Abstract
African savannas are undergoing management intensification, and decision makers are increasingly challenged to balance the needs of large herbivore populations with the maintenance of vegetation and ecosystem diversity. Ensuring the sustainability of Africa's natural protected areas requires information on the efficacy of management decisions at large spatial scales, but often neither experimental treatments nor large-scale responses are available for analysis. Using a new airborne remote sensing system, we mapped the three-dimensional (3-D) structure of vegetation at a spatial resolution of 56 cm throughout 1640 ha of savanna after 6-, 22-, 35-, and 41-year exclusions of herbivores, as well as in unprotected areas, across Kruger National Park in South Africa. Areas in which herbivores were excluded over the short term (6 years) contained 38%–80% less bare ground compared with those that were exposed to mammalian herbivory. In the longer-term (> 22 years), the 3-D structure of woody vegetation differed significantly between protected and accessible landscapes, with up to 11-fold greater woody canopy cover in the areas without herbivores. Our maps revealed 2 scales of ecosystem response to herbivore consumption, one broadly mediated by geologic substrate and the other mediated by hillslope-scale variation in soil nutrient availability and moisture conditions. Our results are the first to quantitatively illustrate the extent to which herbivores can affect the 3-D structural diversity of vegetation across large savanna landscapes.
Keywords: ecological sustainability, ecosystem heterogeneity, Kruger National Park, park management, protected areas, South Africa, vegetation structure
The 3-dimensional (3-D) structure of vegetation is central to the functioning of African savannas, providing habitat for a wide variety of plants and animals (1–4). Like many regions of the world, African savannas are under increasing pressure from humans, and thus increased emphasis is being placed on natural protected areas to preserve biological diversity (5, 6). The sustainability of these protected areas rests on the management plans that affect habitat and wildlife communities, yet most management decisions are formulated with relatively little information on the large-scale ecosystem responses to those decisions. Ground-based ecological monitoring of management outcomes usually lacks regional-scale generality, a problem arising from the enormous vertical complexity and spatial heterogeneity of the vegetation. Nowhere is this deficiency more problematic than in African savannas, where topo-edaphic, climatic, and biological conditions vary at multiple scales, resulting in local, landscape, and regional variability in vegetation 3-D structure (7–9).
The Kruger National Park (KNP) is a premier natural protected area for South Africa and the world, with roughly 2,646 plant and animal species protected on about 2 million ha. KNP has undergone distinct phases in its management history, including periods of elephant culling, large-scale water augmentation, and fire manipulation. Management has recently shifted toward strategic adaptive management approaches that aim to maintain biodiversity and vegetation heterogeneity for the inhabitants of the park (10–12). The efficacy of these management actions has been both highly variable and difficult to quantify over the large geographic areas for which they were intended to serve.
Today in particular, the scales and geographic locations at which KNP's large herbivores impact vegetation and ecosystem processes remain highly uncertain. Herbivores are both a major agent of disturbance and a core focus for conservation (13), so altering herbivore populations in an effort to maintain whole-system biodiversity presents a paradox in a highly managed park such as KNP: Too many or too few herbivores can lead to the loss of ecological functioning through alterations in vegetation composition and structure (14–17). Elephant, buffalo, giraffe, zebra, and many other ungulates contribute to the marked structural changes that have been locally observed in different African landscapes (18–24), yet few experimental studies have been undertaken at a geographic scale that can resolve the impact of herbivores on the overall diversity of the landscape.
Four hillslope experiments have restricted animal access to large areas of savanna in KNP, providing a chance to compare vegetation 3-D structure with and without the presence of herbivores. Two enclosures, 220 and 230 ha in size, were constructed 36 and 41 years ago, respectively, for the breeding of rare and endangered antelope. In 1986, the larger enclosure was extended by another 72 ha to incorporate more lowland habitat. While protecting small numbers of rare antelope (0.01–0.1 animals ha−1) from predators, the mesh fencing of these enclosures effectively excludes all other mammalian herbivores larger than hares (± 5 kg). These 2 enclosures facilitate a large-scale analysis of vegetation structure on both granite and basalt substrates in areas protected from herbivores over the long term (22–41 years). In 2002, KNP constructed 2 additional 129- and 139-ha fenced areas on granite hillslopes adjacent to the Sabie and Letaba Rivers. Although these exclosures have been in place for only 6 years, they complement the 2 long-term enclosures, providing a way to assess the short-term responses of vegetation structure to herbivore exclusion [see supporting information (SI) Table S1].
These large-scale experimental treatments in KNP provide a highly unique opportunity to address many issues surrounding the impact of herbivores on African savannas, but only if the response measurements can be made at a geographic scale commensurate with the broad movements of the animals being manipulated. We deployed a new airborne remote-sensing system to map the 3-D structure of vegetation across the herbivore exclosures and enclosures, as well as the control areas surrounding and adjacent to the treatments. Our total flight coverage for this study was 790 ha of treatment and 850 ha as a comparable control. The data were collected at a spatial resolution of 56 cm, and our analyses explored differences between herbivore treatment and control areas across both upland and lowland hillslope positions (see SI Materials and Methods). We determined the large-scale responses of vegetation and ecosystem structure to herbivore presence/absence using the following remotely sensed measurements: fractional canopy cover of live and senescent/dead herbaceous vegetation, bare soil extent, vegetation height, and the 3-D vertical profile of the woody canopies. These measurements provide a nearly complete structural inventory as defined in classical savanna ecology (25).
Results and Discussion
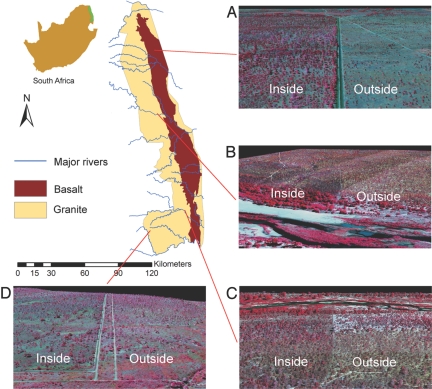
The impact of herbivores on vegetation structure varied by topographic position, geologic substrate, and treatment age. The most obvious effects were seen in the long-term treatment sites on basalt substrate (Fig. 1A); other sites were more difficult to assess by visual inspection alone (Fig. 1 B–D). However, the collection of many thousands of measurements from the air provided a means to explore statistical differences among treatments and topo-edaphic conditions.
Fig. 1.
Airborne 3-D imaging of the 4 herbivore treatments across the KNP in South Africa. (A) Long-term basalt (Nwashitsumbe). (B) Short-term granite (Letaba). (C) Short-term granite (Nkuhlu). (D) Long-term granite (Hlangwine). A map of the park is shown in the upper left, with the 2 major geologic substrates and river systems. Each zoom image shows a portion of each large-scale treatment area, with color-infrared spectroscopy highlighting vegetation canopies (red) and dry/senescent vegetation and bare soil (blue to gray) overlain on the 3-D structure of each woody plant at a spatial resolution of 56 cm.
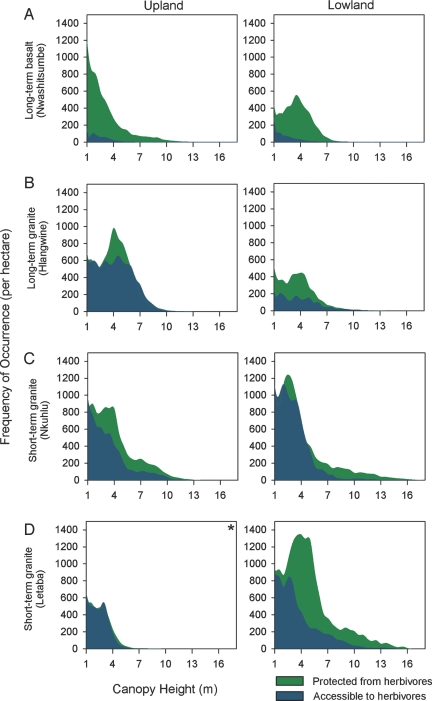
Comparing vegetation height distributions using the Kolmogorov–Smirnov (K-S) test, we found a statistically significant effect of herbivore exclusion in all long-term treatment areas and across both lowland and upland areas (P < .01) (Fig. 2). Although herbivores were excluded from the long-term granite and basalt substrate sites for similar periods, the differences between protected and accessible landscapes were of significantly greater magnitude in the basalt sites. This was true for woody height distribution (Fig. 2A) and also for the percentage of woody cover, which was 7- to 11-fold greater inside than outside of the protected areas (Table 1). Although height distributions differed significantly between protected and accessible areas in the long-term granite sites, mean canopy height did not (Table S2). Taken together, these findings indicate that the diversity of vegetation structure, expressed here in terms of the distribution of woody canopy heights, rather than the average structure (e.g., mean height), is the primary ecological response to herbivory. Moreover, the differing responses by geologic substrate indicate the potential importance of soil nutrient availability in determining the response of vegetation to herbivores. Herbivores use the landscape in a patch-specific manner, and their impact is greatest in nutrient-rich areas offering the best-quality forage (26, 27). In this landscape, the clay soils in the basalt substrate areas are rich in nutrients and have greater water-holding capacity than the sandy soils on granite substrates (28, 29), predisposing the basalt areas to greater herbivory, driven by higher-quality forage (28).
Fig. 2.
Frequency histograms of vegetation canopy height derived from 1640 ha (56-cm resolution) of airborne LiDAR observations. (A and B) Long-term treatments. (C and D) short-term treatments. In all panels, the left column is for uplands and the right column is for lowlands. Values are normalized by both site area and percentage of woody cover at each site. All panels except (D), uplands (*), show significantly different distributions using K-S tests (P < .01; n = 27,000).
Table 1.
Total percentage cover of woody canopies, live and dead/senescent herbaceous canopies, and bare soil across the four treatments in both lowland and upland landscape positions in KNP
| Site | Protected from herbivores |
Accessible to herbivores |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Woody cover (%) | Live herbaceous canopy (%) | Dead/senescent herbaceous canopy (%) | Bare soil (%) | Woody cover (%) | Live herbaceous canopy (%) | Dead/senescent herbaceous canopy (%) | Bare soil (%) | ||
| Long-term basalt (Nwashitsumbe) | Upland | 15.6 | 32.4 | 56.7 | 10.9 | 1.4 | 26.4 | 64.2 | 9.3 |
| Lowland | 12.7 | 26.7 | 69.8 | 3.6 | 1.7 | 26.0 | 68.5 | 5.5 | |
| Long-term granite (Hlangwine) | Upland | 25.5 | 39.3 | 60.0 | 0.7 | 22.6 | 53.1 | 43.6 | 3.3 |
| Lowland | 12.7 | 42.2 | 57.3 | 0.4 | 5.8 | 55.8 | 42.3 | 1.9 | |
| Short-term granite (Nkuhlu) | Upland | 26.9 | 25.1 | 67.7 | 7.1 | 17.2 | 25.0 | 63.5 | 11.5 |
| Lowland | 30.9 | 28.5 | 64.7 | 6.8 | 24.7 | 20.0 | 46.4 | 33.6 | |
| Short-term granite (Letaba) | Upland | 10.0 | 22.7 | 69.6 | 7.3 | 9.5 | 25.1 | 58.6 | 16.3 |
| Lowland | 48.4 | 33.9 | 60.0 | 6.1 | 20.6 | 21.1 | 53.2 | 25.8 | |
| Hillslope comparison | Upland | 18.2 | 29.6 | 65.2 | 5.2 | 11.1 | 31.3 | 59.7 | 9.0 |
| Lowland | 22.3 | 32.1 | 64.3 | 3.6 | 13.2 | 29.0 | 53.1 | 17.9 | |
| Total | 19.8 | 30.0 | 65.2 | 4.8 | 11.8 | 30.3 | 55.6 | 14.1 | |
″Protected″ and ″accessible″ indicate portions of the landscape without and with herbivore activity, respectively. Woody canopy cover values are the mean percentage cover of vegetation > 1 m tall, as defined through airborne laser point cloud classification. Other values are the fractional contribution of live herbaceous, dead or senescent herbaceous, and bare soil cover, as defined from the spectral mixture analysis of hyperspectral imagery (see SI Materials and Methods).
The influence of nutrient distribution was also evident at the hillslope scale, where the greatest effects of herbivore exclusion were found in the lowland areas (Table 1). Water and nutrients are locally more abundant in lowland areas, giving rise to better-quality forage compared with the adjacent upland areas (28, 30, 31). This topographic effect is obvious in the lowland basalt substrate treatment habitat, even when protected from herbivores for only half as long as the upland habitat (22 vs. 41 years) (Table S1). The lowland basalt areas demonstrated a major change, from mainly short, statured woody canopies in the areas accessible to herbivores, to canopies in the 4–5 m range above ground in the protected areas (Fig. 2A).
In contrast to the long-term exclusion sites, the short-term (6-year) sites demonstrated more subtle changes in the distribution of woody vegetation heights (Fig. 2C and D). Total woody canopy cover was greater by an average of 55% in the short-term sites, depending on substrate and topographic position (Table 1). The 6-year-old Letaba exclosure exhibited the most profound changes in the diversity of woody canopy height (Fig. 2D), as well as the greatest difference in woody canopy cover after herbivore exclusion (135%; Table 1). This area is a known hotspot for elephant bulls and entire herds (12), and these exclusion patterns show the major impact of high elephant densities on vegetation height distributions in this landscape.
Our airborne system also provided quantitative measurements of the fractional cover of live photosynthetic vegetation (PV) and dead/senescent nonphotosynthetic vegetation (NPV) among herbaceous plants and their litter, as well as bare soil. Comparisons of the areas protected from and accessible to herbivores showed statistically different fractional cover distributions of the herbaceous layer in upland and lowland areas (P < .01; K-S test) (Fig. S1). In contrast to the woody canopy height distribution results, in which the differences were most pronounced in the long-term treatment areas, some of the major differences in the distribution of herbaceous cover were measured in the short-term treatment areas. Moreover, bare soil cover was 38%–80% lower in the areas protected from herbivores (Table 1). The lowland positions contained the highest bare soil fractions in unprotected areas, where herbivores could access an herbaceous layer supported by relatively high nutrient and moisture conditions.
The fractions of NPV and litter were much higher in the areas protected from herbivores. This directly increases fuel load (32), providing a large herbaceous biomass to support hot fires. Over time, the effects of fire on the woody vegetation structure in the protected areas likely will increase significantly as a result of these increased fuel loads. Interestingly, although some research has shown that areas exposed to grazing can experience increased woody establishment and encroachment due to decreased competition with the herbaceous layer and decreased fire intensity (15, 33, 34), we found no evidence of shrub encroachment in the accessible areas. Much debate in savanna ecology has centered on the relative importance of herbivores and fire in shaping vegetation structure (16, 19, 35, 36). Although these 2 key drivers of vegetation dynamics cannot be viewed in isolation from one another, the net effect of herbivore consumption mapped throughout the 4 large savanna areas is lower woody canopy cover and height, not woody encroachment, despite lower herbaceous cover.
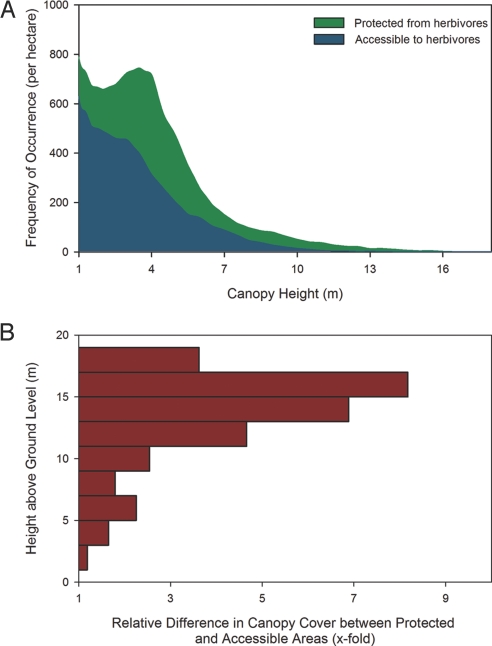
Our results suggest at least 2 scales of ecosystem response to herbivore consumption, one broadly mediated by geologic substrate and the other mediated by hillslope-scale variation in soil nutrient availability and moisture conditions. Despite these scale-dependent mediators of herbivore impact, combining our data across all sites revealed that herbivore exclusion universally increased the height of woody canopies (Fig. 3A). The greatest absolute increases were observed in vegetation ranging from 1 to 5 m in height, although relative differences were greatest among taller trees. Independent of substrate, topographic position, or treatment age, there was also a universal increase in woody canopy cover after herbivore exclusion (Fig. 3B); relative increases ranged from 50% to 800%, depending on vegetation height class. Thus, although herbivores are often considered to be locally selective in their foraging strategies, their impacts on the 3-D structure and diversity of vegetation are clearly evident at much broader scales.
Fig. 3.
Large-scale effects of herbivores on vegetation 3-D structure and structural diversity. (A) Frequency histograms of vegetation canopy height derived from 1640 ha (56-cm resolution) of airborne LiDAR observations showing significant differences between protected and accessible areas (P < .01; K-S test). (B) Height class–specific differences between protected and accessible areas.
Our findings concur in part with those of Pringle et al. (37), who reported a greater impact of herbivores on vegetation in low-productivity areas. Plant productivity is driven primarily by rainfall in savanna systems (25, 38–40); we found greater differences in vegetation structure at the long-term basalt site, which receives ≈30% less rainfall than the long-term granite site that had the least structural differences. Similarly, of the 2 granite sites, the Letaba is drier than the Nkuhlu (Table S1) and exhibited a greater structural response to herbivore exclusion. Our findings thus support the trend toward greater herbivore impact in areas of low rainfall and/or productivity, yet these high-impact areas are where substrate nutrient availability is highest (28). Moreover, our findings at the hillslope scale contradict the high-impact/low-productivity pattern reported by Pringle et al. (37), because the greatest impact of herbivores was apparent in the productive lowlands, where water and nutrient availability is high. Thus, we posit that the impact of herbivores on vegetation structure is most strongly mediated by the distribution of nutrients on the landscape at different scales, rather than by rainfall or productivity.
Conclusion
Herbivores are key agents of vegetation change in savannas, but their impact ranges from subtle to obvious at any given locale and is very challenging to measure at the landscape level because of the great vegetation structural heterogeneity of these areas. Like many natural protected areas, KNP is mandated to “maintain biodiversity in all its facets and fluxes” (11). Biodiversity in this sense encompasses 3 core components: composition, structure, and function (41). We combined a unique airborne mapping system with the KNP large-scale herbivore treatment areas to quantify the effects of herbivore exclusion on 3-D vegetation structure, one of the 3 core components of biodiversity.
In the short term (6 years), the effects of herbivore exclusion appear as greater herbaceous cover, with a few measurable differences in the 3-D structure of woody plants, particularly in lowland, nutrient-rich areas. In the longer term (22–41 years), however, herbivore exclusion manifests at a much larger scale, with both upland and lowland areas experiencing increased woody canopy cover and 3-D structural diversity. These differences in turn affect the diversity and richness of animal species, as well as the ecological functioning of these systems. Greater canopy structural diversity enhances the habitat available for a wide range of organisms beyond the herbivore communities (2, 3, 37, 42) and alters such ecological processes as nutrient cycling, seed dispersal, and germination (2, 4, 43, 44). Our findings highlight the trade-offs that managers must grapple with when attempting to sustain biodiversity among plant and various faunal communities.
In both the long- and short-term treatment areas, the effects of herbivore exclusion on vegetation structure were greatest in locations of high soil nutrient status. Larson and Paine (45) put forth the hypothesis that ecosystems with a low intrinsic primary production capacity are more susceptible to anthropogenic modifications, based on herbivore exclusion findings in eastern Africa (37). We caution that this interpretation may hold true only at broad scales, because our data reveal significant changes in vegetation structure at finer scales in the highly productive lowland zones.
Ensuring the sustainability and successful conservation of biodiversity and ecological functioning within KNP and other savanna parks throughout Africa requires explicit understanding of the spatial and temporal trends in 3-D vegetation structure at multiple scales. Isolated field studies provide a necessarily limited view of the changes incurred by management decisions, including herbivore densities, over large natural protected areas. New approaches that integrate high-resolution imaging spectroscopy and light detection and ranging (LiDAR) can provide large-scale, quantitative insight into system structure and dynamics, allowing managers to make more informed decisions regarding the sustainability of their actions.
Materials and Methods
The Carnegie Airborne Observatory (CAO) integrates high-fidelity imaging spectrometers (HiFIS) with waveform LiDAR sensors for regional-scale ecological research (46). The HiFIS subsystem provides detailed canopy spectroscopic reflectance signatures that express plant chemistry and other ecosystem components, such as NPV cover and bare soils. The LiDAR subsystem provides 3-D structural information on canopies and the terrain. The CAO HiFIS and LiDAR are physically and digitally co-aligned and packaged with a high-performance inertial navigation system that provides highly accurate determinations of aircraft position and the location of ground targets in 3 dimensions (see SI Materials and Methods). The HiFIS, LiDAR, and inertial navigation data were processed together to identify woody, herbaceous, and bare soil based on their unique spectral and structural properties (Fig. S2). The same aircraft data were then used to develop maps of canopy height and 3-D structure using data fusion algorithms. These methods of estimating fractional cover have been validated in previous studies (47, 48), but nonetheless were tested against field transects because of the narrower spectral range of the CAO HiFIS system (see SI Materials and Methods). Vegetation height was validated for this study via a series of randomly selected points on the ground (Fig. S3).
Supplementary Material
Acknowledgments.
This research was funded by a grant from the Andrew Mellon Foundation. The Carnegie Airborne Observatory is supported by the W. M. Keck Foundation and William Hearst III. We thank I. Smit, D. Pienaar, and the entire SANParks staff for their outstanding logistical and scientific support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810637106/DCSupplemental.
References
- 1.Dean WRJ, Milton SJ, Jeltsch F. Large trees, fertile islands, and birds in arid savanna. J Arid Environ. 1999;41:61–78. [Google Scholar]
- 2.Cumming DHM, et al. Elephants, woodlands and biodiversity in southern Africa. S Afr J Sci. 1997;93:231–236. [Google Scholar]
- 3.Fenton MB, et al. Bats and the loss of tree canopy in African woodlands. Conserv Biol. 1998;12:399–407. [Google Scholar]
- 4.Belsky AJ, Canham CD. Forest gaps and isolated savanna trees. BioScience. 1994;44:77–84. [Google Scholar]
- 5.Scholes RJ, Biggs R. A biodiversity intactness index. Nature. 2005;434:45–49. doi: 10.1038/nature03289. [DOI] [PubMed] [Google Scholar]
- 6.Sinclair A, Mduma S, Arcese P. Protected areas as biodiversity benchmarks for human impact: Agriculture and the Serengeti avifauna. Proc R Soc Lond B. 2002;269:2401–2405. doi: 10.1098/rspb.2002.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belsky AJ. Spatial and temporal landscape patterns in arid and semi-arid African savannas. In: Hansson L, Fahrig L, Merrian G, editors. Mosaic Landscapes and Ecological Processes. London: Chapman & Hall; 1995. pp. 31–49. [Google Scholar]
- 8.Pickett STA, Cadenasso ML, Benning TL. Biotic and abiotic variability as key determinants of savanna heterogeneity at multiple spatiotemporal scales. In: du Toit JT, Biggs HC, Rogers KH, editors. The Kruger Experience: Ecology and Management of Savanna Heterogeneity. Washington, DC: Island Press; 2003. pp. 23–40. [Google Scholar]
- 9.Mills AJ, Rogers KH, Stalmans M, Witkowski EDTF. A framework for exploring the determinants of savanna and grassland distribution. BioScience. 2006;56:579–589. [Google Scholar]
- 10.Biggs HC, Rogers KH. An adaptive system to link science, monitoring, and management in practice. In: du Toit JT, Biggs HC, Rogers KH, editors. The Kruger Experience: Ecology and Management of Savanna Heterogeneity. Washington DC: Island Press; 2003. pp. 61–82. [Google Scholar]
- 11.Rogers KH. Adopting a heterogeneity paradigm: Implications for management of protected savannas. In: du Toit JT, Biggs HC, Rogers KH, editors. The Kruger Experience: Ecology and Management of Savanna Heterogeneity. Washington DC: Island Press; 2003. pp. 41–58. [Google Scholar]
- 12.Whyte IJ, Biggs HC, Gaylard A, Braack LEO. A new policy for the management of the Kruger National Park's elephant population. Koedoe. 1999;42:111–132. [Google Scholar]
- 13.Shannon G, et al. The utilization of large savanna trees by elephant in southern Kruger National Park. J Trop Ecol. 2008;24:281–289. [Google Scholar]
- 14.Augustine D, McNaughton S, Frank D. Feedbacks between soil nutrients and large herbivores in a managed savanna ecosystem. Ecol Appl. 2003;13:1325–1337. [Google Scholar]
- 15.Roques KG, O'Connor TG, Watkinson AR. Dynamics of shrub encroachment in an African savanna: Relative influences of fire, herbivory, rainfall and density dependence. J Appl Ecol. 2001;38:268–280. [Google Scholar]
- 16.van Langevelde F, et al. Effects of fire and herbivory on the stability of savanna ecosystems. Ecology. 2003;84:337–350. [Google Scholar]
- 17.van de Koppel J, et al. Spatial heterogeneity and irreversible vegetation change in semiarid grazing systems. Am Nat. 2002;159:209–218. doi: 10.1086/324791. [DOI] [PubMed] [Google Scholar]
- 18.Barnes RFW. Woodland changes in Ruaha National Park (Tanzania) between 1976 and 1982. Afr J Ecol. 1985;23:215–221. [Google Scholar]
- 19.Ben-Shahar R. Woodland dynamics under the influence of elephants and fire in northern Botswana. Plant Ecol. 1996;123:153–163. [Google Scholar]
- 20.Beuchner HK, Dawkins HC. Vegetation change induced by elephants and fire in Murchison Falls National Park, Uganda. Ecology. 1961;42:752–766. [Google Scholar]
- 21.Dublin HT. Vegetation dynamics in the Serengeti-Mara ecosystem: The role of elephants, fire, and other factors. In: Sinclair ARE, Arcese P, editors. Serengeti II: Dynamics, Management, and Conservation of an Ecosystem. Chicago, IL: Univ. Chicago Press; 1995. pp. 71–90. [Google Scholar]
- 22.van de Vijver CADM, Foley CA, Olff H. Changes in the woody component of an east African savanna during 25 years. J Trop Ecol. 1999;15:545–564. [Google Scholar]
- 23.Western D. A half a century of habitat change in Amboseli National Park, Kenya. Afr J Ecol. 2007;45:302–310. [Google Scholar]
- 24.Mosugelo DK, Moe SR, Ringrose S, Nellemann C. Vegetation changes during a 36-year period in northern Chobe National Park, Botswana. Afr J Ecol. 2002;40:232–240. [Google Scholar]
- 25.Scholes RJ, Walker BH. An African Savanna: Synthesis of the Nylsvley Study. Cambridge, UK: Cambridge Univ Press; 1993. [Google Scholar]
- 26.Levick SR, Rogers KH. Patch and species specific responses of savanna woody vegetation to browser exclusion. Biol Conserv. 2008;141:489–498. [Google Scholar]
- 27.Turner MG, Pearson SM, Romme WH, Wallace LL. In: Landscape Heterogeneity and Ungulate Dynamics: What Spatial Scales Are Important? Bissonette JA, editor. New York: Springer; 1997. pp. 331–348. [Google Scholar]
- 28.Grant CC, Scholes MC. The importance of nutrient hot-spots in the conservation and management of large wild mammalian herbivores in semi-arid savannas. Biol Conserv. 2006;130:426–437. [Google Scholar]
- 29.Venter FJ, Scholes RJ, Eckhardt HC. The abiotic template and its associated vegetation pattern. In: du Toit JT, Biggs HC, Rogers KH, editors. The Kruger Experience: Ecology and Management of Savanna Heterogeneity. Washington DC: Island Press; 2003. pp. 83–129. [Google Scholar]
- 30.Naiman RJ, Rogers KH. Large animals and system-level characteristics in river corridors. BioScience. 1997;47:521–529. [Google Scholar]
- 31.Jacobs SM, et al. Nutrient vectors and riparian processing: A review with special reference to African semiarid savanna ecosystems. Ecosystems. 2007;10:1231–1249. [Google Scholar]
- 32.Varga T, Asner G. Hyperspectral and LiDAR remote sensing of fire fuels in Hawaii Volcanoes National Park. Ecol Appl. 2008;18:613–623. doi: 10.1890/07-1280.1. [DOI] [PubMed] [Google Scholar]
- 33.Wiegand K, Ward D, Saltz D. Multi-scale patterns and bush encroachment in an arid savanna with a shallow soil layer. J Veg Sci. 2005;16:311–320. [Google Scholar]
- 34.Bond WJ, Midgley JJ. Ecology of sprouting in woody plants: The persistence niche. Trends Ecol Evol. 2001;16:45–51. doi: 10.1016/s0169-5347(00)02033-4. [DOI] [PubMed] [Google Scholar]
- 35.Trollope WSW, Trollope LA, Biggs HC, Pienaar D, Potgieter ALF. Long-term changes in the woody vegetation of the Kruger National Park, with special reference to the effects of elephants and fire. Koedoe. 1998;41:103–112. [Google Scholar]
- 36.Dublin HT, Sinclair ARE, McGlade J. Elephants and fire as causes of multiple stable states in the Serengeti-Mara woodlands. J Anim Ecol. 1990;59:1147–1164. [Google Scholar]
- 37.Pringle R, Young T, Rubenstein D, McCauley D. Herbivore-initiated interaction cascades and their modulation by productivity in an African savanna. Proc Natl Acad Sci USA. 2007;104:193–197. doi: 10.1073/pnas.0609840104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sankaran M, et al. Determinants of woody cover in African savannas. Nature. 2005;438:846–849. doi: 10.1038/nature04070. [DOI] [PubMed] [Google Scholar]
- 39.Scanlon T, Albertson J, Caylor K, Williams C. Determining land surface fractional cover from NDVI and rainfall time series for a savanna ecosystem. Remote Sens Environ. 2002;82:376–388. [Google Scholar]
- 40.Scholes R, et al. Trends in savanna structure and composition along an aridity gradient in the Kalahari. J Veg Sci. 2002;13:419–428. [Google Scholar]
- 41.Noss RF. Indicators for monitoring biodiversity: A hierarchical approach. Conserv Biol. 1990;4:355–364. [Google Scholar]
- 42.MacArthur RH, MacArthur JW. On bird species diversity. Ecology. 1961;42:594–598. [Google Scholar]
- 43.Skarpe C, et al. The return of the giants: Ecological effects of an increasing elephant population. Ambio. 2004;33:276–282. doi: 10.1579/0044-7447-33.6.276. [DOI] [PubMed] [Google Scholar]
- 44.Treydte A, Heitkönig I, Prins H, Ludwig F. Trees improve grass quality for herbivores in African savannas. Perspect Plant Ecol Evol System. 2007;8:197–205. [Google Scholar]
- 45.Larson A, Paine R. Ungulate herbivory: Indirect effects cascade into the treetops. Proc Natl Acad Sci USA. 2007;104:5–6. doi: 10.1073/pnas.0610198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asner GP, et al. Carnegie Airborne Observatory: In-flight fusion of hyperspectral imaging and waveform light detection and ranging (wLiDAR) for three-dimensional studies of ecosystems. J Appl Remote Sens. 2007;1:1–21. [Google Scholar]
- 47.Asner G, Elmore A, Flint Hughes R, Warner A, Vitousek P. Ecosystem structure along bioclimatic gradients in Hawai'i from imaging spectroscopy. Remote Sens Environ. 2005;96:497–508. [Google Scholar]
- 48.Asner G, Heidebrecht K. Spectral unmixing of vegetation, soil and dry carbon cover in arid regions: Comparing multispectral and hyperspectral observations. Int J Remote Sens. 2002;23:3939–3958. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.