Abstract
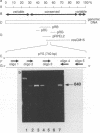
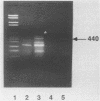
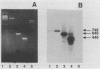
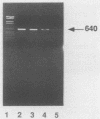
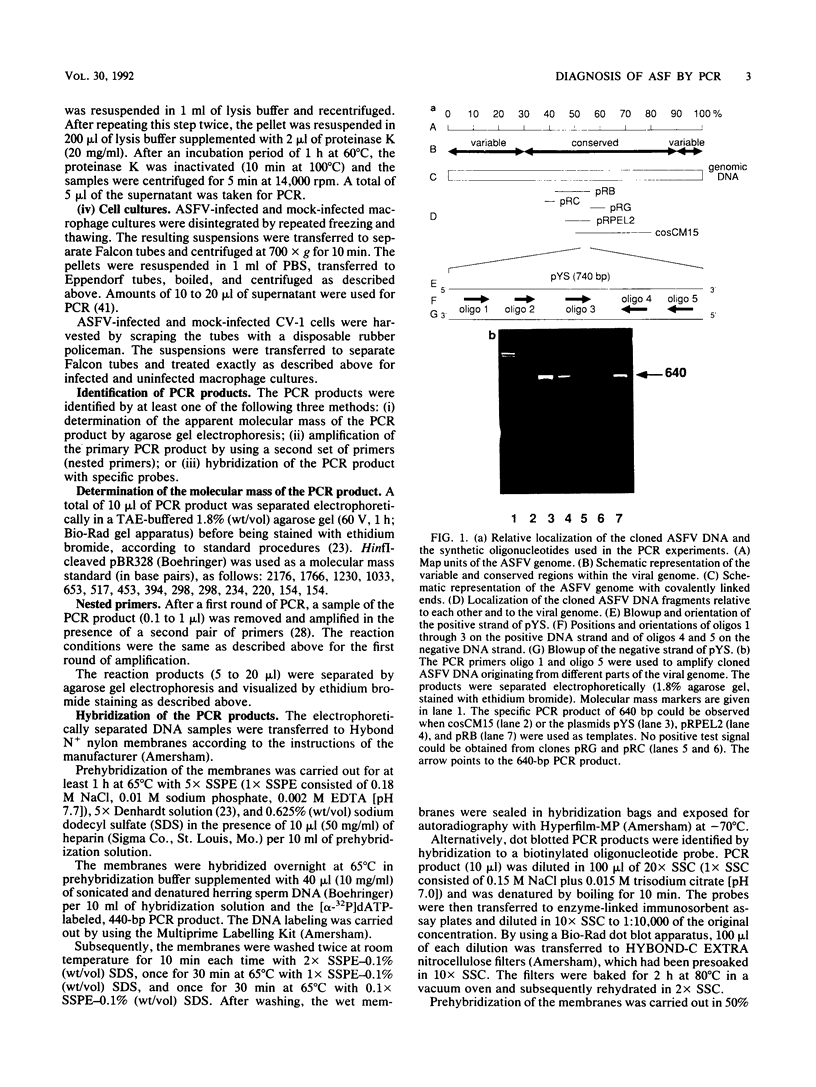
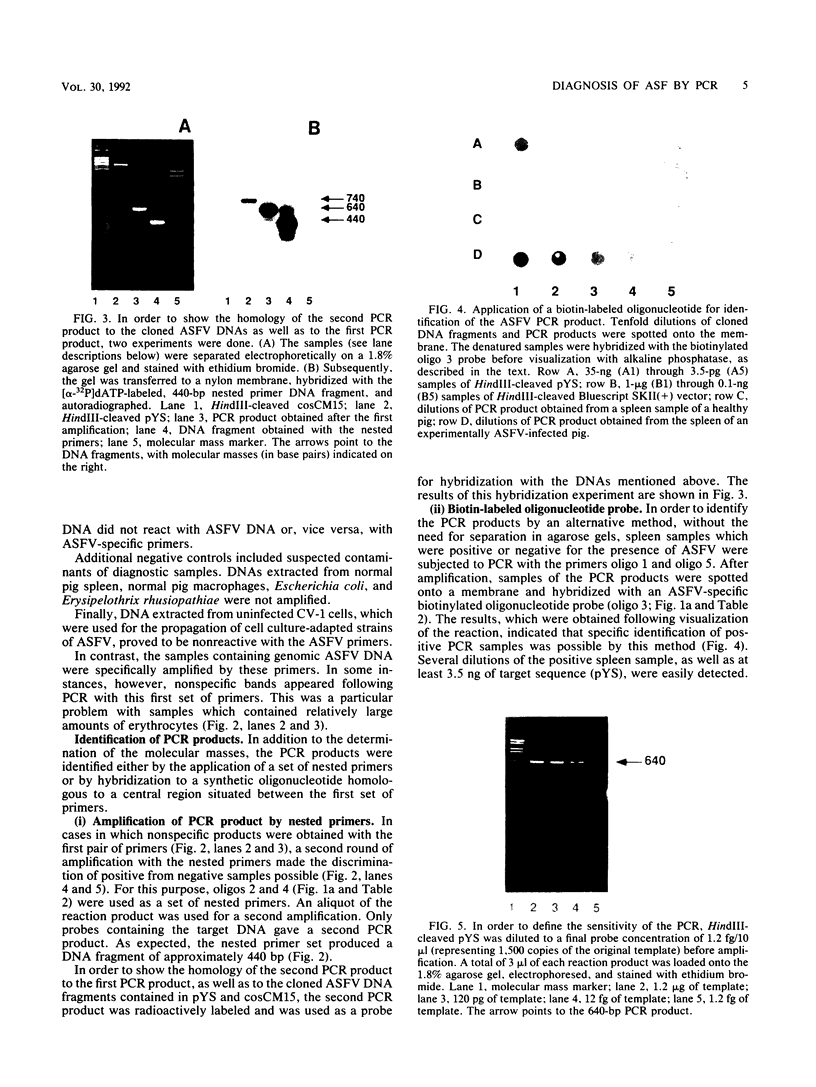
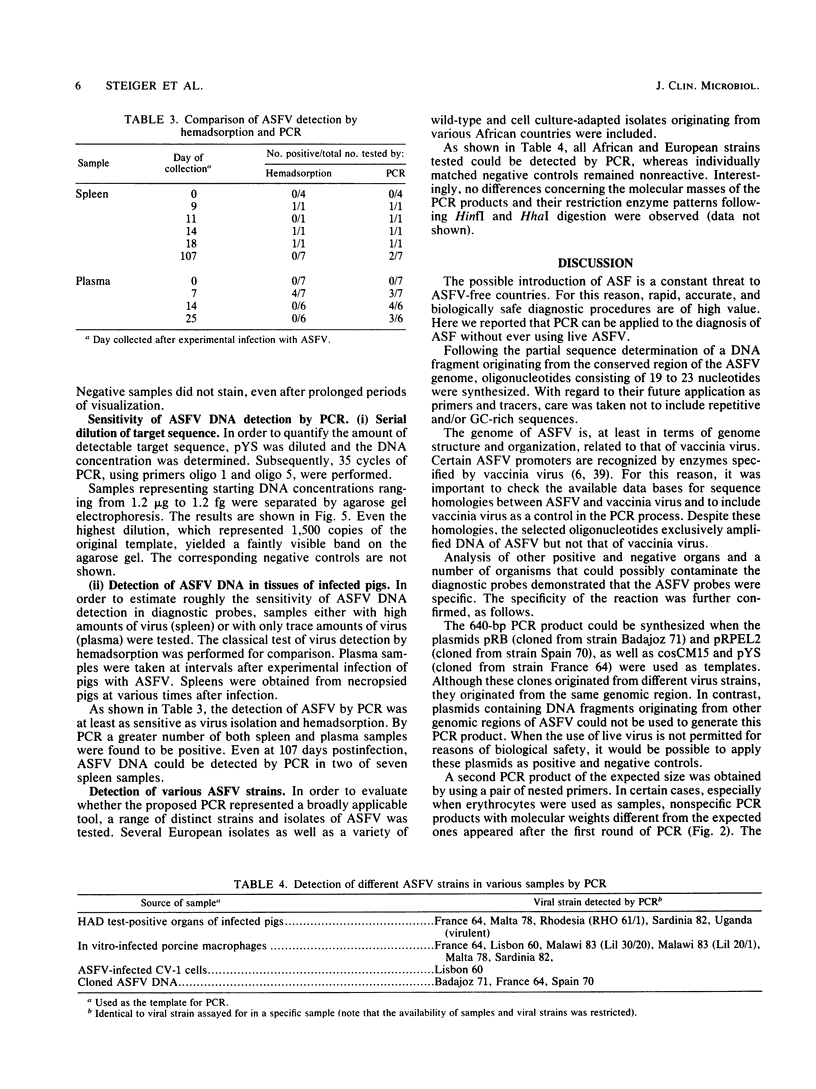
In order to circumvent the need for infectious virus for the diagnosis of African swine fever (ASF), we established the polymerase chain reaction (PCR) technique for the detection of ASF virus (ASFV) DNA. A 740-bp fragment that originated from the conserved region of the viral genome was partially sequenced. From this sequence, four PCR primers and one oligonucleotide probe were designed and synthesized. A specific 640-bp PCR product was amplified by using oligonucleotides 1 and 5 as primers and extracts of the following samples as templates: organs and plasma obtained from ASFV-infected pigs, ASFV-infected cell cultures, and cloned DNA fragments containing the same conserved genomic region as that in the original 740-bp clone. No specific reaction products were observed in the corresponding controls. The identities of the PCR products were confirmed either by a second amplification with nested primers or by hybridization with a specific, biotinylated oligonucleotide probe. PCR proved to be a quicker and more sensitive method than virus isolation followed by the hemadsorption test when spleen and plasma samples from experimentally ASFV-infected pigs were tested. Furthermore, cloned virus DNA could be used as a positive control in the place of a live virus control. This is advantageous whenever the use of live virus is undesirable.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Blasco R., Ley V., Beloso A., Talavera A., Viñuela E. Restriction site map of African swine fever virus DNA. Virology. 1984 Mar;133(2):258–270. doi: 10.1016/0042-6822(84)90393-3. [DOI] [PubMed] [Google Scholar]
- Belák S., Ballagi-Pordány A., Flensburg J., Virtanen A. Detection of pseudorabies virus DNA sequences by the polymerase chain reaction. Arch Virol. 1989;108(3-4):279–286. doi: 10.1007/BF01310940. [DOI] [PubMed] [Google Scholar]
- Blasco R., Agüero M., Almendral J. M., Viñuela E. Variable and constant regions in African swine fever virus DNA. Virology. 1989 Feb;168(2):330–338. doi: 10.1016/0042-6822(89)90273-0. [DOI] [PubMed] [Google Scholar]
- Bostock C. J. Viruses as vectors. Vet Microbiol. 1990 Jun;23(1-4):55–71. doi: 10.1016/0378-1135(90)90136-j. [DOI] [PubMed] [Google Scholar]
- Caballero R. G., Tabares E. Application of pRPEL2 plasmid to detect African swine fever virus by DNA-DNA hybridization. Brief report. Arch Virol. 1986;87(1-2):119–125. doi: 10.1007/BF01310548. [DOI] [PubMed] [Google Scholar]
- DETRAY D. E. AFRICAN SWINE FEVER. Adv Vet Sci. 1963;8:299–333. [PubMed] [Google Scholar]
- Dixon L. K. Molecular cloning and restriction enzyme mapping of an African swine fever virus isolate from Malawi. J Gen Virol. 1988 Jul;69(Pt 7):1683–1694. doi: 10.1099/0022-1317-69-7-1683. [DOI] [PubMed] [Google Scholar]
- Enjuanes L., Carrascosa A. L., Moreno M. A., Viñuela E. Titration of African swine fever (ASF) virus. J Gen Virol. 1976 Sep;32(3):471–477. doi: 10.1099/0022-1317-32-3-471. [DOI] [PubMed] [Google Scholar]
- González A., Talavera A., Almendral J. M., Viñuela E. Hairpin loop structure of African swine fever virus DNA. Nucleic Acids Res. 1986 Sep 11;14(17):6835–6844. doi: 10.1093/nar/14.17.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig A., Plowright W. The excretion of two virulent strains of African swine fever virus by domestic pigs. J Hyg (Lond) 1970 Dec;68(4):673–682. doi: 10.1017/s0022172400042613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haresnape J. M., Wilkinson P. J., Mellor P. S. Isolation of African swine fever virus from ticks of the Ornithodoros moubata complex (Ixodoidea: Argasidae) collected within the African swine fever enzootic area of Malawi. Epidemiol Infect. 1988 Aug;101(1):173–185. doi: 10.1017/s0950268800029332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Ley V., Almendral J. M., Carbonero P., Beloso A., Viñuela E., Talavera A. Molecular cloning of African swine fever virus DNA. Virology. 1984 Mar;133(2):249–257. doi: 10.1016/0042-6822(84)90392-1. [DOI] [PubMed] [Google Scholar]
- MALMQUIST W. A., HAY D. Hemadsorption and cytopathic effect produced by African Swine Fever virus in swine bone marrow and buffy coat cultures. Am J Vet Res. 1960 Jan;21:104–108. [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Pastor M. J., Escribano J. M. Evaluation of sensitivity of different antigen and DNA-hybridization methods in African swine fever virus detection. J Virol Methods. 1990 Apr;28(1):67–77. doi: 10.1016/0166-0934(90)90088-w. [DOI] [PubMed] [Google Scholar]
- Pini A. Isolation and segregation of non-haemadsorbing strains of African swine fever virus. Vet Rec. 1976 Dec 11;99(24):479–480. doi: 10.1136/vr.99.24.479. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Almendral J. M., Talavera A., Viñuela E. Terminal and internal inverted repetitions in African swine fever virus DNA. Virology. 1984 Mar;133(2):271–275. doi: 10.1016/0042-6822(84)90394-5. [DOI] [PubMed] [Google Scholar]
- Sommer R., Tautz D. Minimal homology requirements for PCR primers. Nucleic Acids Res. 1989 Aug 25;17(16):6749–6749. doi: 10.1093/nar/17.16.6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarés E. Detection of DNA viruses by radioactive and non radioactive DNA probes: application to African swine fever virus. Arch Virol. 1987;92(3-4):233–242. doi: 10.1007/BF01317480. [DOI] [PubMed] [Google Scholar]
- Tabarés E., Olivares I., Santurde G., Garcia M. J., Martin E., Carnero M. E. African swine fever virus DNA: deletions and additions during adaptation to growth in monkey kidney cells. Arch Virol. 1987;97(3-4):333–346. doi: 10.1007/BF01314431. [DOI] [PubMed] [Google Scholar]
- Wilkinson P. J., Lawman M. J., Johnston R. S. African swine fever in Malta, 1978. Vet Rec. 1980 Feb 2;106(5):94–97. doi: 10.1136/vr.106.5.94. [DOI] [PubMed] [Google Scholar]
- Zanoni R., Pauli U., Peterhans E. Detection of caprine arthritis-encephalitis- and maedi-visna viruses using the polymerase chain reaction. Experientia. 1990 Mar 15;46(3):316–319. doi: 10.1007/BF01951776. [DOI] [PubMed] [Google Scholar]