Abstract
Reduced expression of sarcoplasmic reticulum calcium ATPase-2 (SERCA2) and other genes in the adult cardiac gene program has raised consideration of an impaired responsiveness to thyroid hormone (T3) that develops in the advanced failing heart. Here we show that human and murine cardiomyopathy hearts have increased expression of Friend of GATA-2 (FOG-2), a cardiac nuclear hormone receptor co-repressor protein. Cardiac-specific overexpression of FOG-2 in transgenic mice led to depressed cardiac function, activation of the fetal gene program, congestive heart failure, and early death. SERCA2 transcript and protein levels were reduced in FOG-2 transgenic hearts, and FOG-2 overexpression impaired T3-mediated SERCA2 expression in cultured cardiomyocytes. FOG-2 physically interacts with thyroid hormone receptor-α1 and abrogated even high levels of T3-mediated SERCA2 promoter activity. These results demonstrate that SERCA2 is an important target of FOG-2 and that increased FOG-2 expression may contribute to a decline in cardiac function in end-stage heart failure by impaired T3 signaling.
Keywords: Friend of GATA-2, thyroid hormone receptor, sarcoplasmic reticulum calcium-activated ATPase-2, heart failure
INTRODUCTION
Congestive heart failure (CHF) is a lethal condition and represents the final common endpoint of many forms of heart disease. A hallmark feature of failing hearts is a downregulation of sarcoplasmic reticulum calcium ATPase-2 (SERCA2) and alpha myosin heavy chain (αMHC), which are critical components of the myocyte excitation-contraction coupling machinery1,2. Although the regulatory mechanisms that affect these changes are poorly understood, a similar pattern of gene expression seen in the hypothyroid heart suggests that impaired T3-responsiveness may contribute to this transcriptional switch3. Indeed, T3 is required for the physiological increase of SERCA2 and αMHC after birth4. Low SERCA2 and αMHC expression levels in the hypothyroid or failing heart are recovered following thyroid hormone replacement5,6. In heart failure models, decreased circulating T3 levels7, accelerated myocardial T3 turnover8 and altered thyroid hormone receptor (TR) isoform expression9 have been implicated mechanisms of cardiac-restricted T3 resistance.
T3 regulates transcription through interaction with the ligand-binding domain of high affinity nuclear TR proteins that recognize T3 response elements (TRE) on target genes10. Three major TR isoforms, TRα1, TRα2 and TRβ1, are expressed in the heart. TRα1 and TRα2 are abundantly expressed in the ventricular compact zone, which contributes the greatest force output from the heart, while TRβ1 expression is limited to the peripheral ventricular conduction system11. TRα1, but not TRβ1, is required for normal SERCA2 and αMHC expression in vivo, and mouse TR gene deletion models support a predominant role for TRα1 in the regulation of cardiac contractile and electrophysiological functions12. Without T3, TR proteins bind TREs and decrease basal gene expression through the recruitment of co-repressor complexes. Upon T3 binding to TR, these complexes are displaced and co-activator proteins are recruited that modulate chromatin structure and enhance gene expression13. Because an imbalance favoring TR co-repressor binding impairs T3-responsiveness14–16, we considered whether a cardiac-enriched co-repressor protein may mediate local cardiac T3 resistance in CHF.
Friend of GATA-2 (FOG-2) is a multi-zinc finger nuclear co-repressor protein necessary for normal development of the heart and in particular the left ventricular compact zone17,18. FOG-2 was originally identified as a GATA4 interacting protein19,20 but also interacts with nuclear hormone receptors, chicken ovalbumin upstream protein transcription factor-2 (COUP-TF2)21 and retinoid-X receptor-α (RXRα)22. In models of load-induced heart failure, GATA4, COUP-TF2, and RXRα regulate components of the cardiac gene program that affect natriuretic peptide expression21,22, cardiac metabolism23, and myocyte architecture24. While FOG-2 represses the transcriptional activities of GATA419, COUP-TF221 and ligand-bound RXRα 22 in cultured cardiomyocytes, the role of FOG-2 in the adult heart remains undefined.
Here, we report that FOG-2 levels are increased in human end-stage heart failure and in a mouse model of dilated cardiomyopathy. Transgenic mice with targeted overexpression of FOG-2 in the heart have reduced systolic and diastolic function. Diminished expression of SERCA2 is one primary finding associated with the cardiomyopathy created by FOG-2 overexpression. We demonstrate that FOG-2 binds TRα1 and impairs T3-mediated transcription of the SERCA2 promoter. Taken together, we identify a novel mechanism of cardiac resistance to T3-signaling caused by increased FOG-2 expression that may contribute directly to heart failure progression.
METHODS
Mice
αMHC-CREB-S133A transgenic mice25 and Fog-2(+/−) (heterozygous null) mice17 were described previously. αMHC-FOG-2 transgenic mice were produced following injection of a transgene including the full-length mouse FOG-2 cDNA (bp 132 to 4770) downstream of the murine αMHC promoter26 into newly fertilized ICR (Taconic) mouse embryos. Founders identified by Southern blot analysis with FOG-2 probe (nt 132-1207) were bred with ICR mice. Histological analysis was performed on hearts fixed in 4% paraformaldehyde and processed into paraffin blocks. Cardiac functional analysis was performed by echocardiography and cardiac catheterization (Supplemental Methods). Protein and total RNA extracts for gene expression analysis were prepared using standard methods (Supplemental Methods). Studies were approved by the Institutional Review Board for Animal Care and Use Committee at Tufts Medical Center and Harvard University.
Gene expression analysis
Northern blotting of total RNA was performed with [32P]-labeled FOG-2 cDNA template (nt 132 to 1207) and 18S probes (Supplemental Methods). Quantitative real time RT-PCR (qRT-PCR) was performed using cDNA synthesized from total RNA and amplified in triplicate with custom primer sequences (Supplementary Methods). Western immunoblotting was performed with SERCA2 (Affinity BioReagents), calsequestrin (Upstate), GAPDH (Research Diagnostics), and FOG-2 (Santa Cruz Biotechnology) antibodies or FOG-2 antiserum26 followed by a species appropriate HRP-conjugated secondary antibody. Membranes incubated with ECL Plus (Amersham) were imaged on a Typhoon 9410 System (Amersham) and band densities quantified using ImageQuant software (Amersham).
Adenovirus infection
Full-length mouse FOG-2 cDNA (nt 132 to 4770) was subcloned to generate a recombinant adenovirus expressing both FOG-2 and EGFP (Ad-FOG-2) using the AdEasy Adenoviral Vector System (Stratagene)27. Control vector expressing β-galactosidase and EGFP (Ad-LacZ) was provided by the Adenoviral Core Facility, Tufts Medical Center. Primary cultures of neonatal rat ventricular myocytes (NRVM) prepared as previously described28 were grown to 70–80% confluency. Media was exchanged with DMEM containing 2% charcoal-stripped fetal bovine serum. NRVM cultures were infected with Ad-LacZ or Ad-FOG-2 for 12h, and grown in media supplemented with vehicle (100 nM NaOH) or T3 (Sigma). Infection at an approximate multiplicity of infection (MOI) of 8 resulted in a >95% cellular EGFP expression at 72h (Nikon Eclipse E800). Cells were harvested for RNA at 48h and for protein at 72h using standard methods (Supplemental Methods).
Interaction assays
All plasmid constructs are detailed in Supplemental Methods. HEK-293 cells were transfected with equal parts pcDNA3-FOG-219 with either pCR3-rTRα1 or pCR3-myc-rTRα1 using Polyfect (Qiagen). Cellular extract was diluted in an immunoprecipitation buffer, precipitated with FOG-2 antiserum26, myc epitope antibody (Tufts University Core Facility) or no antibody (Supplemental Methods) and immunoblotted with FOG-2 antiserum26. GST-pulldown assays were performed with fusion proteins containing either zinc fingers 5 and 6 (aa 497–831) or zinc fingers 7 and 8 (aa 752–1151)21 and recombinant rat TRα1 and TRα2 proteins labeled with [35S|-methionine (Amersham) using the TnT T7 Coupled Reticulocyte System (Promega) (Supplemental Methods).
Transient transfection assays
The p0.6kb-SERCA2-tk-luciferase, containing the proximal rat SERCA2 promoter fused to the thymidine kinase (tk) promoter was described previously29. All other plasmid constructs are described in Supplemental Methods. Embryonic chick ventricular myocytes (CVM) were prepared as previously described30 and grown to 90–95% confluency. Media was replaced with DMEM containing 2% charcoal-stripped horse serum (HS). Triplicate wells were each transfected with 150 ng of luciferase reporter plasmid, 400 ng of each expression vector (pcDNA3.1-hTRα1, pcDNA-mFOG-2 or pCR3 to normalize amounts of transfected plasmid) and 10 ng of CMV-β- galactosidase control vector using Lipofectamine 2000 (Invitrogen). After 18h, the media was replaced with DMEM/2% charcoal-stripped HS supplemented with vehicle or T3. After 24h the cells were lysed and luciferase (Promega) and β-galactosidase (Applied Biosystems) activity were measured using a luminometer (Thermo Labsystems). Fold activation is expressed as a ratio of luciferase activity to β- galactosidase activity and normalized across experiments to either the empty vector or FOG-2 reference condition.
Statistical analysis
Microarray expression differences were determined by Significance Analysis of Microarrays (SAM)31. Statistically significant differences between Tg and NTg groups were determined by an unpaired t-test or log-rank survival analysis when appropriate. All other statistical analyses were done by two-way ANOVA followed by a Bonferroni post test using GraphPad Prism version 4.0 software. Data are expressed as mean±SEM. A minimum value of p<0.05 was accepted as statistically significant.
RESULTS
FOG-2 protein is increased in failing hearts
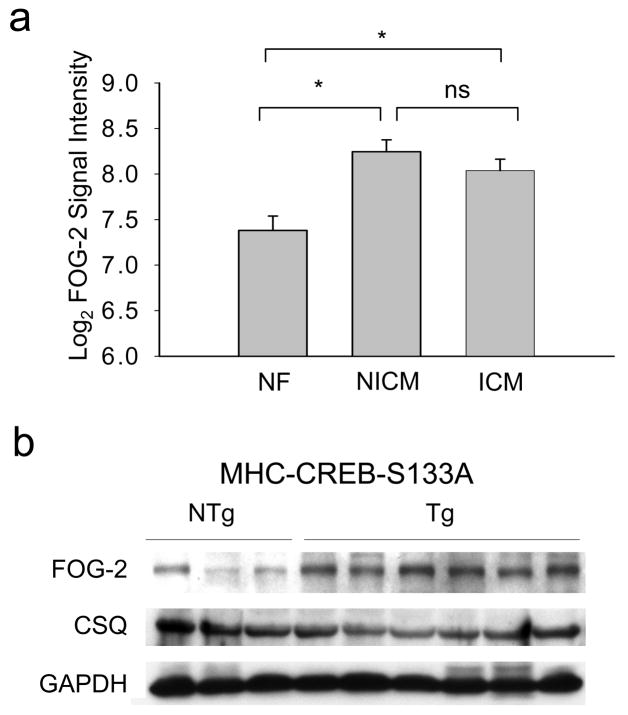
To explore FOG-2 expression in the setting of heart failure, we analyzed microarray data (Gene Expression Omnibus GDS1362) from 37 human cardiac tissue samples taken from non-failing (NF, n=6), non-ischemic cardiomyopathy (NICM, n=21), and ischemic dilated cardiomyopathy (ICM, n=10)32. SAM analysis identified FOG-2 as a differentially expressed gene among cardiomyopathy samples with a local false discovery rate (FDR) of 0.06%. FOG-2 expression was increased 1.8 fold in NICM and 1.6 fold in ICM samples, respectively compared with NF control (Figure 1A).
Figure 1. Increased FOG-2 protein in end-stage heart failure.
(a) Mean±SEM log-transformed FOG-2 probe intensities in NF (n=6), ICM (n=10), and NICM (n=21) and human heart samples. One-way ANOVA followed by Holm-Sidak analysis, *, p<0.01. (b) Immunoblot analysis of ventricular FOG-2 expression in 12 wk CREB-S133A Tg mice and NTg littermate controls. GAPDH and calsequestrin (CSQ) are protein loading controls.
To observe whether increased FOG-2 expression is associated with heart failure independent of concomitant therapy, we studied CREB-S133A mice, a well-characterized model of human dilated cardiomyopathy. These mice develop progressive chamber dilatation and contractile dysfunction by 8 wk of age and begin to die from heart failure by 10 wk of age25. While 6 wk old CREB-S133A mice did not have significant differences in FOG-2 expression compared with nontransgenic (NTg) littermate controls (1.51±0.39 fold difference, p=0.19), a 2.53±0.45 fold increase in FOG-2 protein (p<0.05) was observed at 12 wk of age (Figure 1B). Furthermore, increased myocardial FOG-2 protein expression was also observed in mice subjected to 14 d of transverse aortic constriction (TAC) at which timepoint, left ventricular fractional shortening was significantly depressed (Supplemental Figure 1). Thus, increased FOG-2 expression is associated with decompensated heart function.
FOG-2 transgenic mice develop heart failure
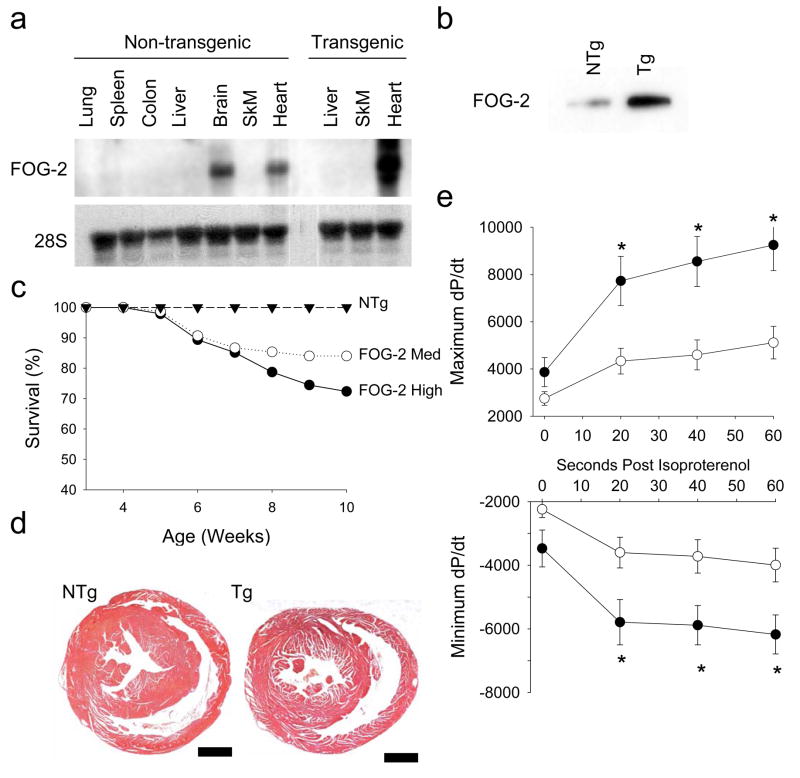
To determine the consequence of increased FOG-2 expression in the adult heart, we generated five lines of cardiac-specific transgenic (Tg) mice with FOG-2 expression driven by the αMHC promoter33. One line did not express FOG-2. The remaining four lines were found to have low (line 2467, FOG-2-low), medium (line 1711, FOG-2-med) and high (lines 2926 and 4102, FOG-2-high) levels of FOG-2 expression. Northern and Western blot analysis confirmed FOG-2 transgene expression in the heart (Figures 2A, 2B).
Figure 2. Generation and characterization of FOG-2 tran sgenic mice.
(a) Northern and (b) Immunoblot analysis comparing organ and ventricular FOG-2 expression in FOG-2-med Tg and NTg littermate control mice. 28S is an RNA loading control. (c) Kaplan-Meier survival analysis of FOG-2-med Tg (○, n=75), FOG-2-high Tg (●, n=46) and NTg littermate control (▲, n=44) mice. Differences in survival rates between the Tg and NTg groups were significant by the log rank test (p<0.05). (d) Representative coronal sections of hearts from NTg littermate control and FOG-2-med Tg mice showed no gross hypertrophy of the ventricles. Scale bar, 100 μm. (e) Hemodynamic analysis of FOG-2-med Tg and NTg littermate control mice. The mean±SEM maximum (top panel) and minimum (bottom panel) first derivative of left ventricular pressure tracings (dP/dt) measured at baseline and at indicated time points following stimulation with isoproterenol. Reductions in both maximum and minimum dP/dt in the Tg (●, n=11) compared with NTg (○, n=9) were noted following isoproterenol. Two-way ANOVA with repeated measures followed by Bonferroni analysis, *, p<0.05 versus NTg.
Genotypic analysis performed at 3 wk of age revealed equivalent numbers of Tg and NTg pups in all lines suggesting that FOG-2 overexpression from the αMHC promoter did not influence viability during embryonic development. The FOG-2-med and -high lines demonstrated significant morbidity and mortality beginning as early as 6 wk of age (Figure 2C) and were observed to have lethargy, tachypnea, and peripheral edema consistent with overt heart failure.
Hearts from FOG-2 mice displayed grossly normal sized ventricles (Figure 2D) and enlarged atria with mural thrombi (Supplemental Figure 2A). Cardiac valve leaflet structure was unremarkable (Supplemental Figure 2B). A trend towards greater whole heart mass relative to body weight in Tg versus NTg hearts (4.21±0.08 versus 3.97±0.09 mg/g, p=0.06) was due to enlarged atria. The ventricular to body weight (3.5±0.1 versus 3.6±0.1 mg/g, p=NS) and tibia length (5.9±0.2 versus 6.0±0.2 gm/mm, p=NS) ratios were similar as were cardiomyocyte cross-sectional areas (data not shown). Pico Sirius staining showed increased interstitial fibrosis (Supplemental Figure 3) consistent with findings in cardiomyopathy25 and hypothyroidism34.
To evaluate the functional cardiac phenotype underlying the susceptibility to heart failure in FOG-2 mice, we assessed contractile performance in 8–10 wk old FOG-2-med Tg and NTg mice. FOG-2 mice had mildly dilated left ventricular (LV) systolic dimensions by echocardiography with significantly reduced fractional shortening (Table 1). Invasive pressure measurement showed reduced LV systolic pressure in FOG-2 mice (61.8±6.1 versus 82.4±6.7 mmHg, p<0.05), while LV diastolic pressures were similar. At baseline, FOG-2 mice revealed a trend towards depressed systolic function (dP/dtmax 2750±288 versus 3866±615 mmHg/sec, p=0.09) and diastolic relaxation (dP/dtmin −2242±258 versus −3471±578 mmHg/sec, p=0.05) that became significant following isoproterenol challenge (Figure 2E).
Table 1.
Echocardiographic data in FOG-2 transgenic and non-transgenic mice
| NTg (n=16) | Tg (n=9) | P value | |
|---|---|---|---|
| Heart Rate (bpm) | 376±27 | 389±45 | NS |
| Anterior Wall Thickness (mm) | 0.73±0.04 | 0.75±0.04 | NS |
| Posterior Wall Thickness (mm) | 0.70±0.02 | 0.75±0.04 | NS |
| LVEDD (mm) | 3.42±0.01 | 3.86±0.02 | 0.07 |
| LVESD (mm) | 2.28±0.02 | 2.91±0.02 | 0.02 |
| Fractional Shortening (%) | 34±3 | 25±2 | 0.03 |
Data are mean ± SEM. Mice were 8–10 wk of age. LVEDD, left ventricular end-diastolic diameter. LVESD, left ventricular end-systolic diameter. Significance of differences between means was determined by unpaired t-test. p<0.05 was accepted as significant.
SERCA2 is a downstream target of FOG-2
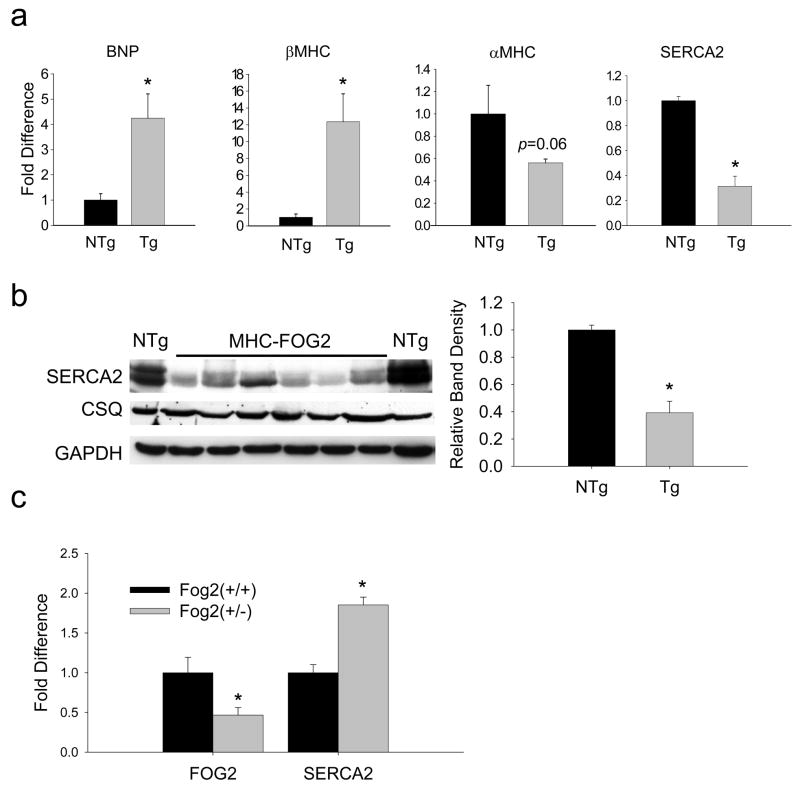
Because FOG-2 mice demonstrated findings consistent with cardiomyopathy, gene expression was evaluated in 4–6 wk old FOG-2-high mice. FOG-2 mouse ventricles had increased B-type natriuretic peptide (BNP), β-myosin heavy chain (βMHC) and atrial natriuretic factor (ANF) (Supplemental Figure 3), borderline significantly decreased αMHC expression (p=0.06) and significantly decreased SERCA2 mRNA levels (Figure 3A) and protein levels (Figure 3B) which were similarly observed in FOG-2-med mice (mRNA, p<0.01). To address potential concerns of overexpression artifact, we also measured SERCA2 protein levels in Fog-2(+/−) (heterozygous null) mice. In this setting of reduced FOG-2 gene dosage, SERCA2 protein levels were increased nearly two-fold (Figure 3C).
Figure 3. FOG-2 mice ventricles show fetal gene expression pattern including repression of SERCA2.
(a) Fold difference of BNP, βMHC and αMHC and SERCA2 mRNA transcripts, each normalized to GAPDH mRNA in 4–6 wk old FOG-2-high Tg mice (n=4) relative to NTg littermate controls (n=4) measured by qRT-PCR. (b) Immunoblot analysis of SERCA2, CSQ and GAPDH protein in ventricular myocardium from FOG-2-high Tg mice. SERCA2 band density normalized to CSQ. (c) Increased SERCA2 protein and reduced FOG-2 protein seen in FOG-2 heterozygote null ventricles. Unpaired t-test, *, p<0.05 versus NTg.
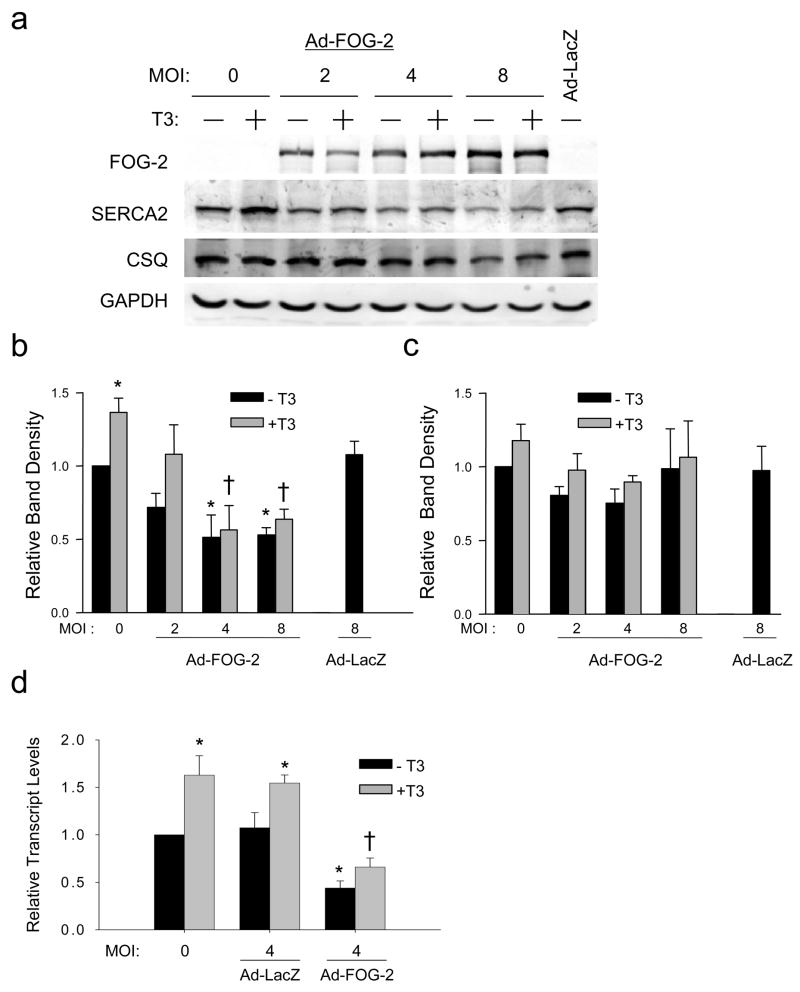
To examine the effects of FOG-2 on endogenous SERCA2 expression independently from the potential secondary effects of heart failure, we infected cultured neonatal rat ventricular cardiomyocytes with a recombinant adenovirus expressing FOG-2. Ad-FOG-2 infection resulted in a dose-dependent increase in FOG-2 protein and a significant dose-dependent decrease in SERCA2 protein with maximal suppression of SERCA2 to levels that were half of that quantified in uninfected cells (Figure 4A and 4B). T3 treatment increased SERCA2 expression, however FOG-2 overexpression repressed this effect at the protein (Figure 4A and 4B) and transcript (Figure 4D) levels. Importantly, FOG-2 did not alter expression of calsequestrin, another calcium handling protein specific to cardiomyocytes (Figure 4C). These results are consistent with SERCA2 being a downstream target of FOG-2 and show that FOG-2 abrogates T3-mediated transcription of the endogenous SERCA2 gene.
Figure 4. SERCA2 is a downstream target of FOG-2.
(a) Immunoblot analysis of neonatal rat ventricular cardiomyocytes lysates following infection with adenoviruses encoding FOG-2 (Ad-FOG2) or LacZ (Ad-LacZ), at the indicated multiplicity of infection (MOI), and stimulation with vehicle (black bars) or T3 (shaded bars). (b) Densitometry analysis of SERCA2 band density normalized to GAPDH. Increased SERCA2 protein seen in T3-treated cells was reduced with increasing Ad-FOG2 (c) Calsequestrin band density normalized to GAPDH did not change with Ad-FOG-2 infection. (d) qRT- PCR of SERCA2 mRNA normalized to GAPDH was significantly reduced by Ad-FOG-2. Two-way ANOVA followed by Bonferroni analysis, *, p<0.05 versus MOI 0/−T3 control; †, p<0.05 versus MOI 0/+T3 control. All data from at least three independent experiments.
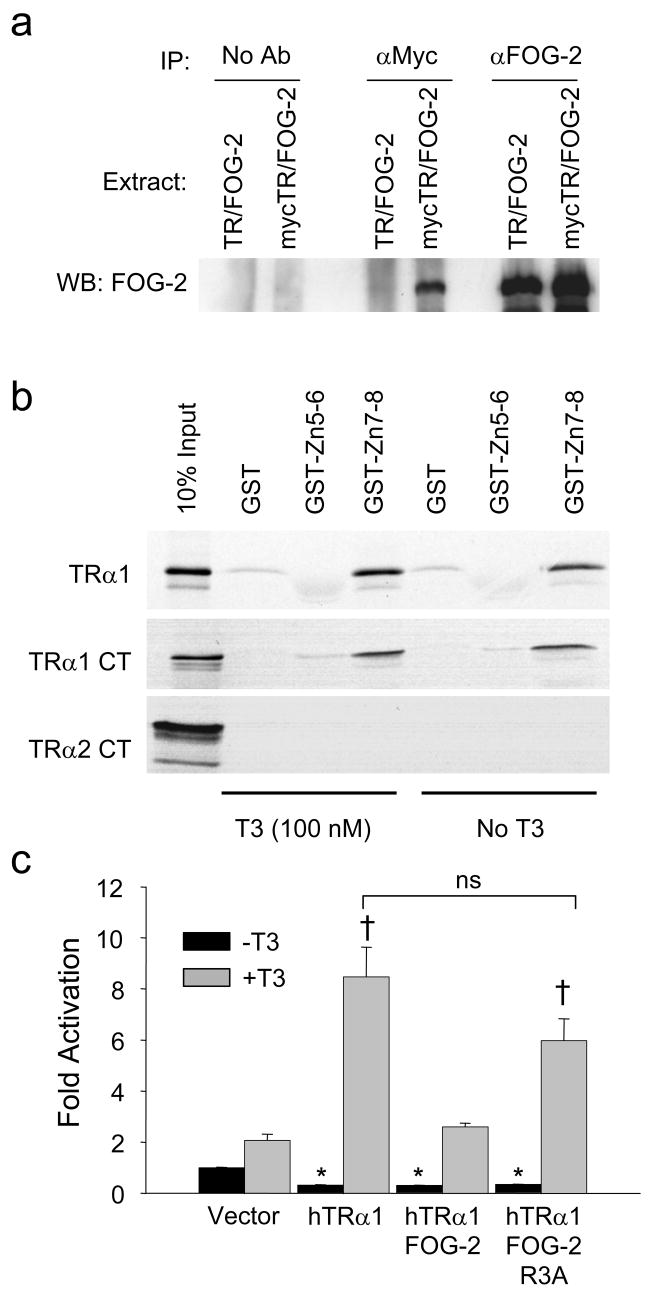
FOG-2 physically interacts with TRα1
Because FOG-2 can bind nuclear hormone receptors21,22 we explored the interaction of FOG-2 with TRα1, the predominant regulator of SERCA2 expression in vivo11. In HEK-293 cells, FOG-2 was overexpressed with either TRα1 or myc-tagged TRα1. A FOG-2 antiserum recovered similar amounts of FOG-2 from both extracts (Figure 5A, right). By comparison, the myc antibody recovered FOG-2 only when co-expressed with the myc-tagged TRα1 (Figure 5A, middle), thus identifying a specific association between FOG-2 and myc-TRα1. Furthermore, in vitro translated TRα1 was retained by GST-FOG-2-Zn7-8, but not by FOG-2-Zn5-6 or GST alone in the presence or absence of T3 (Figure 5B). We also compared the retention of TRα1 with TRα2, an alternative product of the Thra gene that has a distinct C-terminus (CT)35. GST-FOG-2-Zn7-8 selectively retained the TRα1-CT in the presence or absence of T3, but not the TRα2-CT (Figure 5B).
Figure 5. FOG-2 physically interacts with TRα1.
(a) Co-immunoprecipitation assay of HEK-293 cells transiently co-transfected with FOG-2 and either TRα1 or myc-tagged TRα1. Cell extracts precipitated with FOG-2 antiserum (right), myc antibody (center) and no antibody (left) were analyzed by immunoblot for FOG-2. Interaction between FOG-2 and myc-tagged TRα1 (lane 4) was specific and not a result of non-specific binding of FOG-2 to beads (lanes 1, 2) or binding of the myc-antibody to FOG-2 (lane 3). (b) GST pull-down assays of [35S] methionine-labeled full-length TRα1, TRα1-carboxy terminus only (TRα1 CT) or TRα2-carboxy terminus only (TRα2 CT) with immobilized GST-FOG-2 fusion proteins. Representative results from three experiments are shown. (c) Transient transfection assay of a TREx2-tk-luciferase reporter in cultured cardiomyocytes. FOG-2 abrogation of T3-mediated TRα1 transactivation of a plasmid is not found with the inactive FOG-2-R3A mutant. Two-way ANOVA followed by Holm-Sidak analysis, * p<0.05, versus vector control/−T3 (black bar); †, p<0.05 versus vector control/+T3 control (shaded bar).
A functional analysis of FOG-2’s interaction with TRα1 was performed in transient transfection studies of cultured cardiomyocytes using a reporter plasmid regulated by two tandem repeats of the consensus TRE sequence (Figure 5C). Transfection of TRα1 alone significantly repressed basal activity of the consensus TREx2-tk reporter by >50%, while T3 treatment increased activity by approximately 8 fold. While FOG-2 did not further repress activity beyond the level produced by TRα1 in the absence of T3, FOG-2 significantly abrogated transactivation by TRα1 following T3 treatment. Immunoblot analysis of co-transfected COS-7 cell lysates showed preserved if not greater TRα1 protein expression when co-expressed with FOG-2, suggesting that the diminished reporter activity was not caused by lower TRα1 protein levels (data not shown). Furthermore, while the FOG-2-R3A mutant36, which lacks repressive activity, permitted continued repression of basal reporter activity by TRα1 in the absence of T3, the FOG-2-R3A mutant did not abrogateT3-mediated TRα1 transactivation (Figure 5C). Taken together, these results show a ligand-independent interaction between the C-terminus of FOG-2 including zinc-fingers 7–8 (amino acids 752–1151) and the C-terminus of TRα1 and suggest that FOG-2 abrogation of T3-dependent TRα1 transactivation requires the FOG-2 N-terminal repressive domain.
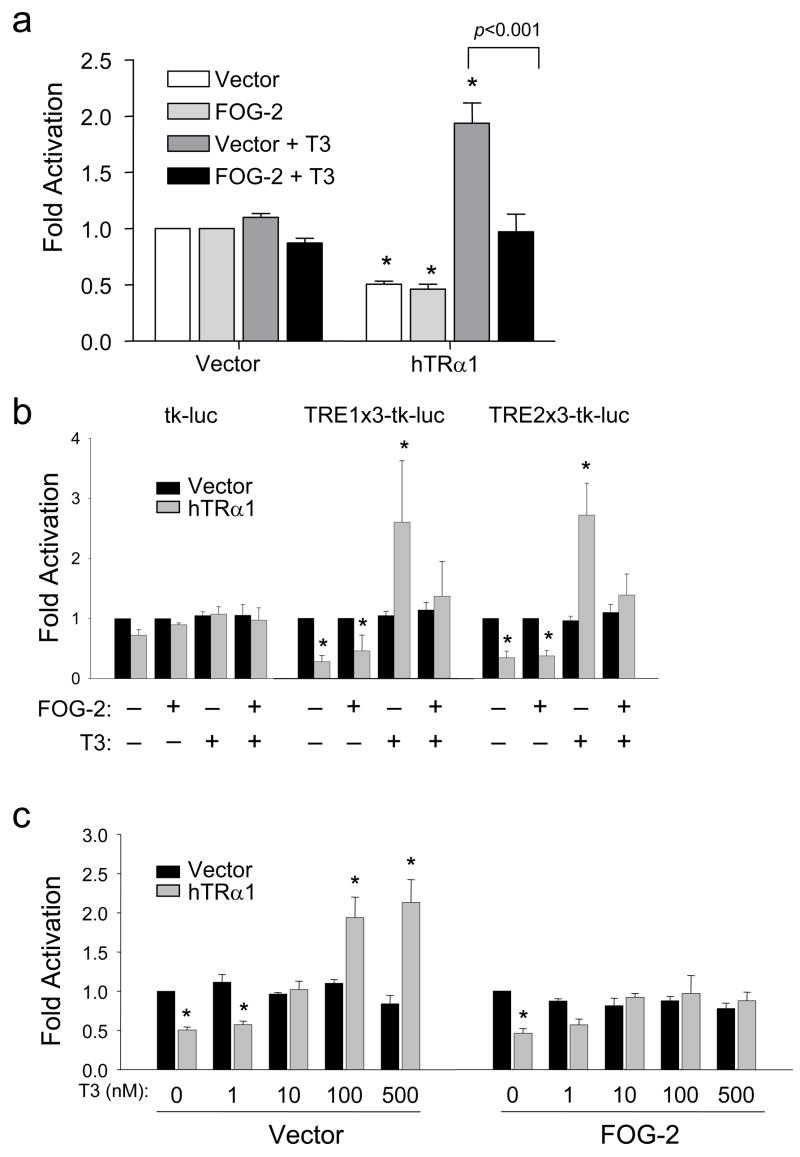
FOG-2 abrogates thyroid hormone-mediated SERCA2 promoter activity
Further transient transfection studies of cultured cardiomyocytes were performed using a T3-responsive fragment of the SERCA2 promoter that includes multiple TREs37. To isolate the effect of FOG-2 on T3-mediated activation, we analyzed relative luciferase activity of the SERCA2-tk reporter29 with respect to the basal effects of FOG-2 in the absence of T3. Similar to its effects on the consensus TREx2-tk reporter, FOG-2 did not further repress activity beyond the level produced by TRα1 in the absence of T3 and significantly abrogated T3-mediated transactivation to basal levels (Figure 6A).
Figure 6. FOG-2 significantly abrogates T3-mediated SERCA2 promoter activity.
Luciferase reporter assays performed in cultured cardiomyocytes cotransfected with empty vector or TRα1 with empty vector or FOG-2 followed by treatment for 24h with vehicle or T3. Fold activation is expressed as relative luciferase activity normalized to either the vehicle-treated, empty vector control, or the vehicle-treated FOG-2 control when appropriate. Two-way ANOVA followed by Bonferroni was used for statistical analysis. (a) Co-transfection of FOG-2 expression plasmid abrogated TRα1 transactivation of the 0.6kb-SERCA2 luciferase reporter following T3-stimulation. * p<0.05, versus vector control (color-matched bar). (b) Assays using reporter constructs driven by tk alone or with three tandem repeats of TRE1 or TRE2 from the rat SERCA2 proximal promoter. TRα1 (shaded bars) silenced reporter activity in the absence of T3 and transactivated the reporter with T3 stimulation. FOG-2 abrogated T3-mediated TRα1 transactivation. * p<0.05, versus vector control (black bar). (c) Transactivation of 0.6kb-SERCA2 reporter by TRα1 with indicated doses of T3 (nM) was abrogated by FOG-2. * p<0.05, versus vector control (black bar). Data are from at least three independent experiments done in triplicate.
Similar abrogation of T3-mediated activity was observed utilizing heterologous tk promoters consisting of 3 tandem repeats of two SERCA2 TREs (TRE1 and TRE2) that preferentially bind TRα137 (Figure 6B). Furthermore, while increasing doses of T3 activated the SERCA2-tk reporter in a dose-dependent manner, doses of T3 as high as 500nM could not overcome the negative effects of FOG-2 (Figure 6C). These results confirm that FOG-2 disrupts T3-mediated TRα1 transactivation of the SERCA2 promoter.
DISCUSSION
Our work demonstrates a novel role for FOG-2 in both adult heart disease and tissue-restricted T3 resistance. Increased FOG-2 expression in end-stage human heart failure, and at a time when CREB-S133A mice are susceptible to dying from heart failure25, and wild-type mice subjected to TAC have decreased systolic function, attests to the fact that FOG-2 upregulation is a novel heart-failure associated feature. We demonstrate that FOG-2 disrupts T3-mediated transcription of the SERCA2 gene, which in cultured cardiomyocytes, cannot be overcome by high doses of T3. By lowering SERCA2 expression, increased FOG-2 expression may therefore contribute to the impaired systolic and diastolic function characteristic of end-stage heart failure. These observations have clinical implications because thyroid hormone therapy, long considered for treating heart disease, has been limited by its cardiotoxic and peripheral side effects38.
Downregulation of SERCA2 is a critical event that accompanies the transition to decompensated heart failure39–41. We observed a novel physical interaction between FOG-2 and TRα1, the principal TR regulating SERCA2 in the myocardium. Furthermore, this interaction occurred in the absence and presence of T3 thus distinguishing FOG-2 from other corepressors whose binding affinities to nuclear hormone receptors are reduced by ligand42. Because the FOG-2-R3A inactive mutant did not abrogate T3-mediated TR-dependent transactivation but permitted intact TR-dependent silencing of a TRE-driven reporter, these results implicate that FOG-2 abrogation occurs via its highly-conserved N-terminus repression motif, which has been shown to exert its effects via interactions with a nucleosome-associated complex 36,43. Alternatively, FOG-2 may impair T3-dependent transcriptional activity through competition with TRα1 for limiting amounts of p30044, a coactivator required for T3-dependent transcriptional activity45.
Repression of endogenous SERCA2 in the absence of T3 suggests that FOG-2 also represses SERCA2 through mechanisms unrelated to T3-signaling. Sequences further upstream of the proximal SERCA2 promoter studied in this report that positively regulate the SERCA2 promoter46,47 are enriched with GATA4 consensus binding sites48 and have severely reduced transcriptional activity in severe LV pressure overload47,48. Multi-protein complexes between FOG-2, GATA4, and other nuclear hormone receptors can cooperatively repress transcription21,22 and may also play a role in SERCA2 repression. Experiments are currently underway to address this question.
Consistent with our in vitro observations, in vivo cardiac overexpression of FOG-2 resulted in decreased expression of SERCA2 mRNA and protein. Furthermore, FOG-2 mouse hearts spontaneously developed multiple features of cardiomyopathy and a reversion to a fetal pattern of gene expression without gross or histologic evidence of hypertrophy, a commonly accepted precursor to load-induced heart failure49. The development of hypertrophy-independent cardiomyopathy in FOG-2 mice is reminiscent of several previously described mouse models of nonhypertrophic cardiomyopathy including the transgenic mutant CREB mouse25 and human idiopathic restrictive cardiomyopathy50. Our findings suggest that FOG-2 may be a key downstream effector of signaling pathways responsible for mediating the transcriptional changes observed with heart failure via mechanisms independent of those which lead to pathologic hypertrophy.
Recent studies supporting GATA4’s necessary role for normal cardiac function and protection against load-induced heart failure51,52 may also shed light on FOG-2’s role in cardiomyopathy. FOG-2 is a powerful repressor of GATA4-mediated gene expression19 and of cardiac myocyte hypertrophy in vitro44. Similar to FOG-2 mice, heterozygous mutant GATA4 mice have systolic and diastolic dysfunction and blunted dP/dTmax and dP/dTmin responses to β-adrenergic stimulation. However, in contrast to FOG-2 mice, they do not develop progressive dysfunction and death despite a nearly 50% reduction of GATA4 protein51. FOG-2 mice also have more severe cardiac dysfunction and higher mortality than GATA4-deficient mice52. These results suggest that the effects of FOG-2 overexpression cannot be explained simply by a loss of GATA4 activity and implicate GATA4-independent functions of FOG-2. We speculate that while GATA4 is necessary for compensatory hypertrophy in response to pathological stress51, increased FOG-2 expression may directly trigger the transition to decompensated heart failure observed in end-stage cardiomyopathy.
Our study indicates that increased cardiac expression of FOG-2 promotes resistance of SERCA2 to T3-stimulation and may render the heart more susceptible to decompensation. This effect may extend broadly to other T3-regulated genes and pathways critical to efficient excitation-contraction coupling. While it remains to be determined whether additional cardiac pathologies are associated with increased FOG-2 expression, our model suggests that therapies designed to decrease FOG-2 activity in the failing heart might slow the progression of heart failure. Defining the signaling pathways that regulate FOG-2 activity and the downstream targets of FOG-2 in cardiac myocytes will provide insight into mechanisms of heart failure progression.
Supplementary Material
Acknowledgments
The authors thank Dr. Jeffrey Leiden for their support of these studies. The authors thank Dr. Hua Lin for cardiac catheterization studies and Dorothy Zhang for histology staining. This work was supported by NIH (R01-AA014140, G.S.H. and T32-HL069770-01A1, R.R.) and American Heart Association (0555877T, G.S.H.).
Footnotes
Publisher's Disclaimer: This is an un-copyedited author manuscript accepted for publication in Circulation Research, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the "Fair Use of Copyrighted Materials" (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at http://circres.ahajournals.org/. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- 1.Lowes BD, Minobe W, Abraham WT, Rizeq MN, Bohlmeyer TJ, Quaife RA, Roden RL, Dutcher DL, Robertson AD, Voelkel NF, Badesch DB, Groves BM, Gilbert EM, Bristow MR. Changes in gene expression in the intact human heart. Downregulation of alpha-myosin heavy chain in hypertrophied, failing ventricular myocardium. J Clin Invest. 1997;100:2315–24. doi: 10.1172/JCI119770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercadier JJ, Lompre AM, Duc P, Boheler KR, Fraysse JB, Wisnewsky C, Allen PD, Komajda M, Schwartz K. Altered sarcoplasmic reticulum Ca2(+)-ATPase gene expression in the human ventricle during end-stage heart failure. J Clin Invest. 1990;85:305–9. doi: 10.1172/JCI114429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–9. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 4.van Tuyl M, Blommaart PE, de Boer PA, Wert SE, Ruijter JM, Islam S, Schnitzer J, Ellison AR, Tibboel D, Moorman AF, Lamers WH. Prenatal exposure to thyroid hormone is necessary for normal postnatal development of murine heart and lungs. Dev Biol. 2004;272:104–17. doi: 10.1016/j.ydbio.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 5.Arai M, Otsu K, MacLennan DH, Alpert NR, Periasamy M. Effect of thyroid hormone on the expression of mRNA encoding sarcoplasmic reticulum proteins. Circ Res. 1991;69:266–76. doi: 10.1161/01.res.69.2.266. [DOI] [PubMed] [Google Scholar]
- 6.Rohrer D, Dillmann WH. Thyroid hormone markedly increases the mRNA coding for sarcoplasmic reticulum Ca2+-ATPase in the rat heart. J Biol Chem. 1988;263:6941–4. [PubMed] [Google Scholar]
- 7.Hamilton MA, Stevenson LW, Luu M, Walden JA. Altered thyroid hormone metabolism in advanced heart failure. J Am Coll Cardiol. 1990;16:91–5. doi: 10.1016/0735-1097(90)90462-x. [DOI] [PubMed] [Google Scholar]
- 8.Wassen FW, Schiel AE, Kuiper GG, Kaptein E, Bakker O, Visser TJ, Simonides WS. Induction of thyroid hormone-degrading deiodinase in cardiac hypertrophy and failure. Endocrinology. 2002;143:2812–5. doi: 10.1210/endo.143.7.8985. [DOI] [PubMed] [Google Scholar]
- 9.Kinugawa K, Minobe WA, Wood WM, Ridgway EC, Baxter JD, Ribeiro RC, Tawadrous MF, Lowes BA, Long CS, Bristow MR. Signaling pathways responsible for fetal gene induction in the failing human heart: evidence for altered thyroid hormone receptor gene expression. Circulation. 2001;103:1089–94. doi: 10.1161/01.cir.103.8.1089. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Lazar MA. The mechanism of action of thyroid hormones. Annu Rev Physiol. 2000;62:439–66. doi: 10.1146/annurev.physiol.62.1.439. [DOI] [PubMed] [Google Scholar]
- 11.Stoykov I, Zandieh-Doulabi B, Moorman AF, Christoffels V, Wiersinga WM, Bakker O. Expression pattern and ontogenesis of thyroid hormone receptor isoforms in the mouse heart. J Endocrinol. 2006;189:231–45. doi: 10.1677/joe.1.06282. [DOI] [PubMed] [Google Scholar]
- 12.Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704–28. doi: 10.1210/er.2003-0033. [DOI] [PubMed] [Google Scholar]
- 13.Jeyakumar M, Tanen MR, Bagchi MK. Analysis of the functional role of steroid receptor coactivator-1 in ligand-induced transactivation by thyroid hormone receptor. Mol Endocrinol. 1997;11:755–67. doi: 10.1210/mend.11.6.0003. [DOI] [PubMed] [Google Scholar]
- 14.Weiss RE, Xu J, Ning G, Pohlenz J, O’Malley BW, Refetoff S. Mice deficient in the steroid receptor co-activator 1 (SRC-1) are resistant to thyroid hormone. Embo J. 1999;18:1900–4. doi: 10.1093/emboj/18.7.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoh SM, Chatterjee VK, Privalsky ML. Thyroid hormone resistance syndrome manifests as an aberrant interaction between mutant T3 receptors and transcriptional corepressors. Mol Endocrinol. 1997;11:470–80. doi: 10.1210/mend.11.4.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safer JD, Cohen RN, Hollenberg AN, Wondisford FE. Defective release of corepressor by hinge mutants of the thyroid hormone receptor found in patients with resistance to thyroid hormone. J Biol Chem. 1998;273:30175–82. doi: 10.1074/jbc.273.46.30175. [DOI] [PubMed] [Google Scholar]
- 17.Svensson EC, Huggins GS, Lin H, Clendenin C, Jiang F, Tufts R, Dardik FB, Leiden JM. A syndrome of tricuspid atresia in mice with a targeted mutation of the gene encoding Fog-2. Nat Genet. 2000;25:353–6. doi: 10.1038/77146. [DOI] [PubMed] [Google Scholar]
- 18.Tevosian SG, Deconinck AE, Tanaka M, Schinke M, Litovsky SH, Izumo S, Fujiwara Y, Orkin SH. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell. 2000;101:729–39. doi: 10.1016/s0092-8674(00)80885-5. [DOI] [PubMed] [Google Scholar]
- 19.Svensson EC, Tufts RL, Polk CE, Leiden JM. Molecular cloning of FOG-2: a modulator of transcription factor GATA-4 in cardiomyocytes. Proc Natl Acad Sci U S A. 1999;96:956–61. doi: 10.1073/pnas.96.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tevosian SG, Deconinck AE, Cantor AB, Rieff HI, Fujiwara Y, Corfas G, Orkin SH. FOG-2: A novel GATA-family cofactor related to multitype zinc-finger proteins Friend of GATA-1 and U-shaped. Proc Natl Acad Sci U S A. 1999;96:950–5. doi: 10.1073/pnas.96.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huggins GS, Bacani CJ, Boltax J, Aikawa R, Leiden JM. Friend of GATA 2 physically interacts with chicken ovalbumin upstream promoter-TF2 (COUP-TF2) and COUP-TF3 and represses COUP-TF2-dependent activation of the atrial natriuretic factor promoter. J Biol Chem. 2001;276:28029–36. doi: 10.1074/jbc.M103577200. [DOI] [PubMed] [Google Scholar]
- 22.Clabby ML, Robison TA, Quigley HF, Wilson DB, Kelly DP. Retinoid X receptor alpha represses GATA-4-mediated transcription via a retinoid-dependent interaction with the cardiac-enriched repressor FOG-2. J Biol Chem. 2003;278:5760–7. doi: 10.1074/jbc.M208173200. [DOI] [PubMed] [Google Scholar]
- 23.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, Hintze TH, Lopaschuk GD, Recchia FA. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–12. doi: 10.1161/01.cir.0000023531.22727.c1. [DOI] [PubMed] [Google Scholar]
- 24.Liang Q, De Windt LJ, Witt SA, Kimball TR, Markham BE, Molkentin JD. The transcription factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J Biol Chem. 2001;276:30245–53. doi: 10.1074/jbc.M102174200. [DOI] [PubMed] [Google Scholar]
- 25.Fentzke RC, Korcarz CE, Lang RM, Lin H, Leiden JM. Dilated cardiomyopathy in transgenic mice expressing a dominant-negative CREB transcription factor in the heart. J Clin Invest. 1998;101:2415–26. doi: 10.1172/JCI2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramaniam A, Jones WK, Gulick J, Wert S, Neumann J, Robbins J. Tissue-specific regulation of the alpha-myosin heavy chain gene promoter in transgenic mice. J Biol Chem. 1991;266:24613–20. [PubMed] [Google Scholar]
- 27.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–14. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, Michael A, Haq S, Nuedling S, Grohe C, Force T, Mendelsohn ME, Karas RH. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 2004;95:692–9. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- 29.Gloss B, Giannocco G, Swanson EA, Moriscot AS, Chiellini G, Scanlan T, Baxter JD, Dillmann WH. Different configurations of specific thyroid hormone response elements mediate opposite effects of thyroid hormone and GC-1 on gene expression. Endocrinology. 2005;146:4926–33. doi: 10.1210/en.2005-0631. [DOI] [PubMed] [Google Scholar]
- 30.Gadbut AP, Toupin DK, Kilbourne EJ, Galper JB. Low density lipoproteins induce parasympathetic responsiveness in embryonic chick ventricular myocytes in parallel with a coordinate increase in expression of genes coding for the M2 muscarinic receptor, G alpha i2, and the acetylcholine-sensitive K+ channel. J Biol Chem. 1994;269:30707–12. [PubMed] [Google Scholar]
- 31.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kittleson MM, Minhas KM, Irizarry RA, Ye SQ, Edness G, Breton E, Conte JV, Tomaselli G, Garcia JG, Hare JM. Gene expression analysis of ischemic and nonischemic cardiomyopathy: shared and distinct genes in the development of heart failure. Physiol Genomics. 2005;21:299–307. doi: 10.1152/physiolgenomics.00255.2004. [DOI] [PubMed] [Google Scholar]
- 33.Subramaniam A, Gulick J, Robbins J. Analysis of the upstream regulatory region of a chicken skeletal myosin heavy chain gene. J Biol Chem. 1990;265:13986–94. [PubMed] [Google Scholar]
- 34.Chen WJ, Lin KH, Lee YS. Molecular characterization of myocardial fibrosis during hypothyroidism: evidence for negative regulation of the pro-alpha1(I) collagen gene expression by thyroid hormone receptor. Mol Cell Endocrinol. 2000;162:45–55. doi: 10.1016/s0303-7207(00)00203-3. [DOI] [PubMed] [Google Scholar]
- 35.Izumo S, Mahdavi V. Thyroid hormone receptor alpha isoforms generated by alternative splicing differentially activate myosin HC gene transcription. Nature. 1988;334:539–42. doi: 10.1038/334539a0. [DOI] [PubMed] [Google Scholar]
- 36.Lin AC, Roche AE, Wilk J, Svensson EC. The N termini of Friend of GATA (FOG) proteins define a novel transcriptional repression motif and a superfamily of transcriptional repressors. J Biol Chem. 2004;279:55017–23. doi: 10.1074/jbc.M411240200. [DOI] [PubMed] [Google Scholar]
- 37.Hartong R, Wang N, Kurokawa R, Lazar MA, Glass CK, Apriletti JW, Dillmann WH. Delineation of three different thyroid hormone-response elements in promoter of rat sarcoplasmic reticulum Ca2+ATPase gene. Demonstration that retinoid X receptor binds 5′ to thyroid hormone receptor in response element 1. J Biol Chem. 1994;269:13021–9. [PubMed] [Google Scholar]
- 38.Degens H, Gilde AJ, Lindhout M, Willemsen PH, Van Der Vusse GJ, Van Bilsen M. Functional and metabolic adaptation of the heart to prolonged thyroid hormone treatment. Am J Physiol Heart Circ Physiol. 2003;284:H108–15. doi: 10.1152/ajpheart.00282.2002. [DOI] [PubMed] [Google Scholar]
- 39.Feldman AM, Weinberg EO, Ray PE, Lorell BH. Selective changes in cardiac gene expression during compensated hypertrophy and the transition to cardiac decompensation in rats with chronic aortic banding. Circ Res. 1993;73:184–92. doi: 10.1161/01.res.73.1.184. [DOI] [PubMed] [Google Scholar]
- 40.Meyer M, Schillinger W, Pieske B, Holubarsch C, Heilmann C, Posival H, Kuwajima G, Mikoshiba K, Just H, Hasenfuss G, et al. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation. 1995;92:778–84. doi: 10.1161/01.cir.92.4.778. [DOI] [PubMed] [Google Scholar]
- 41.Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T, Guerrero JL, Gwathmey JK, Rosenzweig A, Hajjar RJ. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci U S A. 2000;97:793–8. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu X, Lazar MA. Transcriptional repression by nuclear hormone receptors. Trends Endocrinol Metab. 2000;11:6–10. doi: 10.1016/s1043-2760(99)00215-5. [DOI] [PubMed] [Google Scholar]
- 43.Roche AE, Bassett BJ, Samant SA, Hong W, Blobel GA, Svensson EC. The zinc finger and C-terminal domains of MTA proteins are required for FOG-2-mediated transcriptional repression via the NuRD complex. J Mol Cell Cardiol. 2008;44:352–60. doi: 10.1016/j.yjmcc.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirai M, Ono K, Morimoto T, Kawamura T, Wada H, Kita T, Hasegawa K. FOG-2 competes with GATA-4 for transcriptional coactivator p300 and represses hypertrophic responses in cardiac myocytes. J Biol Chem. 2004;279:37640–50. doi: 10.1074/jbc.M401737200. [DOI] [PubMed] [Google Scholar]
- 45.Li Q, Imhof A, Collingwood TN, Urnov FD, Wolffe AP. p300 stimulates transcription instigated by ligand-bound thyroid hormone receptor at a step subsequent to chromatin disruption. Embo J. 1999;18:5634–52. doi: 10.1093/emboj/18.20.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takizawa T, Arai M, Yoguchi A, Tomaru K, Kurabayashi M, Nagai R. Transcription of the SERCA2 gene is decreased in pressure-overloaded hearts: A study using in vivo direct gene transfer into living myocardium. J Mol Cell Cardiol. 1999;31:2167–74. doi: 10.1006/jmcc.1999.1045. [DOI] [PubMed] [Google Scholar]
- 47.Aoyagi T, Yonekura K, Eto Y, Matsumoto A, Yokoyama I, Sugiura S, Momomura S, Hirata Y, Baker DL, Periasamy M. The sarcoplasmic reticulum Ca2+-ATPase (SERCA2) gene promoter activity is decreased in response to severe left ventricular pressure-overload hypertrophy in rat hearts. J Mol Cell Cardiol. 1999;31:919–26. doi: 10.1006/jmcc.1998.0932. [DOI] [PubMed] [Google Scholar]
- 48.Eizema K, Van Heugten HA, Bezstarosti K, Van Setten MC, Lamers JM. In vitro analysis of SERCA2 gene regulation in hypertrophic cardiomyocytes and increasing transfection efficiency by gene-gun biolistics. Ann N Y Acad Sci. 1999;874:111–24. doi: 10.1111/j.1749-6632.1999.tb09229.x. [DOI] [PubMed] [Google Scholar]
- 49.Hunter JJ, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–83. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 50.Ammash NM, Seward JB, Bailey KR, Edwards WD, Tajik AJ. Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation. 2000;101:2490–6. doi: 10.1161/01.cir.101.21.2490. [DOI] [PubMed] [Google Scholar]
- 51.Bisping E, Ikeda S, Kong SW, Tarnavski O, Bodyak N, McMullen JR, Rajagopal S, Son JK, Ma Q, Springer Z, Kang PM, Izumo S, Pu WT. Gata4 is required for maintenance of postnatal cardiac function and protection from pressure overload-induced heart failure. Proc Natl Acad Sci U S A. 2006;103:14471–6. doi: 10.1073/pnas.0602543103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, Molkentin JD. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res. 2006;98:837–45. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.