Abstract
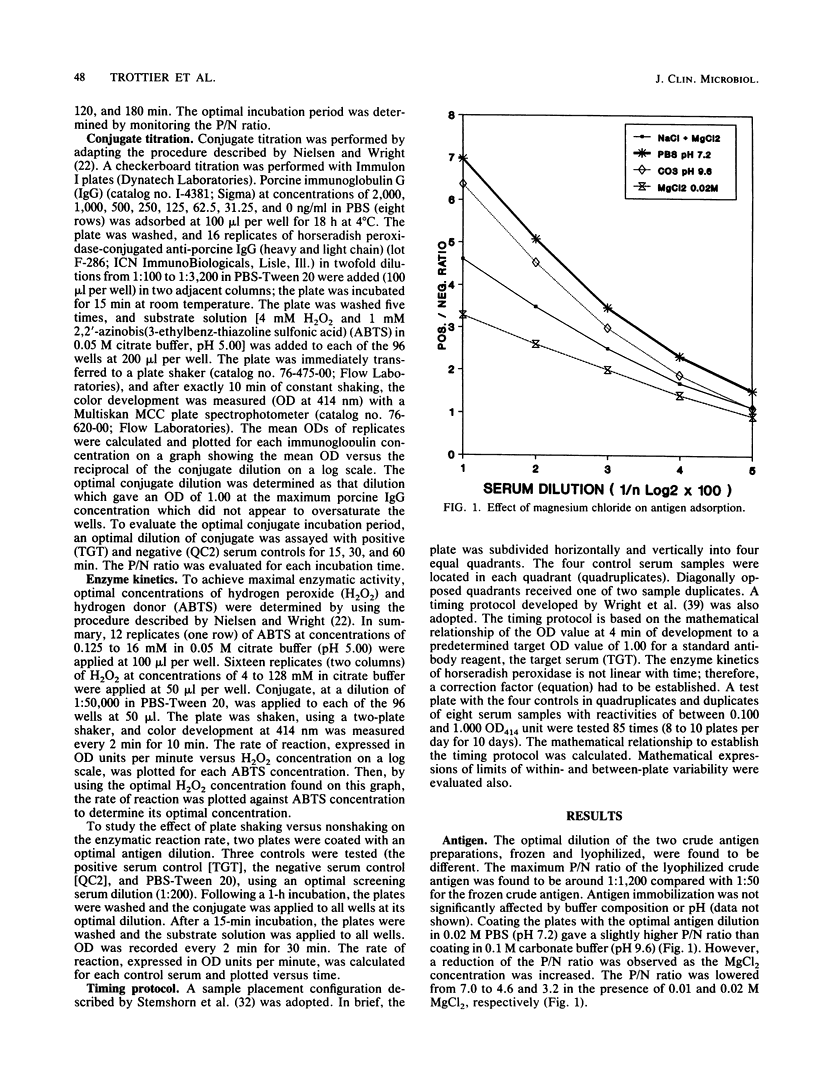
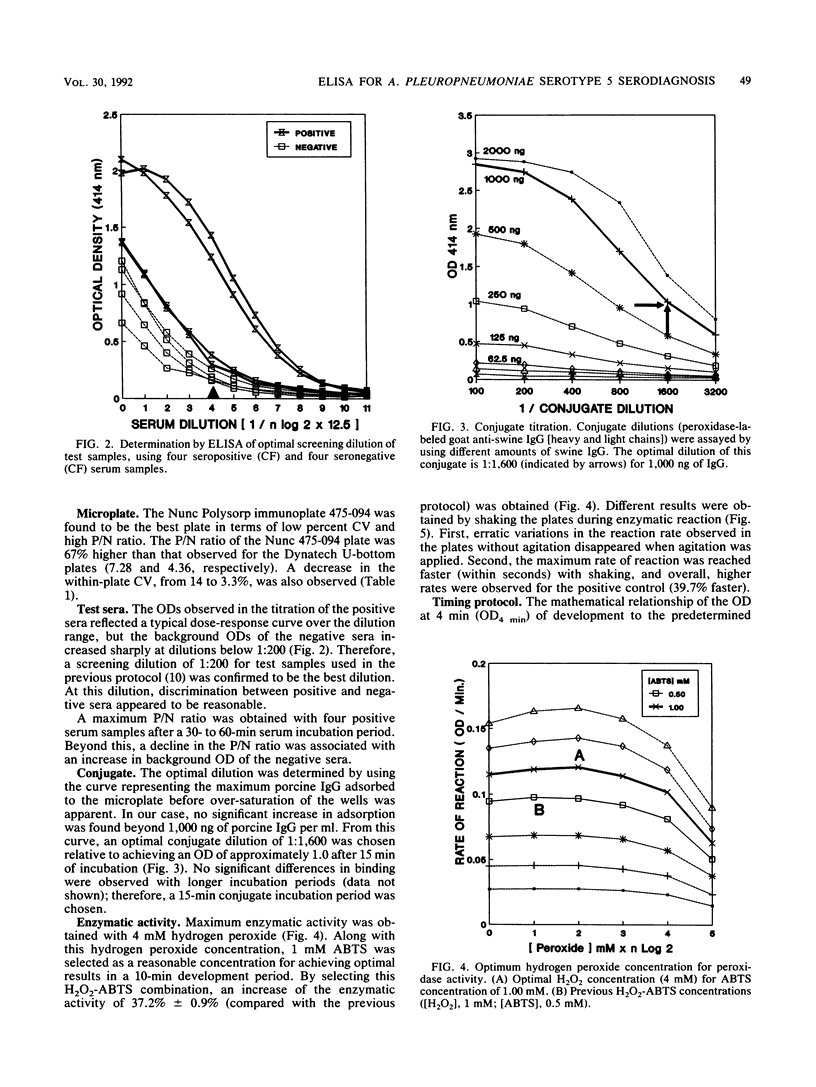
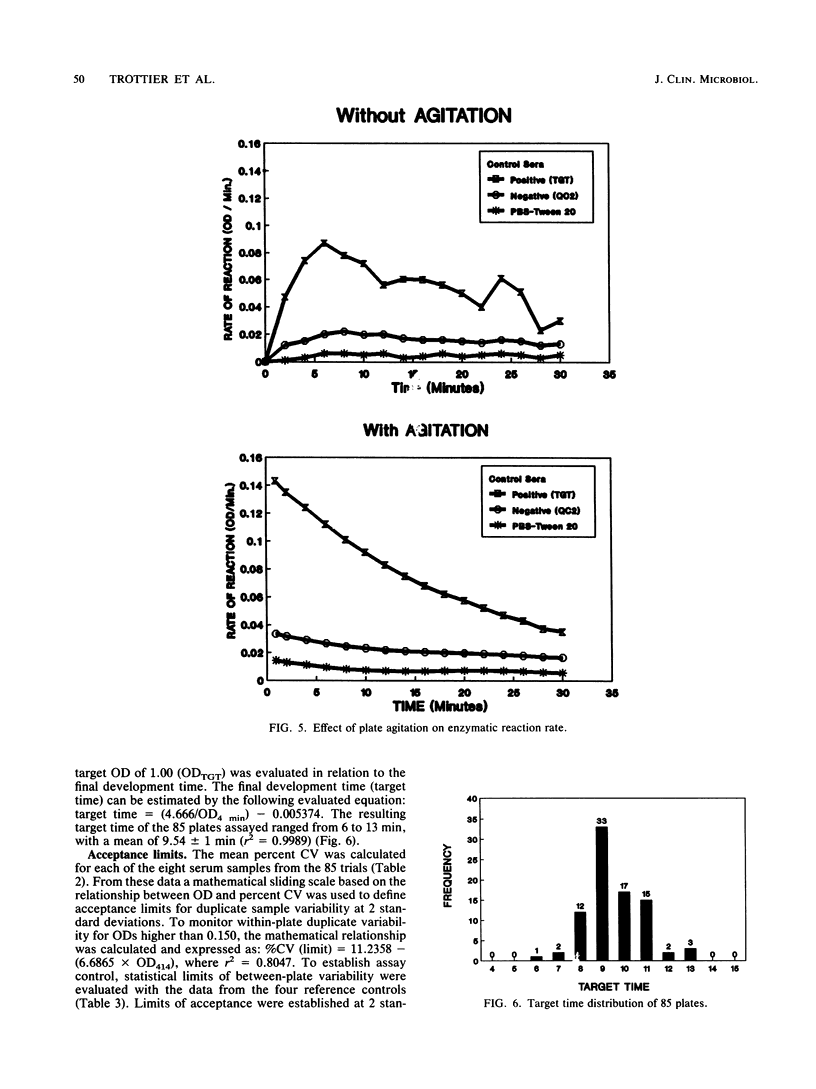
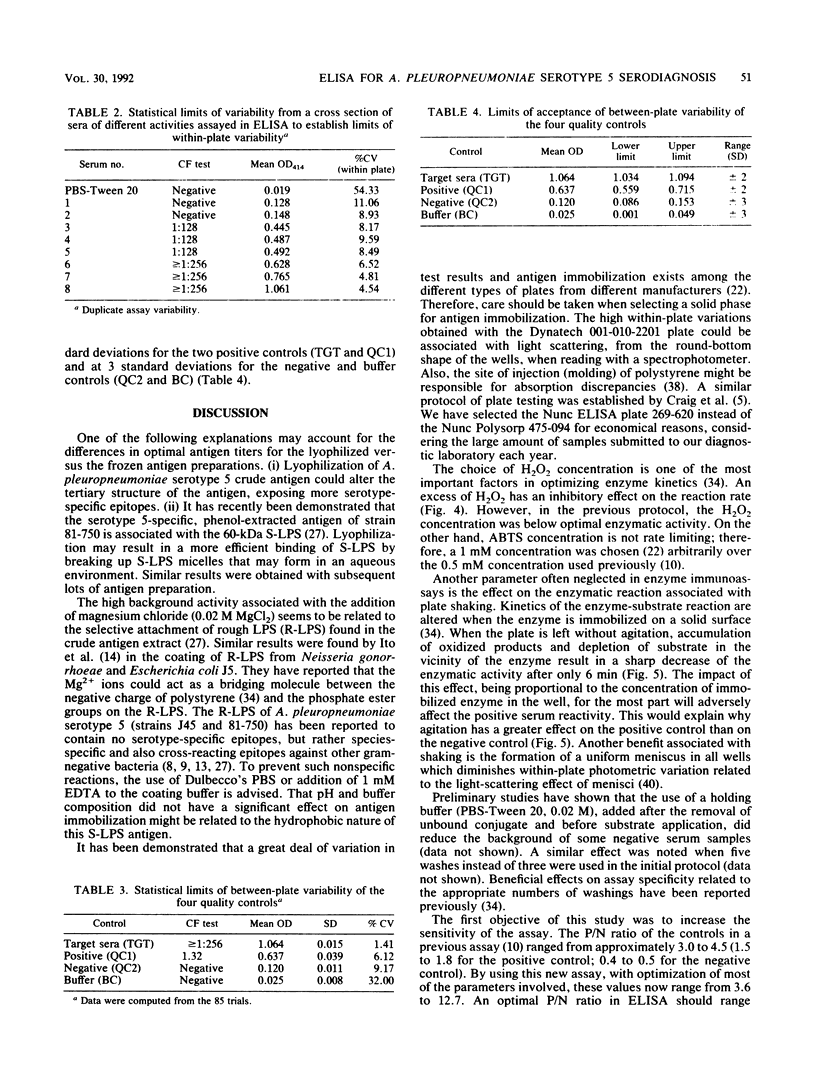
An indirect enzyme-linked immunosorbent assay protocol has been optimized with special emphasis given to assay standardization and quality control. Technical aspects such as choice of a microplate, antigen immobilization, buffer composition, optimal screening dilution of sera, and kinetics of the enzymatic reaction were studied and evaluated in order to design a standard protocol offering maximal analytical sensitivity and specificity, as well as to obtain minimal within- and between-plate variability. Among the 27 plates tested, the Nunc 475-094 and 269-620 immunoplates were found to be the best in terms of high positive-to-negative ratio and low variability. No significant differences in antigen immobilization were found by using buffers of various compositions or pHs; however, the presence of magnesium ions (Mg2+; 0.02 M) resulted in a twofold increase in nonspecific background. An optimal screening dilution of sera was established at 1:200. A 1-h incubation period for test serum was found to be optimal. Maximum enzymatic activity for peroxidase was obtained by adjusting both substrate (H2O2) and hydrogen donor [2,2' -azinobis(3-ethylbenz-thiazoline sulfonic acid)] concentrations to 4 and 1 mM, respectively. To control between-plate variability, a timing protocol was adopted. Within-plate variability was also controlled by using a sample placement configuration pattern. Sliding scales were determined by repeated testing of a cross section of samples to set acceptance limits for both within- and between-plate variability. These limits were used in a quality control program to monitor assay performance. The results obtained suggest that this standardized protocol might be useful in the serodiagnosis of Actinobacillus pleuropneumoniae serotype 5.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman E., Griffith D. W., Perry M. B. Structural studies of the O-chains of the lipopolysaccharides produced by strains of Actinobacillus (Haemophilus) pleuropneumoniae serotype 5. Biochem Cell Biol. 1990 Nov;68(11):1268–1271. doi: 10.1139/o90-188. [DOI] [PubMed] [Google Scholar]
- Bossé J. T., Johnson R. P., Rosendal S. Capsular polysaccharide antigens for detection of serotype-specific antibodies to Actinobacillus pleuropneumoniae. Can J Vet Res. 1990 Jun;54(3):320–325. [PMC free article] [PubMed] [Google Scholar]
- Bossé J. T., Johnson R. P., Rosendal S. Serodiagnosis of pleuropneumonia using enzyme-linked immunosorbent assay with capsular polysaccharide antigens of Actinobacillus pleuropneumoniae serotypes 1, 2, 5 and 7. Can J Vet Res. 1990 Oct;54(4):427–431. [PMC free article] [PubMed] [Google Scholar]
- Craig J. C., Parkinson D., Knowles N. The suitability of different microtitre plates for detection of antibody to virus antigens by indirect ELISA. J Biol Stand. 1989 Apr;17(2):125–135. doi: 10.1016/0092-1157(89)90003-6. [DOI] [PubMed] [Google Scholar]
- Fenwick B. W., Cullor J. S., Osburn B. I., Olander H. J. Mechanisms involved in protection provided by immunization against core lipopolysaccharides of Escherichia coli J5 from lethal Haemophilus pleuropneumoniae infections in swine. Infect Immun. 1986 Aug;53(2):298–304. doi: 10.1128/iai.53.2.298-304.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick B. W., Osburn B. I., Cullor J. S., Henry S. C., Olander H. I. Mortality in swine herds endemically infected with Haemophilus pleuropneumoniae: effect of immunization with cross-reacting lipopolysaccharide core antigens of Escherichia coli. Am J Vet Res. 1986 Sep;47(9):1888–1891. [PubMed] [Google Scholar]
- Fenwick B. W., Osburn B. I. Immune responses to the lipopolysaccharides and capsular polysaccharides of Haemophilus pleuropneumoniae in convalescent and immunized pigs. Infect Immun. 1986 Nov;54(2):575–582. doi: 10.1128/iai.54.2.575-582.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick B. W., Osburn B. I., Olander H. J. Isolation and biological characterization of two lipopolysaccharides and a capsular-enriched polysaccharide preparation from Haemophilus pleuropneumoniae. Am J Vet Res. 1986 Jul;47(7):1433–1441. [PubMed] [Google Scholar]
- Gunnarsson A. Evaluation of different antigens in the complement-fixation test for diagnosis of Haemophilus pleuropneumoniae (parahaemolyticus) infections in swine. Am J Vet Res. 1979 Nov;40(11):1564–1567. [PubMed] [Google Scholar]
- Inzana T. J., Clark G. F., Todd J. Detection of serotype-specific antibodies or capsular antigen of Actinobacillus pleuropneumoniae by a double-label radioimmunoassay. J Clin Microbiol. 1990 Feb;28(2):312–318. doi: 10.1128/jcm.28.2.312-318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Mathison B. Serotype specificity and immunogenicity of the capsular polymer of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1987 Jul;55(7):1580–1587. doi: 10.1128/iai.55.7.1580-1587.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J. I., Jr, Wunderlich A. C., Lyons J., Davis C. E., Guiney D. G., Braude A. I. Role of magnesium in the enzyme-linked immunosorbent assay for lipopolysaccharides of rough Escherichia coli strain J5 and Neisseria gonorrhoeae. J Infect Dis. 1980 Oct;142(4):532–537. doi: 10.1093/infdis/142.4.532. [DOI] [PubMed] [Google Scholar]
- Lagergård T., Trollfors B., Claesson B. A., Schneerson R., Robbins J. B. Comparison between radioimmunoassay and direct and indirect enzyme-linked immunosorbent assays for determination of antibodies against Haemophilus influenzae type b capsular polysaccharide. J Clin Microbiol. 1988 Dec;26(12):2554–2557. doi: 10.1128/jcm.26.12.2554-2557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombin L. H., Rosendal S., Mitchell W. R. Evaluation of the complement fixation test for the diagnosis of pleuropneumonia of swine caused by Haemophilus pleuropneumoniae. Can J Comp Med. 1982 Apr;46(2):109–114. [PMC free article] [PubMed] [Google Scholar]
- MacInnes J. I., Rosendal S. Analysis of major antigens of Haemophilus (Actinobacillus) pleuropneumoniae and related organisms. Infect Immun. 1987 Jul;55(7):1626–1634. doi: 10.1128/iai.55.7.1626-1634.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macinnes J. I., Rosendal S. Prevention and Control of Actinobacillus (Haemophilus) pleuropneumoniae Infection in Swine: A review. Can Vet J. 1988 Jul;29(7):572–574. [PMC free article] [PubMed] [Google Scholar]
- Nicolet J., Paroz P., Krawinkler M., Baumgartner A. An enzyme-linked immunosorbent assay, using an EDTA-extracted antigen for the serology of Haemophilus pleuropneumoniae. Am J Vet Res. 1981 Dec;42(12):2139–2142. [PubMed] [Google Scholar]
- Nicolet J., de Meuron P. A., Bachmann P. Sur l'hémophilose du porc. IV. L'épreuve de déviation du complément, un test de dépistage des infections à Haemophilus parahaemolyticus. Schweiz Arch Tierheilkd. 1971 Apr;113(4):191–200. [PubMed] [Google Scholar]
- Nielsen R. Pleuropneumonia of swine caused by Haemophilus parahaemolyticus. Studies on the protection obtained by vaccination. Nord Vet Med. 1976 Jul-Aug;28(7-8):337–348. [PubMed] [Google Scholar]
- Nielsen R. Serological characterization of Actinobacillus pleuropneumoniae strains and proposal of a new serotype: serotype 12. Acta Vet Scand. 1986;27(3):453–455. doi: 10.1186/BF03548158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R. Serology of Haemophilus (Actinobacillus) pleuropneumoniae serotype 5 strains: establishment of subtypes a and b. Acta Vet Scand. 1986;27(1):49–58. doi: 10.1186/BF03548558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp V. J., Ross R. F. Antibody response of swine to outer membrane components of Haemophilus pleuropneumoniae during infection. Infect Immun. 1986 Dec;54(3):751–760. doi: 10.1128/iai.54.3.751-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Mitchell W. R. Epidemiology of Haemophilus pleuropneumoniae infection in pigs: a survey of Ontario Pork Producers, 1981. Can J Comp Med. 1983 Jan;47(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Sanford S. E., Josephson G. K. Porcine Haemophilus pleuropneumonia epizootic in southwestern Ontario: clinical, microbiological, pathological and some epidemiological findings. Can J Comp Med. 1981 Jan;45(1):2–7. [PMC free article] [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Stemshorn B. W., Buckley D. J., St Amour G., Lin C. S., Duncan J. R. A computer-interfaced photometer and systematic spacing of duplicates to control within-plate enzyme-immunoassay variation. J Immunol Methods. 1983 Jul 29;61(3):367–375. doi: 10.1016/0022-1759(83)90233-8. [DOI] [PubMed] [Google Scholar]
- Willson P. J., Schipper C., Morgan E. D. The Use of an Enzyme-linked Immunosorbent Assay for Diagnosis of Actinobacillus pleuropneumoniae Infection in Pigs. Can Vet J. 1988 Jul;29(7):583–585. [PMC free article] [PubMed] [Google Scholar]
- Wright P. F., Kelly W. A., Gall D. E. Application of a timing protocol to the reduction of inter-plate variability in the indirect enzyme immunoassay for detection of anti-Brucella antibody. J Immunoassay. 1985;6(3):189–205. doi: 10.1080/01971528508063029. [DOI] [PubMed] [Google Scholar]
- Wright P. Enzyme immunoassay: observations on aspects of quality control. Vet Immunol Immunopathol. 1987 Dec;17(1-4):441–452. doi: 10.1016/0165-2427(87)90160-7. [DOI] [PubMed] [Google Scholar]


