Abstract
Oxidative stress has been implicated in the etiology of Parkinson's disease (PD) and in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) animal model of PD. It is known that under conditions of oxidative stress, the transcription factor NF-E2-related factor (Nrf2) binds to antioxidant response element (ARE) to induce antioxidant and phase II detoxification enzymes. To investigate the role of Nrf2 in the process of MPTP-induced toxicity, mice expressing the human placental alkaline phosphatase (hPAP) gene driven by a promoter containing a core ARE sequence (ARE-hPAP) were used. ARE-hPAP mice were injected (30 mg/kg) once per day for 5 days and killed 7 days after the last MPTP injection. In response to this design, ARE-dependent gene expression was decreased in striatum whereas it was increased in substantia nigra. The same MPTP protocol was applied in Nrf2+/+ and Nrf2−/− mice; Nrf2 deficiency increases MPTP sensitivity. Furthermore, we evaluated the potential for astrocytic Nrf2 overexpression to protect from MPTP toxicity. Transgenic mice with Nrf2 under control of the astrocyte-specific promoter for the glial fribillary acidic protein (GFAP-Nrf2) on both a Nrf2+/+ and Nrf2−/− background were administered MPTP. In the latter case, only the astrocytes expressed Nrf2. Independent of background, MPTP-mediated toxicity was abolished in GFAP-Nrf2 mice. These striking results indicate that Nrf2 expression restricted to astrocytes is sufficient to protect against MPTP and astrocytic modulation of the Nrf2-ARE pathway is a promising target for therapeutics aimed at reducing or preventing neuronal death in PD.
Keywords: antioxidant response element, human placental alkaline phosphatase
MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) is a mitochondrial complex I inhibitor that is known to damage the nigrostriatal dopaminergic pathway as seen in Parkinson's disease (PD) (1, 2). PD is a progressive neurodegenerative disease characterized by the selective loss of dopaminergic neurons of the substantia nigra pars compacta. Dopaminergic neuron loss results in reduced striatal dopamine (DA) and the hallmark clinical features of PD (3). Most cases of PD are considered sporadic with unknown cause, and the etiology of sporadic PD is not fully understood. Increasing evidence suggests that mitochondrial dysfunction, oxidative damage, excitotoxicity, and inflammation are contributing factors (4–7).
Evidence for the existence of oxidative stress in PD is derived from post mortem analysis of brain tissue of PD patients that demonstrates increased levels of oxidized proteins, lipids, and nucleic acids (8–13). One mechanism by which cells may combat oxidative insult is through increased transcription of genes containing the antioxidant response element (ARE). The ARE is a cis-acting enhancer sequence that regulates many cytoprotective genes via the transcription factor NF-E2-related factor (Nrf2) (Nrf2 regulation is reviewed in ref. 14). ARE-regulated genes include heme oxygenase-1 (HO-1) (15), NAD(P)H:quinone oxidoreductase-1 (NQO1) (16, 17), and glutathione S-transferases (18) as well as glutathione-synthesizing enzymes glutamate-cysteine ligase catalytic subunit (GCLC) and glutamate-cysteine ligase modifier subunit (GCLM) (19–21).
There is increasing evidence that the Nrf2-ARE pathway is involved in neurodegenerative disease. The expression of ARE-driven genes such as NQO1 and HO-1 is increased in post mortem brain tissue from PD patients (22, 23). These changes could be a neuroprotective response mediated by Nrf2 activation. Indeed, we have demonstrated that Nrf2-dependent transcription can prevent reactive oxygen species-induced apoptosis in neurons and astrocytes in vitro (24–28). In vivo studies show that Nrf2 is protective against intrastriatal administration of the complex II inhibitors malonate or 3-nitroproprionic acid (29, 30), 6-hydroxydopamine (31, 32), and rodent models of cerebral ischemia (33–35). Recent work from our laboratory evaluated Nrf2 overexpression in astrocytes in vivo by generating GFAP-Nrf2 transgenic mice. These mice demonstrated significantly delayed onset of pathology and extended lifespan in genetic models of amyotrophic lateral sclerosis (36). Finally, in other studies using the acute MPTP model, it was shown that Nrf2−/− mice were more sensitive to MPTP (37). The work presented here extends these observations and focuses on the underlying mechanism of Nrf2-mediated neuroprotection in the subchronic models of MPTP exposure. Three lines of genetically engineered mice were used in these experiments: ARE-hPAP reporter mice (38), Nrf2−/− mice (39), and GFAP-Nrf2 transgenic mice (36). The goals of this investigation were to (i) examine how the Nrf2-ARE pathway responds to MPTP exposure; (ii) determine whether the lack of Nrf2 sensitized mice to MPTP; and (iii) evaluate whether mice selectively overexpressing Nrf2 in astrocytes were resistant to MPTP toxicity.
Results
Nrf2-ARE Pathway Is Altered in the Subchronic MPTP Model.
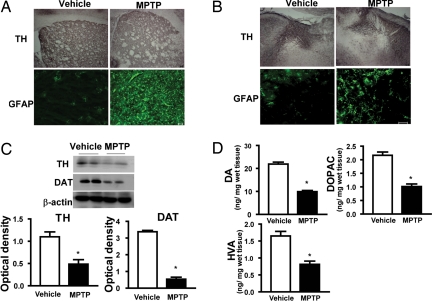
Before examining Nrf2, we confirmed that the MPTP-dosing regimen leads to expected markers of dopaminergic toxicity, including decreases in tyrosine hydroxylase (TH) immunostaining in both striatum (STR) and substantia nigra (SN) (Fig. 1 A and B) and decreases in both TH and dopamine transporter (DAT) protein levels in STR (Fig. 1C). Additionally, DA, dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) were decreased by MPTP in STR (Fig. 1D). Increased GFAP immunostaining, indicative of astrogliosis, was increased in STR and SN (Fig. 1 A and B).
Fig. 1.
Characterization of the subchronic MPTP mouse model of PD. (A and B) Immunohistochemistry for TH and immunofluorescence for GFAP are shown in STR (A) and SN (B). (C) Representative Western blots of TH and DAT proteins). Bar graphs show quantitative data for TH and DAT signals that are normalized to β-actin signal (n = 8–10 per group). (D) Bar graphs show HPLC measurements of DA and metabolites, DOPAC and HVA, after vehicle or MPTP (30 mg/kg per day) treatment (n = 8–10 per group). (Scale bar, 50 μm.) *, P < 0.05 compared with the vehicle-treated group.
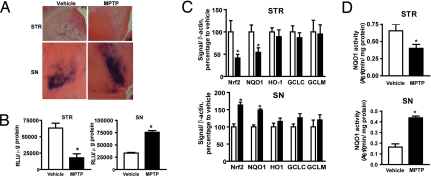
To study Nrf2-ARE pathway activation, ARE-hPAP reporter mice were injected with 30 mg/kg MPTP subchronically. These reporter mice have been used to monitor activation of the Nrf2-ARE pathway in vivo (24, 29, 31, 32). Both histochemical staining and hPAP activity (Fig. 2 A and B) showed that MPTP treatment decreased Nrf2-ARE signaling in the STR but increased it in the SN. To verify the fidelity of our reporter, we measured expression levels of Nrf2, NQO1, HO-1, GCLC, and GCLM as well as NQO1 enzymatic activity. In accordance with the hPAP data, Nrf2 and NQO1 expression as well as NQO1 activity were decreased in STR after MPTP, whereas these measures were increased in the SN (Fig. 2C).
Fig. 2.
Subchronic MPTP treatment alters the Nrf2-ARE pathway. ARE-hPAP reporter mice were given MPTP, and mice were killed 7 days after the last MPTP dose. (A and B) Histochemical staining and hPAP activity assay were performed on the STR (A) and SN (B). (C) Quantitative PCR analyses of Nrf2, NQO1, HO-1, GCLC, and GCLM in STR and SN after vehicle or 30 mg/kg MPTP treatment. (D) NQO1 activity in the STR and the SN after vehicle or MPTP treatments (all data comprise groups totaling 8–10 animals). (Scale bar, 50 μm.) *, P < 0.05 compared with the vehicle-treated group.
Targeted Disruption of Nrf2 Causes Increased MPTP Toxicity in STR and SN.
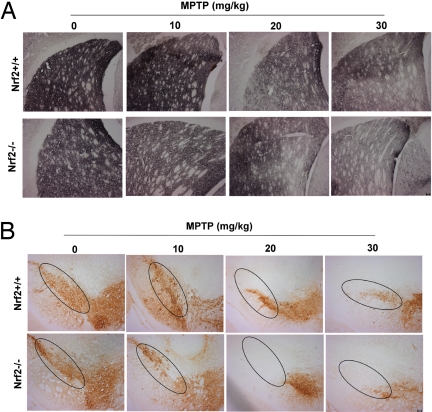
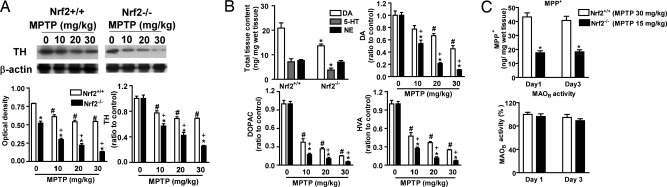
To investigate whether Nrf2 is involved in limiting or preventing MPTP-induced toxicity, MPTP (0, 10, 20, 30 mg/kg) was administered to both Nrf2+/+ and Nrf2−/− mice. TH staining clearly showed that Nrf2−/− mice are more sensitive than Nrf2+/+ mice to MPTP (Fig. 3). These data were confirmed with TH immunoblots and catecholamine analysis. Immunoblots showed that a significantly greater fraction of TH was lost in the Nrf2−/− mice at all doses of MPTP (Fig. 4A Right). Interestingly, Nrf2−/− mice had a lower basal expression of TH (Fig. 4A Left, vehicle-treated bars), which was reflected by reduced basal levels of striatal DA (Fig. 4B), DOPAC (1.6 ± 0.17 vs. 2.5 ± 0.19), and HVA (1.1 ± 0.12 vs. 1.7 ± 0.14) content. There was no significant difference in DOPAC/DA (0.12 ± 0.03 vs. 0.1 ± 0.03) or HVA/DA (0.08 ± 0.03 vs. 0.07 ± 0.02) ratios when comparing Nrf2−/− with Nrf2+/+ mice. Nrf2−/− mice exposed to MPTP showed a more rapid dose-dependent decline in DA, DOPAC, and HVA compared with Nrf2+/+ mice. Using MPTP doses that cause approximately equal damage (15 mg/kg in Nrf2−/−; 30 mg/kg in Nrf2+/+ mice), we measured MPP+ levels 15 min after first and third MPTP injection. The amount of MPP+ generated was ≈50% lower in Nrf2−/− compared with Nrf2+/+ mice (Fig. 4C), indicating that Nrf2 does not alter MPP+ formation. Similarly, MAOB activity was not different between the Nrf2+/+ and Nrf2−/− mice (Fig. 4C). Based on these results, we conclude that loss of Nrf2 potentiates MPTP toxicity without changing MPTP metabolism, and less MPP+ causes equal or greater toxicity in the Nrf2−/− mice. To understand this differential sensitivity in greater mechanistic detail, the expression profile of Nrf2-dependent genes in Nrf2+/+ and Nrf2−/− brains was evaluated after MPTP treatment [supporting information (SI) Fig. S1]. Interestingly, no significant basal difference in NQO1, HO-1, GCLC, and GCLM expression existed between Nrf2+/+ and Nrf2−/− mice. After MPTP treatment, NQO1, HO-1, GCLC, and GCLM genes were all significantly decreased in STR of Nrf2−/− mice (Fig. S1A). In contrast, NQO1 was the only gene reduced in the STR of Nrf2+/+ mice (Fig. S1A). In the SN of Nrf2−/− mice, all genes decreased similarly to STR (Fig. S1B). The exact opposite was seen in the SN of the Nrf2+/+ mice, where the expression of all genes was increased after MPTP treatment (Fig. S1B). NQO1 activity confirmed these quantitative PCR (qPCR) results (Fig. S1C). In both the STR and SN, increasing MPTP dosage leads to more severe gliosis in Nrf2+/+ and Nrf2−/− mice (Fig. S2). These immunohistochemical data were confirmed by quantification of GFAP and Iba-1 expression using qPCR. There was a greater increase of GFAP (astrogliosis) and Iba-1 (microglial activation) mRNA levels in both STR and SN of Nrf2−/− mice after MPTP administration (Fig. S3).
Fig. 3.
Immunohistochemical staining for TH in Nrf2+/+ and Nrf2−/− mice in response to MPTP. (A and B) TH staining of the STR (A) and SN (B) from Nrf2+/+ and Nrf2−/− mice in response to four different doses of MPTP (0, 10, 20, or 30 mg/kg) administered once a day for 5 days. (Scale bar, 50 μm.)
Fig. 4.
Neurochemical analysis of Nrf2+/+ and Nrf2−/− mice in response to MPTP. MPTP was administered to mice at four different doses (0, 10, 20, or 30 mg/kg). (A) (Left) Representative Western blots for striatal TH protein and β-actin signals. Bar graph shows quantification of TH normalized to β-actin. (Right) Bar graph shows the same data normalized to the vehicle-treated group of the same genotype (n = 8–10 per group). HPLC was used to quantify basal levels of DA, serotonin (5-HT), and norepinephrine (NE) in Nrf2+/+ and Nrf2−/− mice (B) (Upper Left) The amount of DA, DOPAC, and HVA was measured in Nrf2+/+ and Nrf2−/− mice in response to the different MPTP doses. Data are normalized to vehicle treatment of the same genotype (n = 8–10 per group) *, P < 0.05 compared with the same dose of Nrf2+/+ mice; #, P < 0.05 compared with vehicle-treated Nrf2+/+ mice; +, P < 0.05 compared with vehicle-treated Nrf2−/− mice. (C) The levels of MPP+ (Upper) and MAOB activity (Lower) were measured 15 min after the first and third MPTP administration on days 1 and 3 of this subchronic protocol. Nrf2+/+ mice were treated with 30 mg/kg MPTP, whereas Nrf2−/− mice were treated with 15 mg/kg (n = 8–10 per group). *, P < 0.05 compared with the same dose of Nrf2+/+ mice.
Striatum Is Protected from MPTP Toxicity in GFAP-Nrf2 Transgenic Mice.
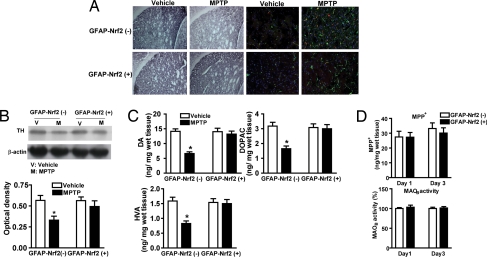
Because mice lacking Nrf2 were more sensitive to MPTP, we investigated whether overexpression of Nrf2 would confer resistance to MPTP. GFAP-Nrf2(+) and GFAP-Nrf2(−) littermates were treated with 30 mg/kg MPTP. TH immunostaining and immunoblots showed that GFAP-Nrf2(+) mice were completely protected from STR TH loss (Fig. 5 A and B). and Immunostaining showed that the extent of astrogliosis (GFAP) and microglial activation (Iba-1) were also dramatically attenuated in the GFAP-Nrf2(+) mice (Fig. 5A and Fig. S4). These data were confirmed by qPCR of GFAP [GFAP-Nrf2(−) mice: vehicle −0.45 ± 0.04 μM, MPTP −0.98 ± 0.03 μM; GFAP-Nrf2(+) mice: vehicle −0.42 ± 0.04 μM, MPTP −0.47 ± 0.09 μM] and Iba-1 [GFAP-Nrf2(−) mice: vehicle −0.04 ± 0.005 μM, MPTP −0.07 ± 0.006 μM; GFAP-Nrf2(+) mice: vehicle −0.03 ± 0.005 μM, MPTP −0.03 ± 0.003 μM] in STR. There was a statistically significant increase in GFAP and Iba-1 in GFAP-Nrf2(−) mice after MPTP that was significantly reduced (GFAP) or eliminated (Iba-1) in the GFAP-Nrf2(+) mice. Similarly, there was no decrease striatial DA, DOPAC, or HVA levels in the GFAP-Nrf2(+) mice (Fig. 5C). Finally, evaluation of MPP+ levels and MAOB activity in GFAP-Nrf2(+) vs. GFAP-Nrf2(−) mice demonstrated that the observed effects were not caused by differences in MPTP metabolism (Fig. 5D). Examination of Nrf2-dependent gene expression revealed that, in contrast to the decrease or no change observed in STR of GFAP-Nrf2(−) mice, all genes were increased by 2- or 3-fold in both the STR of GFAP-Nrf2(+) mice after MPTP treatment (Fig. S5A). Increased Nrf2-driven gene expression in SN by MPTP was greatly enhanced in the GFAP-Nrf2(+) mice (Fig. S5B). Altered NQO1 expression was validated by measuring NQO1 activity (Fig. S5C).
Fig. 5.
Effect of astrocyte-specific Nrf2 overexpression on MPTP toxicity. (A) (Left) Immunohistochemical staining for TH in the STR of GFAP-Nrf2(−) and GFAP-Nrf2(+) mice. (Right) Staining for GFAP (green) and Iba-1 (red) in the STR of GFAP-Nrf2(−) and GFAP-Nrf2(+) mice is shown. Fig. S4 is an enlarged picture of 5A. (B) (Upper) Representative Western blots for TH in the STR of GFAP-Nrf2(−) and GFAP-Nrf2(+) mice. TH signal was normalized to β-actin to account for variations in protein loading. (Lower) Bar graph shows quantification of the TH Western blots (n = 8–10 per group). (C) The amount of DA, DOPAC, and HVA in the STR of GFAP-Nrf2(−) and GFAP-Nrf2(+) mice after vehicle or 30 mg/kg MPTP treatment was determined (n = 8–10 per group). (D) The levels of MPP+ (Upper) and MAOB activity (Lower) were measured in the STR of GFAP-Nrf2(−) and GFAP-Nrf2(+) mice 15 min after MPTP administration (30 mg/kg) on days 1 and 3 of this subchronic protocol (n = 8–10 per group). (Scale bar, 50 μm.) *, P < 0.05 compared with the corresponding vehicle-treated group.
GFAP-Nrf2 Protects Against MPTP in an Nrf2−/− Background.
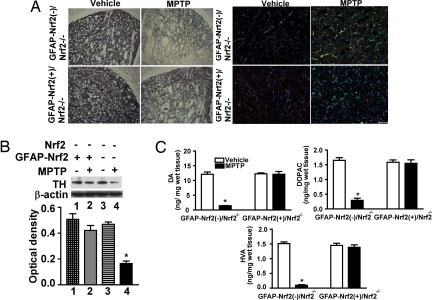
The critical importance of astrocytic expression of Nrf2 in neuroprotection of MPTP toxicity was further demonstrated through the use of GFAP-Nrf2(+)/Nrf2−/− mice. These mice and corresponding littermate controls were challenged with the subchronic MPTP schedule using 30 mg/kg per day. This dose was extremely toxic to the Nrf2−/−, mice leading to 80−90% reductions in TH, DA, and DA metabolites (Fig. 4). Both immunohistochemical and immunoblot analysis of TH levels showed that the GFAP-Nrf2 transgene completely protected from MPTP-induced loss of striatal TH on an Nrf2−/− background (Fig. 6 A and B). Moreover, GFAP-Nrf2(+)/Nrf2−/− mice had less astrogliosis and microglial activation than GFAP-Nrf2(−)/Nrf2−/− mice after MPTP treatment (Fig. 6A and Fig. S6). Catecholamine analysis also demonstrated dramatic Nrf2-mediated protection from MPTP in the GFAP-Nrf2(+)/Nrf2−/− mice. A 90% reduction in DA and DA metabolite levels was entirely reversed in mice where Nrf2 was only overexpressed in the astrocytes (Fig. 6C). To probe the potential mechanism of astrocytic Nrf2-mediated protection, gene expression profiles of selected Nrf2-dependent genes were generated. GFAP-Nrf2(+)/Nrf2−/− mice exhibited increased basal transcription of these genes in STR and SN (Fig. S7 A and B). MPTP treatment decreased all genes in the STR and SN of GFAP-Nrf2(−)/Nrf2−/− mice; however, these genes were increased in GFAP-Nrf2(+)/Nrf2−/− mice (Fig. S7 A and B). MPTP also decreased NQO1 activity in STR and SN of GFAP-Nrf2(−)/Nrf2−/− but increased it in GFAP-Nrf2(+)/Nrf2−/− mice (Fig. S7C).
Fig. 6.
Effect of astrocyte-specific Nrf2 overexpression on MPTP toxicity in a Nrf2−/− background. (A) (Left) Immunohistochemical staining for TH in the STR of GFAP-Nrf2(−)/Nrf2−/− and GFAP-Nrf2(+)/Nrf2−/− mice after vehicle or 30 mg/kg MPTP treatment. (Right) Staining for GFAP (green) and Iba-1 protein (red) in the STR of GFAP-Nrf2(−)/Nrf2−/− and GFAP-Nrf2(+)/Nrf2−/− mice after vehicle or MPTP treatment. Fig. S6 is an enlargement of A. (B) (Upper) Representative Western blots of TH and β-actin. (Lower) TH signal was normalized to β-actin signal for protein loading control, and the bar shows quantification of the TH Western blots (n = 8–10). (C) The amount of DA, DOPAC, and HVA in the STR of GFAP-Nrf2(−)/Nrf2−/− and GFAP-Nrf2(+)/Nrf2−/− mice after vehicle or MPTP treatment (n = 8–10). (Scale bar, 50 μm.) *, P < 0.05 compared with the corresponding vehicle-treated group).
Discussion
Based on these data, we can conclude that Nrf2-mediated neuronal protection against MPTP is caused by astrocytic Nrf2 activation. Previous evidence shows that Nrf2−/− mice are more sensitive to 6-hydroxydopamine and acute MPTP exposure (31, 32, 37). The current study extends these observations by using a subchronic MPTP model and focuses on how Nrf2 may be protective. It also appears that Nrf2−/− mice have lower basal levels of DA than the Nrf2+/+ mice. This contradicts work of Pacchioni et al. (40), who showed no basal difference between Nrf2+/+ and Nrf2−/− mice. This discrepancy may in part be caused by genetic background differences. Pacchioni and colleagues used mice maintained on a C57BL6/129sv background derived from heterozygous mating pairs. In the current study, Nrf2−/− lines are continually back-crossed with C57BL6/SJL F1 wild-type mice, which is advantageous for genetic stability of the colony (41). Regardless, the lower DA levels in the Nrf2−/− mice presented here correlate with lower STR levels of TH protein content compared with Nrf2+/+ mice (Fig. 4). Hence, we speculate that because of insufficient striatal antioxidant defenses, Nrf2−/− mice compensate by decreasing DA levels to lower oxidative stress.
The opposing changes in hPAP activity and Nrf2-mediated gene expression between the STR and SN (Fig. 2) are very interesting. The possible explanation could be that DA terminals are more sensitive to MPTP, and the differential response between STR and SN is related to DA release and striatal innervation of dopaminergic neurons. The Nrf2-ARE pathway can be activated under conditions of oxidative stress. It is known that autooxidation of DA leads to the production of DA (semi)quinones that are easily converted into aminochrome. Aminochrome readily generates superoxide anion (42). Therefore, if MPTP decreases DA release by denervation, striatal tissue would not be subject to oxidative DA byproducts. Denervation may thereby lead to the observed reduction in Nrf2-mediated gene expression. In contrast, dopaminergic neurons in the SN do not receive DA input, but they produce DA. In this case, intracellular DA could potentially generate an oxidative environment (43). The loss of TH staining with concomitant Nrf2 activation in SN may represent an orchestrated attempt to reduce oxidative stress within the neuron. TH loss would reduce the level of DA produced in dopaminergic neurons, whereas Nrf2 activation in surrounding astrocytes may protect the neurons. The neurons themselves could also activate Nrf2 in response to insult. However, our data showing GFAP-Nrf2 protection on an Nrf2−/− background strongly suggest that the Nrf2-mediated dopaminergic neuroprotection is astrocyte-dependent. Because metabolism of MPTP is not different in the GFAP-Nrf2 mice, alternative explanations outside of the increased resistance of dopaminergic neurons could be a greater ability of the astrocyte to detoxify MPP+ and/or reduced transport of MPP+ out of the astrocyte. Experiments are under way to look more closely at the astrocyte–neuron communication and the possible role for Nrf2 in dopaminergic neurons contributing to the protective response.
Inflammation is clearly part of the physiological response to MPTP as indicated by microglial activation; this has been strongly linked to PD. Microglia become persistently activated and maintain elevated production of both cytokines and reactive oxygen species in PD (44). Evidence from post mortem PD brain tissue suggests that this activation of microglia is associated with increased neuronal death (45). A few reports suggest that Nrf2 activation may attenuate microglial activation (36, 46). The lack of Nrf2 leads to an enhanced microglial response in hippocampus after administration of kainic acid (46). In addition, attenuated microglial activation was observed in mouse models of amyotrophic lateral sclerosis by when crossed with the GFAP-Nrf2 mice (36). The data generated herein clearly show an inverse correlation between Nrf2 and microglial activation, thus supporting the concept of Nrf2-mediated attenuation of neuroinflammation. The dramatic suppression of microglial activation by astrocytic Nrf2 also strongly suggests that neuroinflammation is secondary to astrocyte dysfunction and that sustained Nrf2 levels or increased Nrf2 activity in the astrocyte can prevent this secondary event.
In conclusion, we have shown that astrocytic Nrf2 is neuroprotective against MPTP neurotoxicity in mice. The result is of considerable interest in regard to understanding the mechanisms of astrocyte-mediated protection against neurodegeneration. The data strongly support the concept that astrocytic Nrf2 modulation holds great potential for the neuroprotective or therapeutic strategies to treat PD.
Materials and Methods
Animals.
ARE-hPAP transgenic mice were created by using 51 bp of the rat NQO1 promoter upstream of a heat-stable reporter construct (38). Nrf2−/− mice were generated by replacing the basic leucine zipper domain with the lacZ reporter construct as described in ref. 47. GFAP-Nrf2 transgenic mice with Nrf2 expression under the control of the GFAP promoter were developed as described in ref. 36. All mice used for experiments were bred with C57BL6/SJL mice for at least six generations (Jackson Laboratory) (see SI Materials and Methods for details).
Subchronic MPTP Administration.
Mice (age 8–12 weeks, 8–10 per group) received i.p. injections of vehicle or MPTP at indicated doses (free base in PBS; Sigma) once daily for 5 consecutive days. Seven days after the last injection, all mice were killed with CO2.
TH Immunohistochemistry.
Frozen sections (20 μm) were pretreated with 0.3% H2O2 in PBS and incubated with PBS containing 10% normal goat serum for 30 min at room temperature. Sections were then incubated with rabbit polyclonal anti-TH antibody (1:1,000; Chemicon) overnight at 4 °C. Next, the sections were incubated with an avidin–biotin–horseradish peroxidase complex (Vector Laboratories) according to the manufacturer's instructions. The sections were stained with a DAB kit (Vector Laboratories), dehydrated, and cleared with xylenes before coverslipping.
Immunoblotting Analysis.
Tissue was sonicated in 1% SDS buffer, and protein was determined by the BCA assay kit (Pierce). Equal amounts of protein (20–40 μg) were probed by typical Western protocols (see SI Materials and Methods for details).
Isolation of Total RNA and Quantitative PCR.
Isolation of total RNA was performed by using TRIzol according to the manufacturer's instructions (Invitrogen). Quality and quantity of total RNA were measured by using the Agilent 2100 Bioanalyzer. Reverse transcriptase reactions were run on 1 μg of total mRNA by using the reverse transcription system (Promega). Quantitative PCR was performed by using a Light Cycler 480 (Roche) and the SYBR Green I Master (Roche) according to the manufacturer's instructions. Primers for Iba-1 were 5′-GGATTTGCAGGGAGGAAAAG-3′ and 5′-TGGGATCATCGAGGAATTG-3′, and other primers sequences have been described (36).
ARE-hPAP Histochemistry and Activity.
Histochemistry for the hPAP reporter and activity of the hPAP reporter were performed as described in ref. 38.
NQO1 Activity.
Activity of NQO1 was measured in tissue homogenate as described in ref. 48.
Immunofluorescence Staining.
Standard immunohistochemical techniques were performed on slides prepared from frozen tissue. Images were captured by using a Zeiss photomicroscope and analyzed by using Axiovision software (see SI Materials and Methods for details).
MAOB Activity Measurement.
Striatal tissue was homogenized in 50 mM Tris, 5 mM EDTA (pH 7.4). Protein concentration was determined by the BCA assay (Pierce). Samples (100 μL) were used in the Amplex red monoamine oxidase assay kit (Molecular Probes) according to the manufacturer's instructions.
HPLC Determination of Striatal DA and Metabolites.
Mouse striatal tissue was dissected, weighed, and homogenized in perchloric (0.3 N) acid for the HPLC/ED analysis (see SI Materials and Methods for details).
HPLC Determination of Striatal MPP+ Levels.
Striatal tissue was dissected 15 min after the first and third MPTP injections and stored at −80 °C before being analyzed for MPP+ content by HPLC-UV. The UV detector was set at 295 nm for MPP+ detection as described in ref. 49.
Statistical Analysis.
All of the data were represented as mean ± SEM and analyzed by one-way ANOVA followed by unpaired t test analysis by using with the Prism program (GraphPad); P < 0.05 was considered significant.
Supplementary Material
Acknowledgments.
We thank Jon M. Resch, Hoa Anh Phan, and Sara Amirahmadi for maintaining mouse colonies. We also thank Scott Nelson, Marcus J. Calkins, and Neal Burton for editing this manuscript and providing valuable discussion. This work was supported by National Institute of Environment Health Sciences/National Institutes of Health Grant ES10042 and National Institutes of Health Grant NS 39006 (to R.J.S.) M.R.V. is a recipient of the Milton Safenowitz postdoctoral fellowship for amyotrophic lateral sclerosis research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813361106/DCSupplemental.
References
- 1.Chiba K, Peterson LA, Castagnoli KP, Trevor AJ, Castagnoli N., Jr Studies on the molecular mechanism of bioactivation of the selective nigrostriatal toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Drug Metab Dispos. 1985;13:342–347. [PubMed] [Google Scholar]
- 2.Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature. 1988;334:345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- 4.Dawson TM, Dawson VL. Neuroprotective and neurorestorative strategies for Parkinson's disease. Nat Neurosci. 2002;5(Suppl):1058–1061. doi: 10.1038/nn941. [DOI] [PubMed] [Google Scholar]
- 5.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 6.Dunnett SB, Bjorklund A. Prospects for new restorative and neuroprotective treatments in Parkinson's disease. Nature. 1999;399:A32–A39. doi: 10.1038/399a032. [DOI] [PubMed] [Google Scholar]
- 7.Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S49–S60. doi: 10.1002/ana.10481. [DOI] [PubMed] [Google Scholar]
- 8.Alam ZI, et al. A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J Neurochem. 1997;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- 9.Alam ZI, et al. Oxidative DNA damage in the parkinsonian brain: An apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- 10.Castellani RJ, et al. Hydroxynonenal adducts indicate a role for lipid peroxidation in neocortical and brainstem Lewy bodies in humans. Neurosci Lett. 2002;319:25–28. doi: 10.1016/s0304-3940(01)02514-9. [DOI] [PubMed] [Google Scholar]
- 11.Dexter D, et al. Lipid peroxidation as cause of nigral cell death in Parkinson's disease. Lancet. 1986;2:639–640. doi: 10.1016/s0140-6736(86)92471-2. [DOI] [PubMed] [Google Scholar]
- 12.Dexter DT, et al. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem. 1989;52:381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 13.Dexter DT, et al. Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: An HPLC and ESR study. Mov Disord. 1994;9:92–97. doi: 10.1002/mds.870090115. [DOI] [PubMed] [Google Scholar]
- 14.Calkins MJ, et al. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2242. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prestera T, et al. Parallel induction of heme oxygenase-1 and chemoprotective phase 2 enzymes by electrophiles and antioxidants: Regulation by upstream antioxidant-responsive elements (ARE) Mol Med. 1995;1:827–837. [PMC free article] [PubMed] [Google Scholar]
- 16.Favreau LV, Pickett CB. The rat quinone reductase antioxidant response element: Identification of the nucleotide sequence required for basal and inducible activity and detection of antioxidant response element-binding proteins in hepatoma and nonhepatoma cell lines. J Biol Chem. 1995;270:24468–24474. doi: 10.1074/jbc.270.41.24468. [DOI] [PubMed] [Google Scholar]
- 17.Wang B, Williamson G. Detection of a nuclear protein that binds specifically to the antioxidant responsive element (ARE) of the human NAD(P)H:quinone oxidoreductase gene. Biochim Biophys Acta. 1994;1219:645–652. doi: 10.1016/0167-4781(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 18.Rushmore TH, Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene: Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990;265:14648–14653. [PubMed] [Google Scholar]
- 19.Galloway DC, Blake DG, Shepherd AG, McLellan LI. Regulation of human γ-glutamylcysteine synthetase: Coordinate induction of the catalytic and regulatory subunits in HepG2 cells. Biochem J. 1997;328:99–104. doi: 10.1042/bj3280099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galloway DC, McLellan LI. Inducible expression of the γ-glutamylcysteine synthetase light subunit by t-butylhydroquinone in HepG2 cells is not dependent on an antioxidant-responsive element. Biochem J. 1998;336:535–539. doi: 10.1042/bj3360535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulcahy RT, Gipp JJ. Identification of a putative antioxidant response element in the 5′-flanking region of the human γ-glutamylcysteine synthetase heavy subunit gene. Biochem Biophys Res Commun. 1995;209:227–233. doi: 10.1006/bbrc.1995.1493. [DOI] [PubMed] [Google Scholar]
- 22.Schipper HM, Liberman A, Stopa EG. Neural heme oxygenase-1 expression in idiopathic Parkinson's disease. Exp Neurol. 1998;150:60–68. doi: 10.1006/exnr.1997.6752. [DOI] [PubMed] [Google Scholar]
- 23.Yoo MS, et al. Oxidative stress-regulated genes in nigral dopaminergic neuronal cells: correlation with the known pathology in Parkinson's disease. Brain Res Mol Brain Res. 2003;110:76–84. doi: 10.1016/s0169-328x(02)00586-7. [DOI] [PubMed] [Google Scholar]
- 24.Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 26.Lee JM, Shih AY, Murphy TH, Johnson JA. NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem. 2003;278:37948–37956. doi: 10.1074/jbc.M305204200. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Stein TD, Johnson JA. Genetic dissection of systemic autoimmune disease in Nrf2-deficient mice. Physiol Genomics. 2004;18:261–272. doi: 10.1152/physiolgenomics.00209.2003. [DOI] [PubMed] [Google Scholar]
- 28.Shih AY, et al. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calkins MJ, et al. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci USA. 2005;102:244–249. doi: 10.1073/pnas.0408487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shih AY, et al. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem. 2005;280:22925–22936. doi: 10.1074/jbc.M414635200. [DOI] [PubMed] [Google Scholar]
- 31.Jakel RJ, Kern JT, Johnson DA, Johnson JA. Induction of the protective antioxidant response element pathway by 6-hydroxydopamine in vivo and in vitro. Toxicol Sci. 2005;87:176–186. doi: 10.1093/toxsci/kfi241. [DOI] [PubMed] [Google Scholar]
- 32.Jakel RJ, Townsend JA, Kraft AD, Johnson JA. Nrf2-mediated protection against 6-hydroxydopamine. Brain Res. 2007;1144:192–201. doi: 10.1016/j.brainres.2007.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satoh T, et al. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic phase II inducers. Proc Natl Acad Sci USA. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;25:10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett. 2006;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 36.Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burton NC, Kensler TW, Guilarte TR. In vivo modulation of the parkinsonian phenotype by Nrf2. Neurotoxicology. 2006;27:1094–1100. doi: 10.1016/j.neuro.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Johnson DA, Andrews GK, Xu W, Johnson JA. Activation of the antioxidant response element in primary cortical neuronal cultures derived from transgenic reporter mice. J Neurochem. 2002;81:1233–1241. doi: 10.1046/j.1471-4159.2002.00913.x. [DOI] [PubMed] [Google Scholar]
- 39.Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci USA. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pacchioni AM, et al. Nrf2 gene deletion fails to alter psychostimulant-induced behavior or neurotoxicity. Brain Res. 2007;1127:26–35. doi: 10.1016/j.brainres.2006.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolfer DP, Crusio WE, Lipp HP. Knockout mice: Simple solutions to the problems of genetic background and flanking genes. Trends Neurosci. 2002;25:336–340. doi: 10.1016/s0166-2236(02)02192-6. [DOI] [PubMed] [Google Scholar]
- 42.Drukarch B, van Muiswinkel FL. Neuroprotection for Parkinson's disease: A new approach for a new millennium. Expert Opin Investig Drugs. 2001;10:1855–1868. doi: 10.1517/13543784.10.10.1855. [DOI] [PubMed] [Google Scholar]
- 43.Chen L, et al. Unregulated cytosolic dopamine causes neurodegeneration associated with oxidative stress in mice. J Neurosci. 2008;28:425–433. doi: 10.1523/JNEUROSCI.3602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Block ML, Hong JS. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans. 2007;35:1127–1132. doi: 10.1042/BST0351127. [DOI] [PubMed] [Google Scholar]
- 45.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 46.Kraft AD, Lee JM, Johnson DA, Kan YW, Johnson JA. Neuronal sensitivity to kainic acid depends on the Nrf2-mediated actions of the antioxidant response element. J Neurochem. 2006;98:1852–1865. doi: 10.1111/j.1471-4159.2006.04019.x. [DOI] [PubMed] [Google Scholar]
- 47.Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: Detoxification of acetaminophen. Proc Natl Acad Sci USA. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prochaska HJ, Santamaria AB. Direct measurement of NAD(P)H:quinone reductase from cells cultured in microtiter wells: A screening assay for anticarcinogenic enzyme inducers. Anal Biochem. 1988;169:328–336. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- 49.Fornai F, Alessandri MG, Torracca MT, Bassi L, Corsini GU. Effects of noradrenergic lesions on MPTP/MPP+ kinetics and MPTP-induced nigrostriatal dopamine depletions. J Pharmacol Exp Ther. 1997;283:100–107. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.