Abstract
The development of a vaccine for tuberculosis requires a combination of antigens and adjuvants capable of inducing appropriate and long-lasting T cell immunity. We evaluated Mtb72F formulated in AS02A in the cynomolgus monkey model. The vaccine was immunogenic and caused no adverse reactions. When monkeys were immunized with bacillus Calmette–Guérin (BCG) and then boosted with Mtb72F in AS02A, protection superior to that afforded by using BCG alone was achieved, as measured by clinical parameters, pathology, and survival. We observed long-term survival and evidence of reversal of disease progression in monkeys immunized with the prime-boost regimen. Antigen-specific responses from protected monkeys receiving BCG and Mtb72F/AS02A had a distinctive cytokine profile characterized by an increased ratio between 3 Th1 cytokines, IFN-γ, TNF, and IL-2 and an innate cytokine, IL-6. To our knowledge, this is an initial report of a vaccine capable of inducing long-term protection against tuberculosis in a nonhuman primate model, as determined by protection against severe disease and death, and by other clinical and histopathological parameters.
Tuberculosis (TB) causes more than 2 million deaths per year worldwide (1). Bacillus Calmette–Guérin (BCG), an attenuated strain of Mycobacterium bovis, is the only available vaccine against TB, but its efficacy against TB in adults is highly variable (2). Our goal is to develop a defined vaccine against TB that could significantly improve the level of protection afforded by current BCG vaccines. To this end we have developed a comprehensive approach to identify antigens of Mycobacterium tuberculosis (Mtb) recognized by T cells from humans with latent Mtb infection. When appropriately formulated, these antigens, alone or in combination as mixtures or fusion proteins, were capable of inducing protective immunity in small-animal models of TB (mice, guinea pigs, and rabbits). Priority was given to antigens conserved between Mtb and BCG to allow a candidate vaccine to be able to boost a BCG vaccine. Of several dozen antigens screened, Corixa Corp. and GlaxoSmithKline (GSK) developed a candidate polyprotein antigen designated Mtb72F (3), derived from the Mtb proteins Mtb32 (4) and Mtb39 (5). Several studies in animal models indicated that the potent and clinically tested GSK proprietary Adjuvant System AS02A was effective in inducing protection against Mtb when used to formulate Mtb72F. We demonstrated that mice immunized with Mtb72F protein formulated in AS02A were protected against aerosol challenge with M. tuberculosis H37Rv at a level comparable to that of BCG (3). Similarly, Mtb72F/AS02A protected guinea pigs against progressive disease, again to a level comparable to that attained by using BCG (3, 6). Furthermore, when the Mtb72F/AS02A vaccine candidate was mixed with BCG and administered to guinea pigs, survival after aerosol challenge was at least 2 years, compared with 1 year for animals given BCG only (6). These animals had substantial evidence of lesion resolution and very low bacterial numbers in the lungs.
The cynomolgus monkey has been described as a model for human TB (7). When infected intratracheally with a high dose of viable bacteria, these animals develop progressive disease; when infected with a low dose, they develop latent infection (7). Similarities in acute and chronic pathologies regarding the characteristics of the granulomas resulting from infection exist between the cynomolgus monkey and humans. In the present study, we have evaluated the ability of vaccine preparations to induce long-term protection in this nonhuman primate model. Studies included evaluating Mtb72F formulated in AS02A to confer protection, with or without priming with BCG. In the prime-boost experiments, different intervals were tested between administration of BCG and boosting with subunit vaccine, as the interval of the resting period may influence the persistence of the immune response (8). Overall, the best protection, including instances of recovery from positive chest x-rays, was seen in animals receiving the prime-boost regimen. Clinical, immunological, microbiological, and pathological parameters were assessed postinfection. Herein, we describe a well-defined vaccine capable of boosting BCG and inducing significant and durable protection against disease and death from Mtb in nonhuman primates.
Results
Initial safety, immunogenicity, and protection experiments were performed to select antigens to include in a candidate vaccine. Monkeys were immunized with Mtb39 or Mtb39 plus Mtb32 formulated in AS02A (Table S1). In a single experiment (data not shown), it was observed that the level of protection afforded with the combination of Mtb39 plus Mtb32 was superior to that provided by Mtb39 alone. These data, together with those data obtained in extensive small-animal studies (3–6), led us to select these 2 antigens, and a fusion thereof, Mtb72F, linked in tandem in the linear order Mtb32C-Mtb39-Mtb32N, as our lead candidate vaccine antigen.
Safety Evaluations of Mtb72F/AS02A.
Safety data were collected during the course of studies examining the immunogenicity and protective efficacy of Mtb72F/AS02A. In these studies, animals received intramuscular injections of 40 μg of Mtb72F in 500 μL of AS02A (n = 36) or adjuvant alone (n = 30) 3 times at 4-week intervals. The following parameters were measured: (i) body weight over a period of 450 to 700 days (ii) body temperature (at intervals before and after each injection), and (iii) erythrocyte sedimentation rate (ESR, an indirect measure of inflammation) and hematocrit (at intervals before and after each injection) (Table S1). There was no evidence of systemic toxicity in any of the animals receiving the Mtb72F/AS02A vaccine or adjuvant alone. Injection sites were observed for local erythema, induration, and/or tenderness at 1, 9, 24, and 48 hours after injection. There were no apparent local reactions at the injection sites in either naïve animals or in animals primed with BCG followed by Mtb72F/AS02A 16 or 32 weeks later.
Protection Against Tuberculosis by Mtb72f/AS02A.
To examine whether the Mtb72F protein in AS02A given as an adjunct to BCG vaccination would increase the survival or improve clinical symptoms of nonhuman primates infected with Mtb compared with BCG given alone, 2 experiments were performed. In the first of these experiments (Experiment 6, Table S1), the BCG prime was followed by vaccine boosts at 16, 20, and 24 weeks; in the second, the BCG prime was followed by vaccine boosts at 32, 36, and 40 weeks (Experiment 7, Table S1). In each experiment, monkeys were challenged by intratracheal instillation of Mtb Erdman strain 4 weeks after the last immunization (i.e., at 28 or 44 weeks). Clinical parameters (ESR, chest X-rays, weight and survival profiles) were evaluated after challenge with Mtb.
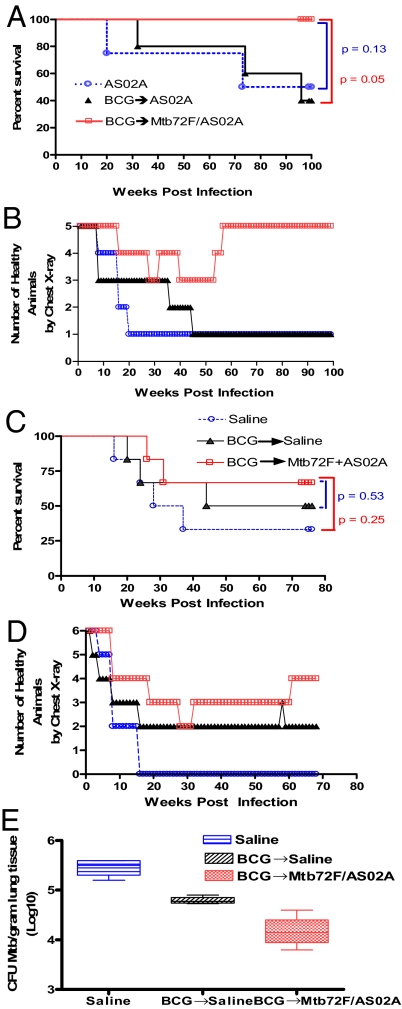
Fig. 1 A and C show the survival after challenge with Mtb in Experiments 6 and 7. In Experiment 6, survival was followed for 100 weeks (700 days) following Mtb challenge. All 5 (100%) of the BCG + Mtb72F/AS02A-immunized monkeys survived to the last time point evaluated, whereas only 2 of 5 (40%, P = 0.17) monkeys in the BCG + AS02A-immunized group and 3 of 5 (60%, P = 0.44) in the AS02A-immunized group survived to this point. The median survival time (MST) for animals in the BCG + Mtb72F/AS02A-immunized group was 700 (min 700, max 700) days, compared with 688 (min 225, max 700, P = 0.05) days in the group that received BCG + AS02A and 700 (min 139, max 700, P = 0.13) days in the group that received AS02A alone (Fig. 1A).
Fig. 1.
Analysis of survival, clinical parameters, and bacterial burden during Mtb infection of immunized monkeys that had been primed with BCG and boosted with Mtb72F/AS02A. In 2 separate experiments, monkeys (5 or 6 per group, respectively) were injected with AS02A alone (A and B) or saline (C–E) or were primed with BCG followed 16 weeks (A and B) or 32 weeks (C–E) later by injections of AS02A (A and B), saline (C–E), or Mtb72F/AS02A (A–E) administered 3 times 4 weeks apart on weeks 16, 20, and 24 (A and B) or on weeks 32, 36, and 40 (C–E). Four weeks after the last immunization, monkeys were challenged by intratracheal instillation of 500 CFU Mtb Erdman strain during week 28 (A and B) or week 44 (C–E). Survival was monitored for 100 weeks or 76 weeks post infection in A and C, respectively. CXR changes were also evaluated monthly for 100 weeks or 68 weeks post infection and are shown in B or D, respectively. At necropsy bacteria were quantified by enumerating the bacteriological burden (CFU) in monkey lungs (E).
In Experiment 7, survival was monitored for 76 weeks (532 days) following Mtb challenge. In this study, the Mtb72F/AS02A-boosted group had 4 of 6 (67%) survivors (Fig. 1C), compared with the BCG-immunized (3 of 6, 50%, P = 1.0) or saline control (2 of 6, 33%, P = 0.57) groups. The MST for animals in the BCG + Mtb72F/AS02A-immunized group was 532 (min 185, max 532) days compared with 419.5 (min 142, max 532, P = 0.53) days in the group that received BCG + saline and 226.5 (min 116, max 532, P = 0.25) days in the group that received saline alone. In terms of survival, these results suggest that the shorter interval (16 weeks) between the BCG prime and Mtb72F/AS02A vaccine boosts elicited superior protection to the 32-week interval against an Mtb challenge.
Radiological changes were first observed 16 weeks after Mtb challenge in monkeys in Experiment 6 (Fig. 1B), whereas infiltrates were observed at only 8 weeks post Mtb challenge in monkeys in Experiment 7 (Fig. 1D). In both experiments, infiltrates were observed even earlier, at 4 weeks post Mtb challenge, in animals in the AS02A-, saline-, and BCG-only control groups (Fig. 1 B and D). Remarkably, all animals in Experiment 6 primed with BCG and boosted 16, 20, and 24 weeks later with Mtb72F/AS02A vaccines either had no radiological changes or recovered from positive chest X-rays before the end of the experiment, whereas none of those receiving BCG or AS02A alone recovered. The proportions with normal chest X-ray at the end of Experiment 6 were 5 of 5 (100%) in those boosted with Mtb72F/AS02A, and 1 of 5 (20%) in both the BCG (P = 0.05) and AS02A (P = 0.05) groups (Fig. 1B). In Experiment 7, 4 of 6 (67%) of the Mtb72F/AS02A-boosted animals had normal chest X-rays at the end of the experiment, compared with 2 of 6 (33%, P = 0.57) of those that received BCG and none (0 of 6, 0%, P = 0.06) of those in the saline-alone group (Fig. 1D).
At necropsy, lung sections from animals in Experiment 7 were taken for determination of colony-forming units (CFU). The lung tissue of animals primed with BCG and boosted with Mtb72f/AS02A showed a significant decrease in the number of CFU and acid-fast bacteria (AFB) counts compared with both animals primed with BCG and boosted with saline and animals who received only saline at both injections. The median number of CFUs per gram of lung tissue in the BCG-primed, Mtb72F/AS02A-boosted group was reduced by approximately 10-fold in comparison with saline controls, with an intermediate level of reduction observed in animals receiving BCG alone (Fig. 1E). The median number of AFBs per mm3 of lung tissue was 0 (min 0, max 0) in the BCG-primed, Mtb72F/AS02A-boosted group, 8.0 × 107 (min 8.0 × 107, max 1.0 × 108, P = 0.03) in the BCG-only group, and 2.0 × 1011 (min 2.0 × 1011, max 2.0 × 1011, P = 0.02) in the saline group (data not shown). Thus, although survival differences did not reach significance in Experiment 7, differences in bacterial counts were highly significant.
Histopathological Findings.
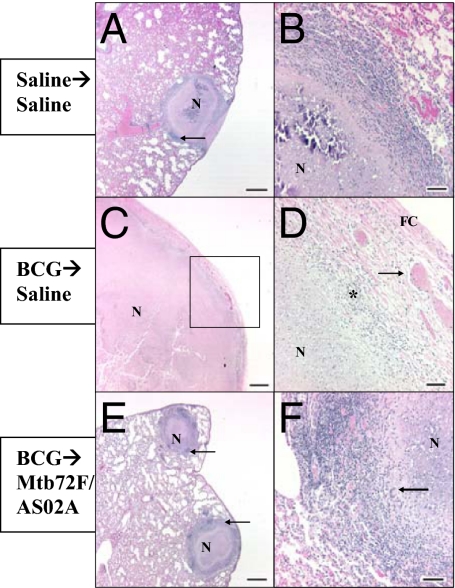
Like human Mtb-containing granulomas, those in cynomolgus monkeys have an organized structure and can have several morphologically distinct forms, including some consisting of organized lymphocytes and macrophages without necrosis, caseous necrosis in the center of a granuloma surrounded by lymphocytes and macrophages, and calcified granulomas. In lungs from control monkeys, the entire lobe submitted for evaluation was almost completely consolidated by the fusion of multiple caseous granulomas (data not shown). The granulomas were incompletely encapsulated, with the epithelioid macrophages extending into the adjacent pulmonary parenchyma. Foci of dystrophic mineralization were present in the caseous centers of the fused lung granulomas. In monkeys injected with saline only, aggregates of lymphocytes were observed (demarcated by arrows in Fig. 2A). The parenchyma in Fig. 2A is also marked by extensive congestion and edema.
Fig. 2.
Histologic appearance of lung tissues harvested from monkeys primed with BCG and immunized with Mtb72F-containing vaccines. Cynomolgus monkeys were injected with saline alone (A and B–Right Lung), BCG followed by saline (C and D–Right Lung), BCG followed by Mtb72F /AS02A (E and F–Right lung), 3 times 4 weeks apart and then infected 4 weeks later with Mtb. D represents high magnification of area outlined by black box in C. Note fibrous capsule (FC), congested blood vessels (arrow) and mixed inflammation—macrophages, lymphocytes, and neutrophils (*). Sections were stained with hematoxylin and eosin and von Kossa. n, necrosis. (Scale bars: A, C, and E; 1 mm; B, D, and F, 100 μm).
In monkeys vaccinated with BCG followed by Mtb challenge, severe, extensive, necrotizing granulomatous pneumonia with no normal tissue visible was observed (Fig. 2 C and D). Fibrous capsules, congested blood vessels, and mixed inflammation—macrophages, lymphocytes, and neutrophils—were also observed in each lung of monkeys in this group.
In monkeys primed with BCG and boosted with Mtb72F/AS02A either 16 or 32 weeks later, granulomas were discrete, individualized entities (moderate, focal to multifocal granulomatous pneumonia) with aggregates of lymphocytes (arrows) with few macrophages, plasma cells, and eosinophils at the margins (Fig. 2 E and F). No significant lesions consisting of necrosis or edema were observed in these monkeys (Figs. 23–4). The spleens, livers, and kidneys from BCG-primed, Mtb72F/AS02A-boosted monkeys were also normal and did not have evidence of infection (Fig. 4 G–I). Organs from BCG-immunized animals (Fig. 4 D–F) showed normal spleens with germinal centers, mild mesangioproliferative glomerulonephritis, and diffuse hepatic fatty changes. Like the lungs, organs from saline-injected animals (Fig. 4 A–C) had large caseous granulomas present in the spleen, renal cortex, and liver.
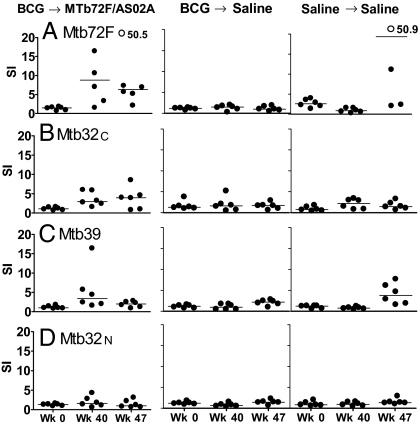
Fig. 3.
Cellular responses to mycobacterial antigens after injection with saline or immunization with BCG followed by 3 boost immunizations with Mtb72F/AS02A or by injection with saline. Monkeys (6 per group) were injected with saline or were primed with BCG (week 0) followed 32 weeks later by injections of Mtb72F/AS02A or saline alone administered 3 times 4 weeks apart on weeks 32, 36, and 40. Lymphoproliferation (stimulation index) of whole blood in response to mycobacterial antigens (PPD, Mtb72F, Mtb32-C, Mtb39, Mtb32-N; each at a concentration of 10 μg/mL) were measured at baseline (week 0), 3 weeks after the last boost immunization (week 43), and 4 weeks postchallenge (week 48). Horizontal lines represent medians; solid circles represent actual values; open circles represent maximum values >20.
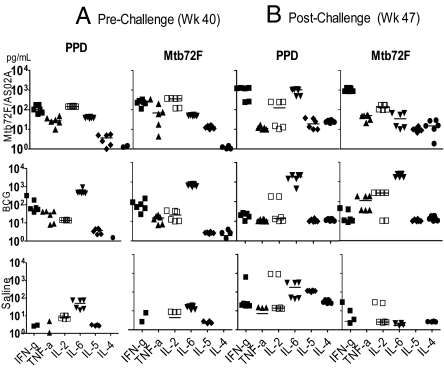
Fig. 4.
Cellular responses to mycobacterial antigens after injection with saline or immunization with BCG followed by 3 boost immunizations with Mtb72F/AS02A or by injection with saline. Cynomolgus monkeys (6 per group) were primed with BCG and boosted 32 weeks later with 3 doses of Mtb72F/AS02A or injected with saline alone. In vitro IFN-γ, TNF-α, IL-2, IL-6, IL-5, and IL-4 (pg/mL) release by PBMC in response to stimulation with PPD or Mtb72F were evaluated by CBA 3 weeks post last immunization with Mtb72F-containing vaccines (week 43) (A) and 4 weeks postchallenge (week 50) (B). Horizontal lines represent median cytokine concentrations.
Acid-fast stains were negative or rarely positive for Mtb in all tissues removed from animals immunized with BCG followed by Mtb72F formulated in AS02A (Fig. 3C). In contrast, multiple clumps of acid-fast bacilli were observed in BCG-vaccinated animals, and 1 to 5 bacilli were seen per high-power field (600×) in saline-injected animals (Fig. 3A).
Immune Responses in Vaccinated Animals.
Immunization with 40 μg of Mtb72F/AS02A induced strong IgG responses to Mtb72F in all Mtb72F/AS02A-immunized animals before challenge with Mtb, and these responses continued to increase after challenge. In contrast, all of the other groups failed to produce appreciable antibodies to these same antigens, even after challenge (data not shown).
To examine whether the Mtb72F protein in AS02A given as a boost to BCG vaccination would enhance the antigen-specific Th1 responses, a total of 33 monkeys (experimental groups 6 and 7, BCG prime-Vaccine boost, in Table S1) was primed with BCG and injected with Mtb72F/AS02A, saline, or adjuvant followed by examination of lymphoproliferation of whole blood cells after in vitro restimulation with vaccine antigens for 114–116 h. Fig. S1 shows the responses of monkeys evaluated in Experiment 7. Proliferation to Mtb72F was also observed in monkeys primed with BCG and boosted with Mtb72F in AS02A when examined at 3 weeks after the third Mtb72F/AS02A boost (week 40), and at 4 weeks post Mtb challenge (week 47). The majority of the proliferative responses were directed to the Mtb39 and Mtb32-C components of the Mtb72F molecule (Fig. S1 B and C). Negligible proliferative responses to Mtb72F and its component antigens were observed at week 0 in all animals in the BCG-primed, Mtb72F/AS02A-boosted group, and in BCG-immunized or saline control animals at weeks 40 and 47 (Fig. S1 A–D).
To measure T cell responsiveness to the vaccine antigens in immunized versus control animals, IFN-γ levels from LPA supernatants were evaluated after 114–116 h of in vitro stimulation of whole blood with Mtb72F, Mtb32C, Mtb32N, and Mtb39 (Fig. S2). Antigen-specific IFN-γ levels evaluated 3 weeks after the last immunization, and compared with the saline control group, were found to be elevated in animals primed with BCG and boosted with Mtb72F/AS02A vaccine and those immunized with BCG only. All of the monkeys immunized with Mtb72F-containing vaccines had positive IFN-γ responses to Mtb72F, Mtb39, and the C terminus of Mtb32, 3 weeks after the last immunization (week 40) (Fig. S2). In contrast to our published results in C57BL/6 mice in that a relatively weak response to Mtb32 was observed after in vitro recall (3), immunization of nonhuman primates with Mtb72F formulated in AS02A resulted in the production of a strong IFN-γ response after in vitro stimulation of whole blood cultures with Mtb72F, Mtb39, and Mtb32C. Similar to our prior observations in mice and guinea pigs, priming of nonhuman primates with BCG followed by immunization with Mtb72F adjuvanted with AS02A stimulated a robust production of IFN-γ (2-fold higher than the BCG only group) against Mtb72F and its components upon in vitro recall (Fig. S2).
To help identify correlates of protection, we analyzed antigen-specific cytokine responses induced by PBMC from immunized and unimmunized monkeys before and after challenge with Mtb (Fig. S3). Cytokine production in response to in vitro stimulation with PPD and Mtb72F was measured by Th1/Th2 cytometric bead array (CBA).
Before challenge with Mtb, PBMC from both immunized groups produced IFN-γ in response to both antigens, whereas those from saline-injected animals did not. However, the group immunized with BCG boosted 32 weeks later with Mtb72F/AS02A vaccine was distinguished from the group immunized with BCG (Experiment 7, Table S1) in that animals that received the booster immunizations with Mtb72F/AS02A produced >10 times more IL-2 (P = 0.004) and 10 times less IL-6 (P = 0.004) in response to both PPD and Mtb72F than those not receiving the defined vaccine boost. The 2 groups produced similar levels of IFN-γ (P = 0.52) and TNF-α (P = 1.0), but the IFN-γ:IL-6 ratios were higher in the group that received the defined vaccine (P = 0.004). Only animals receiving the Mtb72F/AS02A vaccine produced substantial amounts of all 3 Th1- associated cytokines measured, IFN-γ, TNF-α, and IL-2. Prechallenge production of IL-4 and IL-5 was negligible in all 3 groups. The cytokine patterns were similar for the PPD and Mtb72F restimulations.
The cytokine responses 4 weeks postchallenge revealed 2 interesting points: First, saline-injected control monkeys had PBMC responses specific to PPD marked by production of the Th2 cytokines IL-4 and IL-5 at levels higher than either of the immunized groups, and although they also produced moderate levels of IL-2 and IFN-γ in response to antigen stimulation, TNF-α production was low or absent. No Mtb72F-specific response was detected in the control group after challenge with Mtb. Second, both immunized groups had low IL-5 responses. PPD-specific IL-4 levels measured after in vitro restimulation were higher in the saline group than in both immunized groups and higher in the Mtb72F/AS02A group than in the BCG group.
Both immunized groups had positive Mtb72F-specific IL-2, IFN-γ, and TNF-α responses, whereas no increase inTNF-α was measured when the PBMC were restimulated with PPD. After challenge, IFN-γ levels were higher in response to both PPD and Mtb72F in those immunized with BCG + Mtb72F/AS02A, but IL-6 was high in response to PPD only. Consequently the IFN-γ:IL-6 ratio in response to Mtb72F in BCG + Mtb72F/AS02A-immunized animals was much higher than that in BCG-immunized animals (median 40.1 vs. 0.006, P = 0.004).
In vivo cell-mediated immune responses were measured by evaluating the reaction to TB protein in the tuberculin skin test (TST). Conversion from a negative to positive TST was generally observed by 5–6 weeks postchallenge. Tuberculin skin tests after bacterial challenge showed that 20% of monkeys in groups immunized with BCG and boosted with Mtb72F/AS02A vaccines 16 or 32 weeks later were reactive. None of these monkeys was positive 16 weeks post Mtb challenge. In contrast, all (100%) monkeys receiving only saline injections were skin test-positive 4 weeks after challenge; 4 of the 5 remained positive at and after 16 weeks postchallenge (data not shown).
Discussion
Despite the fact that BCG is one of the most widely used vaccines in the world, TB remains a leading cause of disease and death, with an estimated 1 billion people infected and 2–3 million deaths per year (9). Although BCG may confer a significant degree of protection for several years following immunization, it is established that this protection is neither complete nor durable. It is doubtful that TB will be brought under control in the absence of a more effective vaccine. One approach is to develop a vaccine that replaces BCG. However, BCG is among the most widely used of all vaccines under the Expanded Program on Immunization and is unlikely to be discontinued as a TB prevention strategy, nor should this be considered. Another approach is to develop a vaccine that can be safely applied to BCG-immunized individuals and which may function to boost the incomplete immunity afforded by BCG. In this regard, a strategy of repeat immunizations with BCG has not increased protection in either animals or humans (10, 11), perhaps because preexisting immunity prevents the booster dose from persisting in the vaccinee. GSK, in collaboration with Corixa Corporation, has developed a defined subunit vaccine, Mtb72F/AS02A, that is capable of boosting BCG and was shown to confer protection in 3 experimental models of TB, the mouse (3), guinea pig (3, 6), and rabbit (12). In this report, we demonstrate that this vaccine candidate induces long-term protection against disease and death in the cynomolgus monkey model of TB. Optimal protection was obtained by immunizing monkeys first with BCG and then boosting with 3 injections of Mtb72F/AS02A. Animals receiving the prime-boost regimen were protected to a greater degree than animals receiving BCG alone, as measured by clinical, bacterial burden quantification, histological parameters, and by survival. Although Mtb72F/AS02A alone conferred some protection, optimal results were obtained by building on the partial protection conferred by BCG. To our knowledge, this is the first report of a defined vaccine candidate that has achieved long-term protection against TB in a nonhuman primate.
Our approach to TB vaccine development has been a systematic one, consisting of (i) identifying antigens recognized by humans with TB infection (4, 5, 13–15), (ii) identifying effective adjuvant formulations by using candidate antigens for immunogenicity and protection studies in rodents, (iii) confirming safety and protective efficacy in guinea pigs, rabbits, and nonhuman primates (16), and (iv) ongoing clinical trials to evaluate safety and immunogenicity. Mtb72F/AS02A is the only TB vaccine candidate shown to protect in 4 animal models (16, 17). The data presented in this report support the safety and efficacy profile of the vaccine candidate and point out some useful applications of the cynomolgus monkey model.
Immunization with Mtb72F/AS02A resulted in the activation and/or proliferation of Mtb72F-specific cells producing and releasing IFN-γ, IL-2, and TNF-α, which are known to be associated with protection against TB (18–20). TNF-α synergizes with IFN-γ in killing Mtb (21). Thus, the induction of Mtb72F-specific T cells capable of producing multiple cytokines upon antigen recall is likely to be beneficial for control of TB. Of note, boosting with Mtb72F/AS02A enhanced the Th1-type immune responses over immunizing with BCG alone. Boosting BCG with Mtb72F/AS02A also significantly increased PPD- and Mtb72F-specific IL-2 levels when compared with the levels measured in animals receiving BCG alone. Because IL-2 is known to elevate antigen-specific CD8+ and CD4+ T cell numbers by increasing proliferation and decreasing apoptosis, the expansion of antigen-specific memory T cells resulting from the prime-boost strategy used may have contributed to long-term protective immunity.
Another important aspect of the immune responses induced by the Mtb72F-containing vaccines was observed following challenge with virulent Mtb. Whereas nonimmunized monkeys responded to Mtb antigens with a broad range of cytokines, including the Th2-associated IL-4 and IL-5, monkeys immunized with either BCG alone or with BCG followed by Mtb72F/AS02A did not produce IL-5 in response to challenge. The main distinction between cytokine response patterns between immunized groups showing moderate (BCG alone) or more complete (BCG + Mtb72F/AS02A) protection was the greater IFN-γ/IL-6 ratio in the groups having the highest degree of protection. These results provide evidence of an immune response profile that may be indicative of protection against TB in a primate model.
The results described herein support the concept of using Mtb72F/AS02A to increase partial protection afforded by BCG. Boosting BCG with Mtb72F/AS02A protected animals almost completely from the development of pathology. Interestingly, among animals that did develop disease, only those primed with BCG and boosted with Mtb72F/AS02A showed resolution of lung lesions. The animals boosted with Mtb72F/AS02A at 16 weeks after the BCG prime had better survival and were more likely to resolve any lung lesions than those boosted at 32 weeks. Prechallenge immunogenicity data (data not shown) in these animals demonstrated the elicitation of all 3 of the Th1-asssociated cytokines measured, IFN-γ, TNF-α, and IL-2, upon in vitro restimulation with PPD or Mtb72F. The data thus indicate that induction of an appropriate immune response can actually reverse the progression of pathology induced by TB infection.
Extensive congestion, edema, and granulomatous pneumonia and clumps of acid-fast bacilli were observed in the lungs of monkeys injected with saline or BCG alone, whereas in monkeys that received BCG + Mtb72F/AS02A, bacilli were rare, significant decreases in the number of CFU and acid-fast bacteria (AFB) counts were recorded, and granulomas and necrotic lesions were discrete.
A similar tendency was observed with experiments performed in guinea pigs in that BCG vaccination was boosted with vaccines containing Mtb72F (6). In those experiments, BCG-only controls eventually died from overwhelming lung consolidation, whereas animals receiving BCG and Mtb72F had mostly clear lungs with minimal granulomatous lesions and considerable evidence of lesion healing and airway remodeling. Remarkably, very few bacilli were observed in BCG-treated monkeys.
Each of the commonly used animal models has the ability to make useful contributions to the development of a TB vaccine. In our experience, by measuring reduction in lung bacterial burdens following challenge of immunized animals, the mouse model provided a good first step in the process of vaccine candidate selection, with the added advantage of allowing comprehensive immunological analysis. The guinea pig represents a disease model, permitting vaccine candidates to be evaluated for the ability to protect against disease progression and death, whereas a rabbit model has been developed to demonstrate protection against neurological manifestations of the disease. Both of these disease models are valuable for providing histopathological information as well. The cynomolgus monkey is not a model amenable to vaccine screening, but can provide extremely valuable information when used to evaluate late-stage vaccine candidates because in humans, BCG is partially effective in protecting against TB. Most monkeys immunized with BCG alone developed progressive disease. Previous reports have not followed infected monkeys long enough to characterize this partial protection. Thus, the cynomolgus monkey, like the guinea pig, allows the testing of vaccine candidates that can improve on BCG immunization. The ability to follow lung disease by radiological changes as infection progresses and to assess immune responses by using an array of primate reagents is an important aspect of the cynomolgus model.
Although it has been proposed that minimal animal studies are sufficient to progress into clinical trials, this approach does not resolve the issue of how to advance candidate vaccines beyond Phase I clinical studies. In practical terms, only a comprehensive approach based on optimizing protection in animal models, careful examination of possible immune correlates of protection such as our observation of elevated IFN-γ:IL-6 ratios, and demonstration of clinical safety is likely to result in the development of a safe and effective human vaccine against TB.
Materials and Methods
Experimental Vaccine.
The vaccine antigen construct Mtb72F has been described in ref. 3. It was formulated with the GlaxoSmithKline Biologicals proprietary Adjuvant System AS02A (22) before intramuscular (i.m.) inoculation. Doses used were 40 μg of antigen protein and 500 μL of AS02A.
Experimental Animals.
Adult, cynomolgus macaques (Macaca fascicularis) ranging in age from 1.6 to 3.7 years and weighing 1.6 to 3.2 kg were used for these studies. Before the commencement of the studies, the macaques underwent a rigorous battery of diagnostic and clinical procedures [e.g., physical examination, complete blood count (CBC) with differential, erythrocyte sedimentation rate (ESR), serum chemistry profile, thoracic radiography, lymphocyte proliferation assays/whole blood assays (LPAs/WBAs), and tuberculin skin testing (PPD-TST)].
Animals and Tissue Processing.
Cynomolgus macaques were inoculated intratracheally with ≈5 × 102 CFU of virulent M. tuberculosis (Erdman strain). The infection was allowed to proceed until the macaques reached disease states that spanned a spectrum from no apparent disease to advanced disease.
Bacteria and Infection.
M. tuberculosis Erdman strain was grown as described in ref. 3 and used for all infections. To prepare for infection, animals were preanesthetized with acepromazine maleate. Before infection, each animal was anesthetized intramuscularly with Ketamine HCL (0.3–0.5 mg/kg body weight). The M. tuberculosis Erdman inoculum was instilled intratracheally in a 1.0-mL volume.
Radiographic Procedures.
Ventral-dorsal and right lateral thoracic radiographs were taken before infection and monthly or twice a month thereafter. Radiographs were also taken when clinically necessary and immediately before euthanasia and were read by a board-certified thoracic radiologist with extensive experience in pulmonary tuberculosis.
Immunization.
Monkeys were injected 3 times (4 weeks apart) with 40 μg of Mtb72F formulated with the AS02A Adjuvant System or with AS02A Adjuvant System alone. For prime-boost protocols, monkeys were primed with 0.1 mL of BCG (Sanofi Pasteur) or injected with saline delivered intradermally. Starting 16 or 32 weeks later they were injected intramuscularly in the gluteal muscle 3 times (4 weeks apart) with 40 μg of Mtb72F formulated with the AS02A Adjuvant System, AS02A, or saline alone.
Clinical Assessment.
Weight, temperature, CBC, serum chemistry profile, direct fecal examination, rectal culture, thoracic radiography (CXR), PPD-ST, and the Sediplast Westergren ESR were recorded monthly.
Cellular Immune Response Assays.
Monkeys were bled at day 0, and 3 weeks post third Mtb72F/AS02A immunization (weeks 27 or 43 depending on the experiment) to evaluate cellular immune responses. Heparinized blood was diluted with complete RPMI 1:5 dilution for lymphoproliferation (LPA) assays and stimulated with phytohemagglutinin (PHA; 10 μg/mL), PPD (10 μg/mL), Mtb72F, or component antigens at 10 μg/mL, or not stimulated. Cells stimulated with PHA or antigens were incubated at 37 °C for 66–68 h (3 days) or 114–116 h (5 days), respectively, and then [3H]thymidine (1 μCi per well) was added for the final 6 h of incubation. Results are reported as stimulation index, the fold increase in cpm over the unstimulated control.
Supernatants of LPA/whole blood assays (WBA) assays were harvested after 72 h to measure IFN-γ levels by ELISA. Heparinized blood was also used to isolate peripheral blood mononuclear cells (PBMC) using Ficoll–Hypaque density gradient centrifugation. PBMC were incubated with 10 μg/mL each of tuberculin PPD or Mtb72F. Supernatants were collected after 72 h and evaluated for IFN-γ, TNF-α, IL-6, IL-5, IL-4, and IL-2 by using a Th1/Th2 CBA kit (Becton Dickinson).
Necropsy Procedures, Histological Analysis, and Determination of Bacterial Burden.
Before necropsy, animals were anesthetized with ketamine and killed with an i.v. overdose of sodium pentobarbital. The thoracic cavity was entered, and the gross extent of mycobacterial infection was recorded. Each lung lobe was dissected, and the gross dissemination of mycobacterial infection (e.g., number of visible granulomas) and other pathological findings was recorded. Selected pieces of pulmonary, lymphatic, and other organ tissues were preserved in 10% formalin for histopathological examination and/or were flash frozen using liquid nitrogen for quantifying bacteria. The bacteriological burden (CFU) of monkey lungs was determined by homogenizing known weights of lungs in PBS Tween-80 0.05%, plating 5-fold serial dilutions on 7H10 agar plates (Molecular Toxicology Inc.), and counting CFU 3 wks later. Sections were also cut and stained with hematoxylin and eosin (H&E), Ziehl-Neelsen, and Fite's stain (for acid-fast bacilli), and von Kossa stain for calcium.
Bacterial burden was also determined by calculating the concentration of AFB per mm3 of tissue in monkey organs. AFB were located within the macrophages of granuloma mantels, and 5 high power fields (HPF) were counted within each mantel. Counts varied from 0 to 6 organisms per HPF, and the counts were averaged to provide the average number of organisms/HPF. The mantel volume was determined by calculating the volume of the ellipse shaped granuloma measured from the outer surface of the reactive mantel and subtracting the volume of the ellipse volume of the granuloma measured from the inner surface of the reactive mantel. Organism concentrations were determined for the total individual granuloma and for the total organ by multiplying the value for a single granuloma by the equivalent amount of granuloma tissue present in that organ. Sections were analyzed by a board-certified pathologist.
Statistical Analysis.
Statistical analyses were performed using Prism 4 for Windows (version 4.02, GraphPad Software, Inc.) and Stata (version 9.2, StataCorp LP). Nonparametric tests were used: Categorical data were analyzed using Fisher's exact test and continuous data with the Wilcoxon rank sum test for comparison of data in 2 groups. Because sample sizes were small, the statistical power of these studies was limited, and P values less than the customary 0.05 level for statistical significance should not necessarily be interpreted as precluding an important difference between groups.
Supplementary Material
Acknowledgments.
We thank Nicole Stride, Silvia Vidal, and Karen Bernards for excellent technical assistance, and Yannick Vanloubbeeck, DVM, PhD for the critical review. This work was supported in part by National Insitutes of Health Grants UC1 AI049505, R01 AI044373, and N01 AI25479.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712077106/DCSupplemental.
References
- 1.World Health Organization. The World Health Report 2004 - Changing History. Geneva: WHO; 2004. pp. 1–96. [Google Scholar]
- 2.Andersen P, Doherty TM. The success and failure of BCG–implications for a novel tuberculosis vaccine. Nat Rev Mol Cell Biol. 2005;3:656–662. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 3.Skeiky YA, et al. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J Immunol. 2004;172:7618–7628. doi: 10.4049/jimmunol.172.12.7618. [DOI] [PubMed] [Google Scholar]
- 4.Skeiky YA, et al. Cloning, expression, and immunological evaluation of two putative secreted serine protease antigens of mycobacterium tuberculosis. Infect Immun. 1999;67:3998–4007. doi: 10.1128/iai.67.8.3998-4007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dillon DC, et al. Molecular characterization and human T-cell responses to a member of a novel Mycobacterium tuberculosis mtb39 gene family. Infect Immun. 1999;67:2941–2950. doi: 10.1128/iai.67.6.2941-2950.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt L, et al. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect Immun. 2004;72:6622–6632. doi: 10.1128/IAI.72.11.6622-6632.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh GP, et al. The Philippine cynomolgus monkey (Macaca fasicularis) provides a new nonhuman primate model of tuberculosis that resembles human disease. Nat Med. 1996;2:430–436. doi: 10.1038/nm0496-430. [DOI] [PubMed] [Google Scholar]
- 8.Masopust D, Kaech SM, Wherry EJ, Ahmed R. The role of programming in memory T-cell development. Curr Opin Immunol. 2004;16:217–225. doi: 10.1016/j.coi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Dye C, Raviglione M. Monitoring global health: WHO has mandate and expertise. BMJ. 2005;330:195. doi: 10.1136/bmj.330.7484.195-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffin JF, Mackintosh CG, Rodgers CR. Factors influencing the protective efficacy of a BCG homologous prime-boost vaccination regime against tuberculosis. Vaccine. 2006;24:835–845. doi: 10.1016/j.vaccine.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 11.McShane H, Hill A. Prime-boost immunisation strategies for tuberculosis. Microbes andInfect. 2005;7:962–967. doi: 10.1016/j.micinf.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Tsenova L, et al. Evaluation of the Mtb72F polyprotein vaccine in a rabbit model of tuberculous meningitis. Infect Immun. 2006;74:2392–2401. doi: 10.1128/IAI.74.4.2392-2401.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alderson MR, et al. Expression cloning of an immunodominant family of Mycobacterium tuberculosis antigens using human CD4(+) T cells. J Exp Med. 2000;191:551–560. doi: 10.1084/jem.191.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dillon DC, et al. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J Clin Microbiol. 2000;38:3285–3290. doi: 10.1128/jcm.38.9.3285-3290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skeiky YA, et al. T cell expression cloning of a Mycobacterium tuberculosis gene encoding a protective antigen associated with the early control of infection. J Immunol. 2000;165:7140–7149. doi: 10.4049/jimmunol.165.12.7140. [DOI] [PubMed] [Google Scholar]
- 16.Reed SG, Alderson MR, Dalemans W, Lobet Y, Skeiky YA. Prospects for a better vaccine against tuberculosis. Tuberculosis (Edinburgh, Scotland) 2003;83:213–219. doi: 10.1016/s1472-9792(02)00080-x. [DOI] [PubMed] [Google Scholar]
- 17.Reed S, Lobet Y. Tuberculosis vaccine development; from mouse to man. Microbes andInfect. 2005;7:922–931. doi: 10.1016/j.micinf.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Abebe F, Bjune G. The emergence of Beijing family genotypes of Mycobacterium tuberculosis and low-level protection by bacille Calmette-Guerin (BCG) vaccines: Is there a link? Clin Exp Immunol. 2006;145:389–397. doi: 10.1111/j.1365-2249.2006.03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beveridge NE, et al. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur J Immunol. 2007;37:3089–3100. doi: 10.1002/eji.200737504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoff ST, et al. Evaluation of Mycobacterium tuberculosis—Specific antibody responses in populations with different levels of exposure from Tanzania, Ethiopia, Brazil, and Denmark. Clin Infect Dis. 2007;45:575–582. doi: 10.1086/520662. [DOI] [PubMed] [Google Scholar]
- 21.Salgame P. Host innate and Th1 responses and the bacterial factors that control Mycobacterium tuberculosis infection. Curr Opin Immunol. 2005;17:374–380. doi: 10.1016/j.coi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Bojang KA. RTS,S/AS02A for malaria. Expert Rev Vaccines. 2006;5:611–615. doi: 10.1586/14760584.5.5.611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.