Abstract
The HIV-1 coreceptor CCR5 possesses tyrosine sulfate (TYS) residues at its N-terminus (Nt) that are required for binding HIV-1 gp120 and mediating viral entry. Using a 14-residue fragment of CCR5 Nt containing two TYS residues, we recently showed that CCR5 Nt binds gp120 through a conserved region specific for TYS moieties and suggested that this site may represent a target for inhibitors and probes of HIV-1 entry. As peptides containing sulfotyrosines are difficult to synthesize and handle due to limited stability of the sulfo-ester moiety, we have incorporated TYS isosteres into CCR5 Nt analogs and assessed their binding to a complex of gp120-CD4 using Saturation Transfer Difference (STD) NMR and surface plasmon resonance (SPR). STD enhancements for CCR5 Nt peptides containing tyrosine sulfonate (TYSN) in complex with gp120-CD4 were very similar to those observed for sulfated CCR5 Nt peptides indicating comparable modes of binding. STD enhancements for phosphotyrosine-containing CCR5 Nt analogs were greatly diminished consistent with earlier findings demonstrating sulfotyrosine to be essential for CCR5 Nt binding to gp120. Tyrosine sulfonate-containing CCR5 peptides exhibited reduced water solubility, limiting their use in assay and probe development. To improve solubility, we designed, synthesized, and incorporated in CCR5 Nt peptide analogs an orthogonally functionalized azido tris(ethylenoxy) L-alanine (L-ate-Ala) residue. Through NMR and SPR experiments, we show a 19-residue TYSN-containing peptide and its analogs to be a functional, hydrolytically stable CCR5 Nt isostere that was in turn used to develop an SPR-based assay to screen for inhibitors of CCR5 binding to gp120-CD4.
Introduction
The events leading to HIV-1 infection include interactions between the viral surface envelope (Env) glycoprotein gp120 and cellular receptors CD4 and CCR5 or CXCR4.1 CCR5 and CXCR4, also referred to as HIV-1 co-receptors, are 7-transmembrane spanning G-protein coupled receptors that have the unusual feature of containing sulfated tyrosine (TYS) residues in their extracellular N-terminal domains.2 CCR5 N-terminus (Nt) contains four tyrosine residues at positions 3, 10, 14 and 15, and sulfation of at least residues 10 and 14 is essential for mediating viral entry.3 Moreover, CCR5 Nt peptides containing sulfotyrosine residues at positions 10 and 14 inhibit HIV-1 membrane fusion.4 Recently we showed by NMR that a CCR5 Nt peptide comprising residues 2–15 (1, Nt2–15), where Tyr 10 and Tyr 14 are sulfated, binds CD4-activated HIV-1 gp120 (CD4–gp120) but not gp120 or CD4 alone; and that residues 9–15 adopt an ordered, alpha helical structure upon binding.5 Molecular docking of the minimized mean structure showed CCR5 Nt to dock in a single orientation to a conserved region on gp120 specific for sulfotyrosine. Those studies demonstrated that a CCR5 Nt peptide fragment can function as a natural co-receptor mimic.
Despite interesting and important biological roles, Tyr(SO4)-containing peptides are not without liabilities. The ArO-SO3 − bond is prone to hydrolysis.6 Lack of robust protecting groups orthogonal to the sulfate group combined with the need for acid catalyzed cleavage of side chain protecting groups and peptide from resin can make peptide synthesis difficult and yields low. Furthermore, the need to prevent hydrolysis during chromatographic separations can make purification of crude peptides a challenge. Together, these factors place limitations on the use of Tyr(SO4)–containing peptides as versatile biochemical probes and tools. We thus sought to develop a functional, non-hydrolyzable CCR5 Nt peptide analog amenable to orthogonal modifications and whose synthesis can be scaled up for assay development. To this end, we used Saturation Transfer Difference7 (STD) NMR and/or Surface Plasmon Resonance (SPR) techniques to investigate the effects on gp120 binding of CCR5 Nt peptides where (i) sulfotyrosine (referred to hereafter as TYS) residues were replaced by tyrosine phosphate [Tyr(PO3 2−), referred to as TYP] and tyrosine sulfonate [Tyr(CH2SO3−), referred to as TYSN] isosteres (Figure 1); (ii) their length was increased to 17 or 19 residues; (iii) a third TYS or TYSN isostere at residue Tyr3 was incorporated; and/or (iv) a C-terminal biotinylated polyethylene glycol linker was incorporated. On the basis of these results, we have synthesized a functional CCR5 Nt analog (7) bearing chemically stable TYSN residues, and shown that when biotinylated through a PEG linker (12) the TYSN-containing construct can be used to screen in an SPR-based platform for molecules that specifically inhibit CCR5 Nt binding to HIV-1 gp120. Additionally, STD NMR of peptides containing one TYS and one TYP residue validate our model of CCR5 Nt binding to gp120.
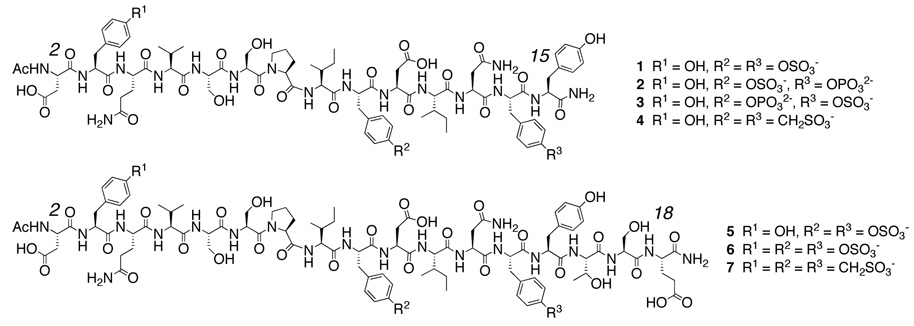
Figure 1.
CCR5 Nt peptides and analogs.
Results and Discussion
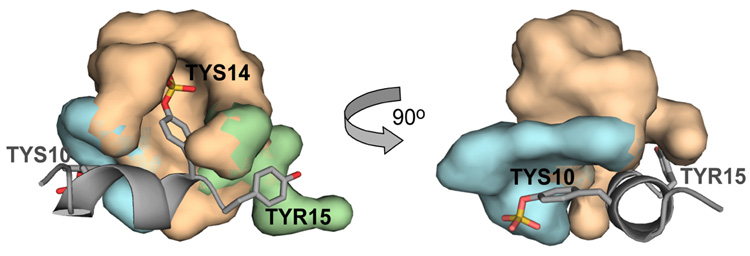
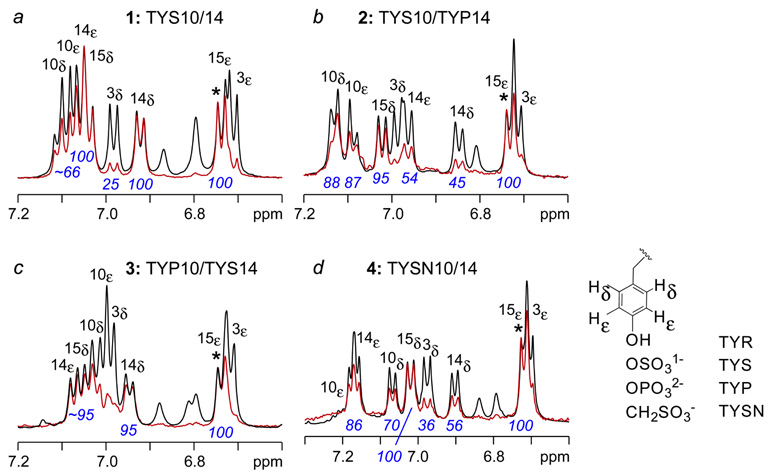
Recently we solved by NMR the solution structure of a functional 14-residue CCR5 Nt peptide comprising residues 2–15 (1) in complex with gp120-CD4.5 When bound to this complex, the backbone of residues 9–15 adopt an alpha helical structure that displays TYS10 and TYS14 from the same face of the helix (Figure 2). Molecular docking of CCR5 Nt to the crystal structure of gp120-CD4 in complex with sulfated monoclonal antibody 412d showed Nt to bind to the base of the third variable loop (V3) in a manner similar to that of 412d. In the model, the side chain of TYS14 makes numerous hydrogen bonds and extensive van der Waal’s interactions with positively charged and hydrophobic residues forming this site (Fig. 2, beige surface), and Tyr15 packs closely to several neighboring hydrophobic residues (Fig. 2, green surface). The side chain of TYS10 on the other hand is positioned further from gp120, possibly making hydrogen bonds through its sulfate group (Fig. 2, blue surface). This model was validated by STD NMR, a technique that gives rise to a difference spectrum whose proton signal intensities are proportional to their proximity to receptor.7 As seen in the expansion of the aromatic region of a 1-D 1H-STD NMR spectrum of 1 binding to gp120-CD4 (Figure 3A), resonances corresponding to Hd and He of TYS14 and Tyr15 respectively exhibited the strongest enhancements within the entire spectrum (normalized to 100%), while those for TYS10 were estimated at ~66% (spectral overlap occurs between TYS10 Hd and TYS10 He), consistent with our docked model. Enhancements corresponding to Tyr 3 were negligible in comparison.
Figure 2.
Close up of model of CCR5 Nt binding to gp120. Tyrosine residues 10, 14 and 15 are labeled. Surfaces for gp120 atoms within 5 Å of TYS10, TYS14 and TYR15 are colored blue, beige and green, respectively.
Figure 3.
Aromatic region of 1H STD-NMR spectra of 14-residue CCR5 Nt2–15 (1) and analogs 2–4. (a) CCR5 Nt2–15 (1) containing TYS10 and TYS14; (b) analog 2 containing TYS10 and TYP14; (c) analog 3 containing TYP10 and TYS14; (d) analog 4 containing TYSN10 and TYSN14. Spectra were recorded at 298 K on samples containing 20 mM gp120-CD4 in the presence of 800 mM peptide at pH 6.8.
Using these group epitope-mapping results from peptide 1 as a reference, we next assessed binding to gp120-CD4 of Nt2–15 peptides bearing a single substitution of TYP in place of TYS at either position 10 or 14. As seen in the expansion of the aromatic region of the STD NMR spectrum for analog 2 (Figure 3b), containing phosphotyrosine at residue 14, STD enhancements for TYP14 Hd and He decrease from 100% in 1 to 45% and 54% in 2, respectively (Figure 3b). Enhancements for Tyr3 and Tyr15 were unaffected by the presence of TYP14, while enhancements for TYS10 increased slightly from 66% in 1 to 88% in 2. In contrast, relative enhancements observed for tyrosine residues in analog 3 (Figure 3c) containing TYP at position 10 and TYS at position 14 were more similar to those of 1 (Figure 3c) with strongest enhancements observed for TYS14 and Tyr15 followed by TYP10. Although overlap between signals for Tyr3 Hd and TYP10 He, and TYP10 He and TYP10 Hd prevented accurate integration of the difference spectrum for 3, a moderate decrease in intensity for signals corresponding to TYP10 Hd and He relative to the same atoms in 1 is apparent from comparison of Figure 3c with 3a. Incorporation of two TYP residues at positions 10 and 14 resulted in complete loss of binding to gp120-CD4 (data not shown), in agreement with previous studies.4 Together, these results indicate that the Tyr14 binding site on CD4-activated gp120 is specific for Tyr(SO4) while that of Tyr10 is less stringent and will accommodate sulfotyrosine isostere Tyr(PO3), albeit with diminished interactions.
At physiological pH, sulfotyrosine and phosphotyrosine bear formal charges of −1 and −2, respectively. We thus reasoned that the decrease in binding observed for Tyr(SO4) versus Tyr(PO4) might be attributed to the difference in charge, and that a non-hydrolyzable phenylmethanesulfonic acid moiety (Tyr-CH2-SO3 −), referred to as tyrosine sulfonate (TYSN), which bears a single negative charge, would be a more suitable isostere for Tyr(SO4).8 Fmoc-protected tyrosine sulfonate was synthesized9 and incorporated by solid phase synthesis into CCR5 Nt analog 4, and its binding to gp120-CD4 was assessed by NMR. The difference spectrum (Figure 3d) for 4 in the presence of gp120-CD4 clearly indicated binding to the complex. In particular, enhancements for the aromatic protons of TYSN10 were comparable to those of TYS10 with values ranging from 70–86%, while slightly reduced binding of tyrosine sulfonate to the gp120 TYS14 binding site was apparent from an enhancement of 86% for He of TYSN14 relative to 100% for TYS14. Nonetheless, direct comparison of values for both Hd and He of TYP versus TYSN shows stronger enhancements for TYSN regardless of position. Finally, strong STD enhancements were also observed for methylene protons in both TYSN units, further indicating that tyrosine sulfonate can act as a functional tyrosine sulfate isostere and bind in close proximity to gp120.
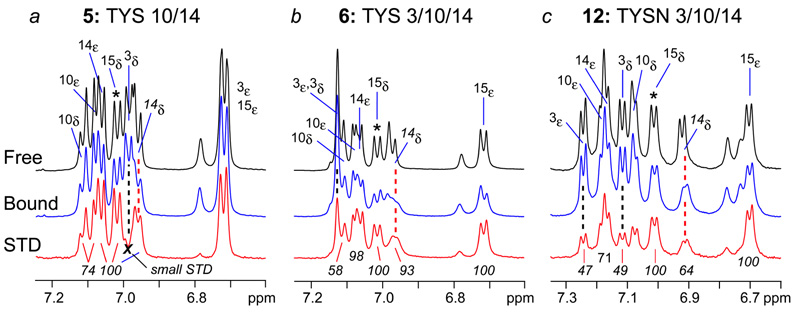
The predicted extracellular region of CCR5 N-terminus includes residues 1–20, where Cys20 forms a disulfide bridge with Cys269 of CCR5’s third extracellular loop. In addition, Tyr3 is has been shown to undergo sulfation.10 To examine the effects on binding gp120 of longer CCR5 Nt peptides bearing two or three sulfotyrosine residues, samples comprising gp120-CD4 in complex with the 17-residue peptides 5 and 6, where sulfotyrosines are present at positions 10/14 and 3/10/14, respectively, were prepared and investigated by NMR using conditions identical to those for 1–4. Relative to 14-mer 1, 1H NMR spectra for both constructs showed increased line broadening in the presence of gp120-CD4, especially for TYS14 and Tyr15 (data not shown). Line broadening persisted even when increasing the ratio of peptide to receptor from 40:1 to 80:1 (Figures 4a, b) indicating that gp120 binds these constructs in slower exchange and with higher affinity than peptides 1–4. 1H STD NMR revealed strong enhancements for TYS10, TYS14 and Tyr15 in Nt2–18 (5). Additionally, the g-methyl (Hg’s) of Thr16 showed appreciable STD enhancements (data not shown), while Tyr3 resonances did not. In triply sulfated CCR5 Nt peptide 6, line broadening of Hd and He resonances of TYS14 and Tyr15 was even greater than in peptide 5 (Figure 4b), suggesting the presence of TYS3 further stabilized the ternary complex of CD4-HIV-1 gp120-CCR5 Nt peptide and effectively reduced the peptide’s off rate from complex. Overlap of several Hd and He peaks of TYS3 and TYS10 prevented quantitative measure of STD enhancements for these residues. However, measurement of line widths in 1H spectra of free versus bound peptides showed that all resolved aromatic signals undergo line broadening ranging from 0.8 to greater than 3.0 Hz in the presence of complex (Table 1) indicating the combination of increasing peptide length and sulfation of Tyr3, Tyr10 and Tyr14 enhances binding to gp120.
Figure 4.
Expansions of NMR spectra of 17-residue CCR5 Nt (2–18) and analogs. 1H NMR spectra of free and bound peptides are shown in black and blue, respectively, and 1H STD spectra of complexes are red. Number and position of TYS and TYSN residues are shown above respective spectra and in Figure 1 and Figure 5.
Table 1.
Increase in line width (Hz) between free and bound peptides.a
| Peptide | 10δ | 10ε | 14δ | 14ε | 15δ | 15ε |
|---|---|---|---|---|---|---|
| 1 | 1.0 | —b | 0.8 | — | — | 0.6 |
| 4 | 1.1 | — | 1.3 | — | 1.0 | — |
| 5 | 0.8 | 0.8 | 2.2 | 1.4 | 1.0 | — |
| 6 | 2.3 | 1.2 | ~3c | 2.8 | 2.9 | 2.8 |
| 11 | 2.4 | — | 2.8 | — | 1.9 | 3.2 |
NMR solutions contained 40:1 peptide:gp120-CD4.
Overlapped signals not measured.
Conservative estimate due to slight overlap between 14d and side chain amide with severe line broadening apparent in Fig. 3b, blue spectrum).
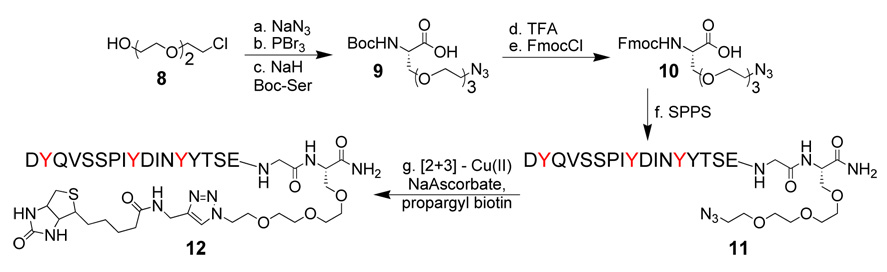
To determine whether a triply sulfonated peptide would show increased binding to complex, we synthesized CCR5 Nt analog 7 where TYSN residues were present at positions 3, 10 and 14. Despite containing three sulfonate residues, analog 7 was only sparingly soluble in water hindering extensive binding studies. To overcome this solubility problem, we next sought to design and synthesize an alpha amino acid that would be compatible with the conditions used during peptide synthesis, and incorporate both an ethylene glycol side chain and an orthogonal functional group amenable to late stage chemical modifications (such as Hüisgen cycloaddition11 or Staudinger ligation12 reactions). Azidation of commercially available chlorohydrin 8 provided the desired masked amino moiety13 compatible with peptide synthesis. Bromination of the azido alcohol gave quantitatively the azido bromide14 that was added directly to Boc L-serine in the presence of sodium hydride15 to give N-protected azido tris(ethylenoxy) L-alanine 9 (Boc L-ate-Ala). This 3-step sequence of reactions thus allowed synthesis of multigram quantities of an unnatural amino acid suitable for peptide synthesis using Boc chemistry. Alternatively, TFA deprotection of 9 followed by treatment with FmocCl afforded 10 for use in Fmoc-based solid phase synthesis. For our needs, the synthesis of a second generation and water-soluble CCR5 TYSN analog 11 was initiated with 10 at the C-terminus. Inclusion of L-ate-Ala increased more than 25-fold water solubility of triply sulfonated peptides, allowing for preparations of concentrated stock solutions exceeding 30 mM compared to a maximum solubility of 0.4 mM observed for peptide 7. To further demonstrate the utility of the L-ate-Ala linker, [2+3] Hüisgen cyclization of propargyl biotin onto analog 11 readily provided 12 for immobilization or labeling.
Once in hand, CCR5 Nt tyrosine sulfonate analogs 11 and 12 were first evaluated for binding to gp120-CD4 using NMR. Similar to the 1H spectra for peptide 6 in the presence of complex, line broadening of Hd and He signals was observed for both sulfonated peptides 11 and 12 (Figure 4c). (We note that STD NMR spectra of 11 and 12 were indistinguishable from those of 7, demonstrating that neither the presence of L-ate-Ala nor biotin effects binding of Nt pepides to gp120-CD4.) Once again, the strongest STD enhancements were observed for Hd and He of TYSN 14 and Tyr15, and in contrast to all other sulfated peptides studied, the Hd and He resonances of TYS3 in analog 12 are completely resolved and show an ~50% STD enhancement relative to Tyr15. Moreover, STD enhancements of the aryl sulfonate methylenes were observed for all sulfonate residues (data not shown), clearly demonstrating the utility of TYSN as a TYS isostere in the context of CCR5 Nt peptides.
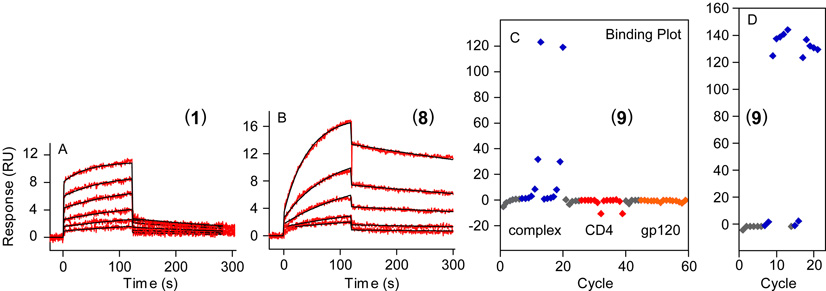
The enhanced binding observed by NMR for longer and triply sulfated or sulfonated CCR5 Nt peptides was corroborated by surface plasmon resonance experiments. To assess the binding of Nt peptides to gp120-CD4, CD4 was first immobilized onto a carboxymethylated dextran surface. Prior to each injection of Nt peptide, HIV-1 gp120 was introduced to the CD4 surface to assure generation of fresh complex. As seen in Figure 5a, CCR5 Nt peptide 1 bound gp120-CD4 in a concentration dependent manner and in fast exchange with sensorgrams characteristic of fast association and fast dissociation. Steady-state analysis of the binding curves of 1 using Michaelis-Menton kinetics provided an equilibrium dissociation constant of 17±2mM. Triply sulfonated CCR5 Nt analog 11 exhibited a vastly different kinetic profile. As seen in Figure 5b, the association of 11 to gp120-CD4 is slower than 1; and the ternary complex between gp120, CD4 and 11 was more stable than that formed with 1. This was apparent from the slower dissociation relative to 1 and the higher level of late-stage binding of peptide 11 to complex. Least squares best fitting of the binding curves to a 1:1 Langmuir binding kinetic model gave an equilibrium dissociation constant of 1.92±0.02mM for sulfonated Nt peptide 11. These SPR observations were consistent with the variations in binding profiles seen in our NMR experiments where 17- and 19-residue triply sulfonated Nt peptides exhibited greater line broadening than the 14-residue peptides. Finally, immobilization of biotinylated Nt analog 12 onto a streptavidin-coated chip allowed utilization of this chemically stable CCR5 Nt analog as a tool to screen for inhibitors of CCR5 Nt binding to CD4-captured gp120. In accord with our NMR experiments, immobilized 12 bound neither CD4 nor gp120 on their own, but showed concentration dependent binding to gp120-CD4 as shown in the Rmax plot in Figure 5c and Supporting Information. CCR5 Nt and the sulfated antibody 412d bind the same site on gp120 located at the base of the V3 loop.5 Consistent with this mode of binding, 412d was able to compete with binding of 12 to CD4-activated gp120 in a dose dependent manner (Figure 5d), further validating the use of sulfonate analog 12 as a CCR5 Nt isostere.
Figure 5.
Surface plasmon resonance experiments of CCR5 Nt peptides binding to gp120-CD4. Doubly referenced sensorgrams for (a) doubly sulfated Nt peptide 1 and (b) triply sulfonated Nt peptide 11 binding to CD4-captured HIV-1 gp120 with peptide concentrations ranging from 3.1 to 50mM. Rmax plots for immobilized peptide 12 binding to (c) gp120-CD4 (complex), CD4, and gp120, with protein concentrations ranging from 39 nM to 5.0 mM; and (d) gp120-CD4 in the presence of 10-fold serial dilutions of 412d ranging from 100 nM through 0.001 nM. Response units (RUs) are shown on the y-axis with doubly referenced sensorgrams plotted on the same scale for panels a and b.
In summary, we have analyzed by 1H NMR, STD NMR and surface plasmon resonance a series of 14-, 17- and 19-residue CCR5 Nt peptides containing variations such as single Tyr(PO3) residues, and two versus three Tyr(SO4) or Tyr(CH2SO3) residues. Results from these studies demonstrate that tyrosine sulfonate can function as a sulfotyrosine isostere, allowing construction of non-hydrolyzable sulfonated CCR5 Nt peptides that can be used to screen for inhibitors to the CCR5 Nt sulfotyrosine binding-site on gp120. In addition to increased stability, incorporation of an unnatural amino acid bearing an azido moiety and a tri-ethylene glycol segment (ate-Ala) increased solubility of these peptides to levels approaching 100 mg per mL. The orthogonal azido group facilitated late-stage biotinylation through [2+3] Hüisgen cyclization without side reactions from the other functionalities. We anticipate that sulfonated, chemically stable CCR5 Nt peptide analogs such as 12 will enable the rapid discovery of small molecule inhibitors of HIV-1 gp120 and CCR5 interactions.
Supplementary Material
Scheme 1.
Synthesis of the alpha amino acid azido tris(ethylenoxy) L-alanine (L-ate-Ala). (a) NaN3, DMF, quant (b) PBr3, THF, 85% (c) N-Boc L-Ser, NaH, DMF, 75% (d) TFA, TIS, CH2Cl2 (e) FmocCl, DIPEA, H2O/dioxane, 75% 2 steps (f) SPPS (g) Cu(II)SO4, Na ascorbate, propargyl biotin, H2O/MeOH.
Acknowledgment
We thank J. Lloyd for HRMS data. This work was supported by the NIDDK Intramural Research Program and the Intramural AIDS Targeted Antiviral Program (IATAP) of the Office of the Director, NIH (C.A.B.).
References
- 1.(a) Wyatt R, Sodroski J. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]; (b) Berger EA, Murphy PM, Farber JM. Annu. Rev. Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 2.Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard NP, Sodroski J, Choe H. Cell. 1999;96:667–676. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- 3.Farzan M, Chung S, Li W, Vasilieva N, Wright PL, Schnitzler CE, Marchione RJ, Gerard C, Gerard NP, Sodroski J, Choe H. J. Biol. Chem. 277:40397–40402. doi: 10.1074/jbc.M206784200. [DOI] [PubMed] [Google Scholar]
- 4.(a) Cormier EG, Persuh M, Thompson DAD, Lin SW, Sakmar TP, Olson WC, Dragic T. Proc. Nat. Acad. Sci. 2000;97:5762–5767. doi: 10.1073/pnas.97.11.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Farzan M, Vasilieva N, Schnitzler CE, Chung S, Robinson J, Gerard C, Choe H, Sodroski J. J. Biol. Chem. 2000;275:33516–33521. doi: 10.1074/jbc.M007228200. [DOI] [PubMed] [Google Scholar]
- 5.Huang C-C, Lam SN, Acharya P, Shazad-ul-Hussan S, Stanfield RL, Tang M, Xiang S-H, Robinson J, Sodroski J, Wilson I, Wyatt R, Bewley CA, Kwong PD. Science. 2007;317:1930–1934. doi: 10.1126/science.1145373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hydrolysis of Tyr-SO4
- 7.Mayer M, Meyer B. Angew. Chem. Int. Ed. 1999;38:1784–1788. doi: 10.1002/(SICI)1521-3773(19990614)38:12<1784::AID-ANIE1784>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Muniz R, Cornille F, Bergeron F, Ficheux D, Pothier J, Durieux C, Roques BP. Int. J. Pept. Prot. Res. 1991;37:331–340. doi: 10.1111/j.1399-3011.1991.tb00747.x. [DOI] [PubMed] [Google Scholar]
- 9.Miranda MTM, Liddle RA, Rivier JE. J. Med. Chem. 1993;36:1681–1688. doi: 10.1021/jm00064a001. [DOI] [PubMed] [Google Scholar]
- 10.Seibert C, Cadene M, Sanfiz A, Chait BT, Sakmar TP. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11031–11036. doi: 10.1073/pnas.172380899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kolb HC, Finn MG, Sharpless KB. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. (b) For a recent review of reactions involving organic azides: Bräse S, Gil C, Knepper K, Zimmermann V. Angew. Chem. Int. Ed. 2005;44:5188–5240. doi: 10.1002/anie.200400657.
- 12.Saxon E, Armstrong JI, Bertozzi CR. Org. Lett. 2000;2:2141–2143. doi: 10.1021/ol006054v. [DOI] [PubMed] [Google Scholar]; (b) Kohn M, Breinbauer R. Angew. Chem. Int. Ed. 2004;43:3106–3116. doi: 10.1002/anie.200401744. [DOI] [PubMed] [Google Scholar]
- 13.Iyer SS, Anderson AS, Reed S, Swanson B, Schmidt JG. Tetrahedr. Lett. 2004;45:4285–4288. [Google Scholar]
- 14.Roy BC, Santos M, Mallik S, Campiglia AD. J. Org. Chem. 2003;68:3999–4007. doi: 10.1021/jo026833k. [DOI] [PubMed] [Google Scholar]
- 15.Hwang J, Deming TJ. Biomacromolecules. 2001;2:17–21. doi: 10.1021/bm005597p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.