Abstract
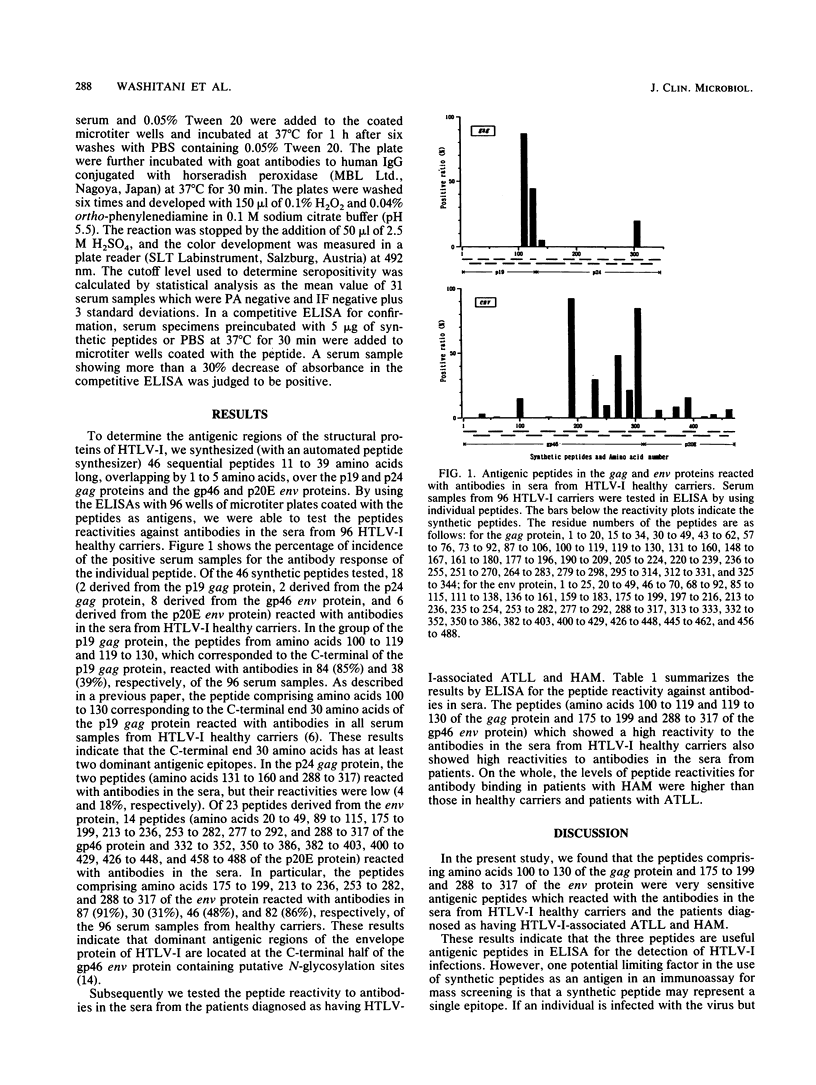
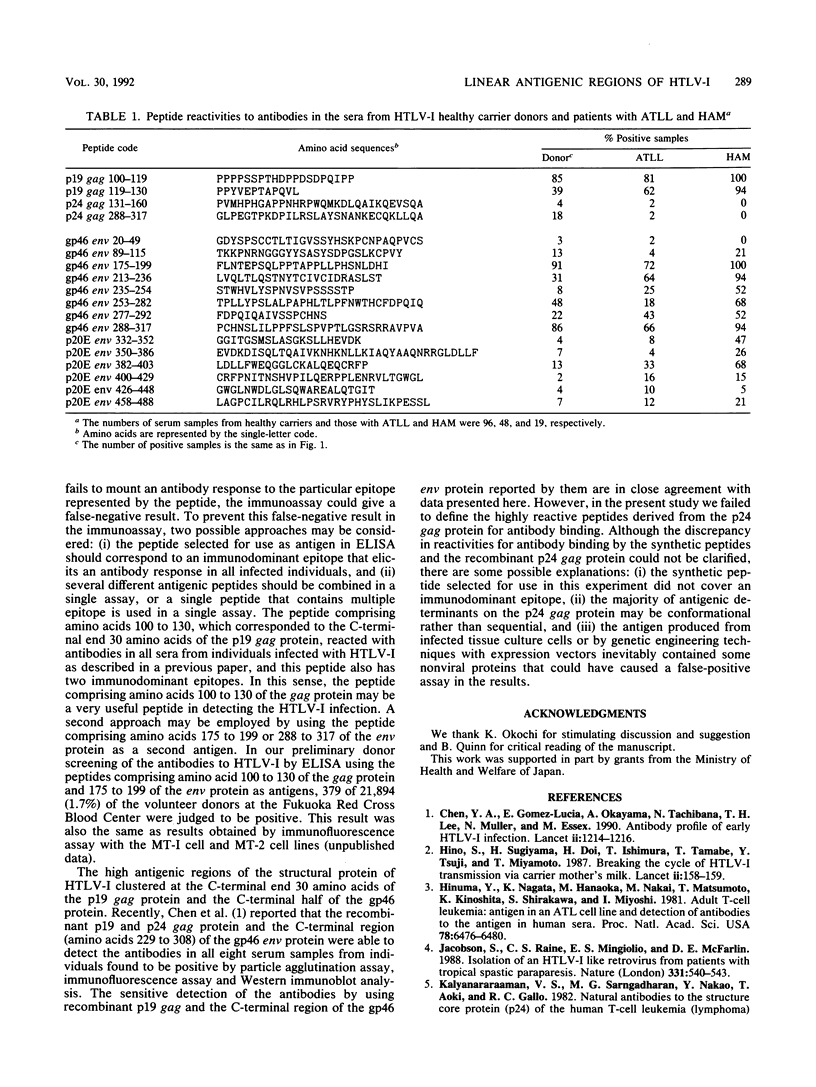
We synthesized 46 sequential peptides 21 to 39 amino acids long over the structural protein of human T-cell leukemia virus type I (HTLV-I; the p19 and p24 gag protein and the gp46 and p20E env proteins) and tested their reactivities against antibodies in sera from HTLV-I healthy carriers and patients diagnosed as having human T-cell leukemia-lymphoma (ATLL) and myelopathy (HAM) by using an enzyme-linked immunosorbent assay. Of the 46 synthetic peptides, 18 peptides (2 corresponding to the p19 gag protein, 2 corresponding to the p24 gag protein, 8 corresponding to the gp46 env protein, and 6 corresponding to the p20E env protein) reacted with antibodies in the sera from HTLV-I healthy carriers. In particular, the peptides comprising amino acids 100 to 119 and 119 to 130 of the gag and 175 to 199, 213 to 236, 253 to 282, and 288 to 317 of the env proteins reacted with antibodies in sera from more than 30% of HTLV-I healthy carriers. These peptides also showed high reactivities to the antibodies in the sera from patients with ATLL and HAM. The results indicate that the predominant antigenic regions of the structural protein of HTLV-I were located at the C-terminal end of the p19 gag protein and the C-terminal half of the gp46 env protein, and the corresponding peptides proved to be useful antigens in detecting antibodies in the sera from individuals infected with HTLV-I.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y. M., Gomez-Lucia E., Okayama A., Tachibana N., Lee T. H., Mueller N., Essex M. Antibody profile of early HTLV-I infection. Lancet. 1990 Nov 17;336(8725):1214–1216. doi: 10.1016/0140-6736(90)92833-4. [DOI] [PubMed] [Google Scholar]
- Hino S., Sugiyama H., Doi H., Ishimaru T., Yamabe T., Tsuji Y., Miyamoto T. Breaking the cycle of HTLV-I transmission via carrier mothers' milk. Lancet. 1987 Jul 18;2(8551):158–159. doi: 10.1016/s0140-6736(87)92358-0. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S., Raine C. S., Mingioli E. S., McFarlin D. E. Isolation of an HTLV-1-like retrovirus from patients with tropical spastic paraparesis. Nature. 1988 Feb 11;331(6156):540–543. doi: 10.1038/331540a0. [DOI] [PubMed] [Google Scholar]
- Kuroda N., Washitani Y., Shiraki H., Kiyokawa H., Ohno M., Sato H., Maeda Y. Detection of antibodies to human T-lymphotropic virus type I by using synthetic peptides. Int J Cancer. 1990 May 15;45(5):865–868. doi: 10.1002/ijc.2910450514. [DOI] [PubMed] [Google Scholar]
- Okochi K., Sato H., Hinuma Y. A retrospective study on transmission of adult T cell leukemia virus by blood transfusion: seroconversion in recipients. Vox Sang. 1984;46(5):245–253. doi: 10.1111/j.1423-0410.1984.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Osame M., Usuku K., Izumo S., Ijichi N., Amitani H., Igata A., Matsumoto M., Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986 May 3;1(8488):1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Nakao Y., Notake K., Ito Y., Sliski A., Gallo R. C. Natural antibodies to human retrovirus HTLV in a cluster of Japanese patients with adult T cell leukemia. Science. 1982 Feb 19;215(4535):975–978. doi: 10.1126/science.6760397. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima K., Tominaga S., Suchi T., Kawagoe T., Komoda H., Hinuma Y., Oda T., Fujita K. Epidemiological analysis of the distribution of antibody to adult T-cell leukemia-virus-associated antigen: possible horizontal transmission of adult T-cell leukemia virus. Gan. 1982 Dec;73(6):893–901. [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]


