell. 2005;20:699–708. doi: 10.1016/j.molcel.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Jariel-Encontre I, Bossis G, Piechaczyk M. Ubiquitin-independent degradation of proteins by the proteasome. Biochim Biophys Acta. 2008;1786:153–177. doi: 10.1016/j.bbcan.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Sorokin AV, Selyutina AA, Skabkin MA, Guryanov SG, Nazimov IV, Richard C, Th’ng J, Yau J, Sorenson PH, Ovchinnikov LP, Evdokimova V. Proteasome-mediated cleavage of the Y-box-binding protein 1 is linked to DNA-damage stress response. EMBO J. 2005;24:3602–3612. doi: 10.1038/sj.emboj.7600830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahlmann B, Becher B, Sobek A, Ehlers C, Kopp F, Kuehn L. In vitro activation of the 20S proteasome. Enzyme Protein. 1993;47:274–284. doi: 10.1159/000468685. [DOI] [PubMed] [Google Scholar]
- 18.Kisselev AF, Kaganovich D, Goldberg AL. Binding of hydrophobic peptides to several non-catalytic sites promotes peptide hydrolysis by all active sites of 20 S proteasomes. Evidence for peptide-induced channel opening in the alpha-rings. J Biol Chem. 2002;277:22260–22270. doi: 10.1074/jbc.M112360200. [DOI] [PubMed] [Google Scholar]
- 19.Walters KJ, Lech PJ, Goh AM, Wang Q, Howley PM. DNA-repair protein hHR23a alters its protein structure upon binding proteasomal subunit S5a. Proc Natl Acad Sci USA. 2003;100:12694–12699. doi: 10.1073/pnas.1634989100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan X, Simpson P, McKeown C, Kondo H, Uchiyama K, Wallis R, Dreveny I, Keetch C, Zhang X, Robinson C, Freemont P, Matthews S. Structure, dynamics and interactions of p47, a major adaptor of the AAA ATPase, p97. EMBO J. 2004;23:1463–1473. doi: 10.1038/sj.emboj.7600152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prakash S, Tian L, Ratliff KS, Lehotzky RE, Matouschek A. An unstructured initiation site is required for efficient proteasome-mediated degradation. Nat Struct Mol Biol. 2004;11:830–837. doi: 10.1038/nsmb814. [DOI] [PubMed] [Google Scholar]
- 22.Piwko W, Jentsch S. Proteasome-mediated protein processing by bidirectional degradation initiated from an internal site. Nat Struct Mol Biol. 2006;13:691–697. doi: 10.1038/nsmb1122. [DOI] [PubMed] [Google Scholar]
- 23.Hiyama H, Yokoi M, Masutani C, Sugasawa K, Maekawa T, Tanaka K, Hoeijmakers JH, Hanaoka F. Interaction of hHR23 with S5a. The ubiquitin-like domain of hHR23 mediates interaction with S5a subunit of 26 S proteasome. J Biol Chem. 1999;274:28019–28025. doi: 10.1074/jbc.274.39.28019. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Talis AL, Howley PM. Identification of HHR23A as a substrate for E6-associated protein-mediated ubiquitination. J Biol Chem. 1999;274:18785–18792. doi: 10.1074/jbc.274.26.18785. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Dai X, Haas AL, Wen R, Wang D. Proteasome-dependent down-regulation of activated Stat5A in the nucleus. Blood. 2006;108:566–574. doi: 10.1182/blood-2005-12-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciechanover A, Finley D, Varshavsky A. Ubiquitin dependence of selective protein degradation demonstrated in the mammalian cell cycle mutant ts85. Cell. 1984;37:57–66. doi: 10.1016/0092-8674(84)90300-3. [DOI] [PubMed] [Google Scholar]
- 27.Finley D, Ciechanover A, Varshavsky A. Thermolability of ubiquitin-activating enzyme from the mammalian cell cycle mutant ts85. Cell. 1984;37:43–55. doi: 10.1016/0092-8674(84)90299-x. [DOI] [PubMed] [Google Scholar]
- 28.Chowdary DR, Dermody JJ, Jha KK, Ozer HL. Accumulation of p53 in a mutant cell line defective in the ubiquitin pathway. Mol Cell Biol. 1994;14:1997–2003. doi: 10.1128/mcb.14.3.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nurtjahja-Tjendraputra E, Fu D, Phang JM, Richardson DR. Iron chelation regulates cyclin D1 expression via the proteasome: a link to iron deficiency-mediated growth suppression. Blood. 2007;109:4045–4054. doi: 10.1182/blood-2006-10-047753. [DOI] [PubMed] [Google Scholar]
- 30.Asher G, Shaul Y. p53 proteasomal degradation: poly-ubiquitination is not the whole story. Cell Cycle. 2005;4:1015–1018. doi: 10.4161/cc.4.8.1900. [DOI] [PubMed] [Google Scholar]
- 31.Basbous J, Jariel-Encontre I, Gomard T, Bossis G, Piechaczyk M. Ubiquitin-independent- versus ubiquitin-dependent proteasomal degradation of the c-Fos and Fra-1 transcription factors: Is there a unique answer? Biochimie. 2008;90:296–305. doi: 10.1016/j.biochi.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Brooks P, Fuertes G, Murray RZ, Bose S, Knecht E, Rechsteiner MC, Hendil KB, Tanaka K, Dyson J, Rivett J. Subcellular localization of proteasomes and their regulatory complexes in mammalian cells. J Biochem. 2000;346:155–161. [PMC free article] [PubMed] [Google Scholar]
- 33.Tanahashi N, Murakami Y, Minami Y, Shimbara N, Hendil KB, Tanaka K. Hybrid proteasomes. Induction by interferon-gamma and contribution to ATP-dependent proteolysis. J Biol Chem. 2000;275:14336–14345. doi: 10.1074/jbc.275.19.14336. [DOI] [PubMed] [Google Scholar]
- 34.Liu CW, Li X, Thompson D, Wooding K, Chang TL, Tang Z, Yu H, Thomas PJ, DeMartino GN. ATP binding and ATP hydrolysis play distinct roles in the function of 26S proteasome. Mol Cell. 2006;24:39–50. doi: 10.1016/j.molcel.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeuchi J, Chen H, Coffino P. Proteasome substrate degradation requires association plus extended peptide. EMBO J. 2007;26:123–131. doi: 10.1038/sj.emboj.7601476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- 37.Pacifici RE, Kono Y, Davies KJ. Hydrophobicity as the signal for selective degradation of hydroxyl radical-modified hemoglobin by the multicatalytic proteinase complex, proteasome. J Biol Chem. 1993;268:15405–15411. [PubMed] [Google Scholar]
- 38.Dunand-Sauthier I, Walker C, Wilkinson C, Gordon C, Crane R, Norbury C, Humphrey T. Sum1, a component of the fission yeast eIF3 translation initiation complex, is rapidly relocalized during environmental stress and interacts with components of the 26S proteasome. Mol Biol Cell. 2002;13:1626–1640. doi: 10.1091/mbc.01-06-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoareau Alves K, Bochard V, Rety S, Jalinot P. Association of the mammalian proto-oncoprotein Int-6 with the three protein complexes eIF3, COP9 signalosome and 26S proteasome. FEBS Lett. 2002;527:15–21. doi: 10.1016/s0014-5793(02)03147-2. [DOI] [PubMed] [Google Scholar]
- 40.Hoppe T, Matuschewski K, Rape M, Schlenker S, Ulrich HD, Jentsch S. Activation of a membrane-bound transcription factor by regulated ubiquitin/proteasome-dependent processing. Cell. 2000;102:577–586. doi: 10.1016/s0092-8674(00)00080-5. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell TJ, John S. Signal transducer and activator of transcription (STAT) signalling and T-cell lymphomas. Immunology. 2005;114:301–312. doi: 10.1111/j.1365-2567.2005.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meedel TH, Levine EM. Regulation of protein synthesis in human diploid fibroblasts: reduced initiation efficiency in resting cultures. J Cell Physiol. 1978;94:229–242. doi: 10.1002/jcp.1040940212. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Barton LF, Chi Y, Clurman BE, Roberts JM. Ubiquitin-independent degradation of cell-cycle inhibitors by the REGgamma proteasome. Mol Cell. 2007;26:843–852. doi: 10.1016/j.molcel.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Amazit L, Long W, Lonard DM, Monaco JJ, O’Malley BW. Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway. Mol Cell. 2007;26:831–842. doi: 10.1016/j.molcel.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 45.Mathes E, O’Dea EL, Hoffmann A, Ghosh G. NF-kappaB dictates the degradation pathway of IkappaBalpha. EMBO J. 2008;27:1357–1367. doi: 10.1038/emboj.2008.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rivett AJ, Bose S, Brooks P, Broadfoot KI. Regulation of proteasome complexes by gamma-interferon and phosphorylation. Biochimie. 2001;83:363–366. doi: 10.1016/s0300-9084(01)01249-4. [DOI] [PubMed] [Google Scholar]
- 47.Wojcik C, DeMartino GN. Intracellular localization of proteasomes. Int J Biochem Cell Biol. 2003;35:579–589. doi: 10.1016/s1357-2725(02)00380-1. [DOI] [PubMed] [Google Scholar]
- 48.Kondo H, Rabouille C, Newman R, Levine TP, Pappin D, Freemont P, Warren G. p47 is a cofactor for p97-mediated membrane fusion. Nature. 1997;388:75–78. doi: 10.1038/40411. [DOI] [PubMed] [Google Scholar]
- 49.Lomakin IB, Hellen CU, Pestova TV. Physical association of eukaryotic initiation factor 4G (eIF4G) with eIF4A strongly enhances binding of eIF4G to the internal ribosomal entry site of encephalomyocarditis virus and is required for internal initiation of translation. Mol Cell Biol. 2000;20:6019–6029. doi: 10.1128/mcb.20.16.6019-6029.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brignone C, Bradley KE, Kisselev AF, Grossman SR. A post-ubiquitination role for MDM2 and hHR23A in the p53 degradation pathway. Oncogene. 2004;23:4121–4129. doi: 10.1038/sj.onc.1207540. [DOI] [PubMed] [Google Scholar]
- 51.Johnson KR, Merrick WC, Zoll WL, Zhu Y. Identification of cDNA clones for the large subunit of eukaryotic translation initiation factor 3. Comparison of homologues from human, Nicotiana tabacum, Caenorhabditis elegans, and Saccharomyces cerevisiae. J Biol Chem. 1997;272:7106–7113. doi: 10.1074/jbc.272.11.7106. [DOI] [PubMed] [Google Scholar]
- 52.Glickman MH, Rubin DM, Fried VA, Finley D. The regulatory particle of the Saccharomyces cerevisiae proteasome. Mol Cell Biol. 1998;18:3149–3162. doi: 10.1128/mcb.18.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.