Abstract
Both mu opioid (MOP)† and type 2 cholecystokinin (CCK2) receptors are present in areas of the central nervous system that are involved in modulation of pain processing. We conducted bioluminescence resonance energy transfer (BRET) studies on COS cells coexpressing MOP and CCK2 receptors to determine whether receptor heterodimerization is involved in such modulation. These studies revealed the absence of constitutive or monovalent ligand-induced heterodimerization. Heterodimerization of MOP and CCK2 receptors therefore is unlikely to be responsible for the opposing effects between morphine and CCK in the CNS. However, association was induced, as indicated by a positive BRET signal, on exposure of the cells to bivalent ligands containing mu-opioid agonist and CCK2 receptor antagonist pharmacophores linked through spacers containing 16 to 22 atoms, but not with a shorter (9-atom) spacer. These studies demonstrate for the first time that an appropriately designed bivalent ligand is capable of inducing association of G protein-coupled receptors. The finding that opioid tolerance studies with these ligands in mice showed no correlation with the BRET data is consistent with the absence of association of MOP and CCK2 receptors in vivo.
Introduction
Both opioid (MOP) and cholecystokinin (CCK) receptors are members of the Family-A G protein-coupled receptor (GPCR) superfamily. Opioid receptors constitute a family of four receptor types, namely MOP (mu), DOP (delta), KOP (kappa), and NOP (nociceptin) having ∼60% sequence homology. They are present in the central nervous system (CNS) and peripherally. Interaction of members of the opioid peptide family with opioid receptors trigger activation mainly of Gi or Go which mediate neuro- or immunomodulation.1,2
There are two types of CCK receptors,3 CCK1 and CCK2, having sequence homology of approximately 50%.4, 5, 6, 7, 8 CCK1 receptors are distributed principally in the peripheral alimentary tract, while CCK2 receptors are found mainly in the CNS.9 Activation of both CCK receptors appears to be coupled primarily to Gq, resulting in the stimulation phospholipase C and intracellular calcium, which lead to stimulation of gallbladder contraction, pancreatic exocrine secretion, enteric motility, gastric acid secretion, and modulation of neurotransmission.3-9
Areas of the CNS that are involved in pain processing contain overlapping populations of CCK2 and opioid receptors.10,11,12 Activation of MOP or DOP receptors promotes the release of CCK which in turn acts to reduce opioid receptor-mediated analgesia through interaction with the CCK2 receptor. CCK2 receptor knockout mice display enhanced opioid-induced analgesia and upregulation of the endogenous opioid receptor system.13,14 The rostral ventromedial medulla (RVM) is the key area where this effect takes place and, in this regard, increased CCK activity in the RVM during exposure to morphine leads to morphine tolerance.15,16
Since antagonism of CCK2 receptors is known to block morphine tolerance, the combined use of a CCK2 antagonist and opioid agonist represents a potentially important strategy for the treatment of chronic pain states.17 In this regard, given the overlapping distribution of MOP and CCK2 receptors in the brain, an important question concerning the opposing roles of these receptors relates to their physical organization, particularly in view of the relatively large number of opioid receptor heterodimers that are already known to exist.18,19,20
We have investigated the possible existence of heterodimeric MOP/CCK2 receptors through the careful application of bioluminescence resonance energy transfer (BRET) technology in cultured cells that contain both MOP and CCK2 receptors. Our BRET data indicates the absence of constitutive or monovalent ligand-stimulated association of MOP and CCK2 receptors. However, in the presence of bivalent ligands that contain a mu-opioid agonist pharmacophore linked through an appropriate length spacer to a CCK2 antagonist pharmacophore, the association of MOP and CCK2 receptors was observed.
Design considerations for bivalent ligands
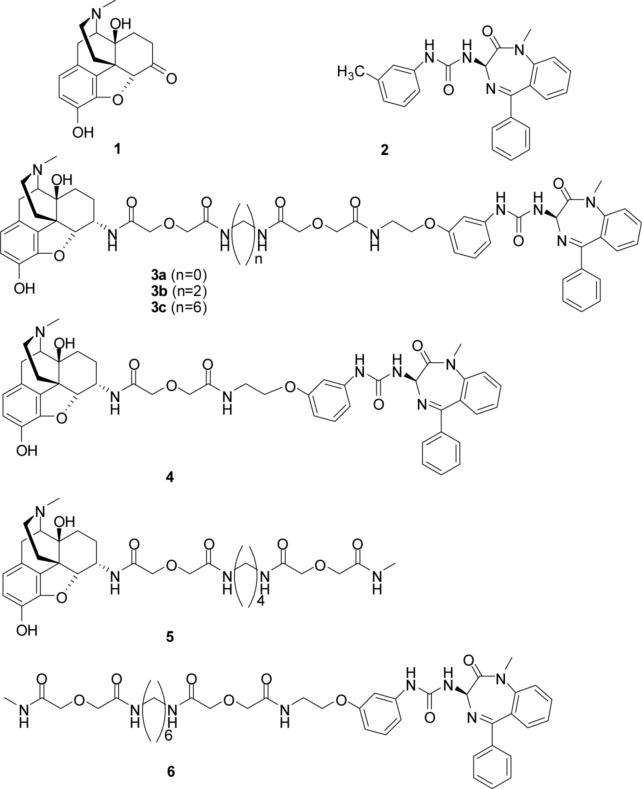
The design approach for the bivalent ligands 3a-c and 4 employed in this study consisted of the tethering of a mu-opioid agonist pharmacophore with a CCK2 antagonist pharmacophore using a spacer. Nonpeptidic pharmacophores were employed because it has been established that they bind within the cavity of the heptahelical (seven transmembrane segments) domain of opioid and CCK receptors, and would permit a better estimate of the spacer length required for bridging the two recognition sites in a putative MOP/CCK2 receptor heterodimer. 21,22,23,24 Spacers of varied length ranging from 9 to 22 atoms were employed in order to optimize the length for bridging of these receptors and, in this regard, efforts were focused on 16 to 22 atoms because other reported bivalent ligands that target dimeric opioid receptors are believed to bridge in this range. 25 The mu-opioid-selective agonist pharmacophore was derived from 1 (oxymorphone),26 whereas the CCK2 antagonist pharmacophore is closely related to 2 (L-365,260).27 The combination of agonist and antagonist pharmacophores in the bivalent ligand was based on reports that such pharmacologic interaction results in enhancement of analgesia with concomitant loss of tolerance.28,29 If this effect were to arise from the association of receptors as heterodimers, such a bivalent ligand could potentially serve as a valuable pharmacological tool. Monovalent ligands 530 and 6 served as controls in our studies.
Chemistry
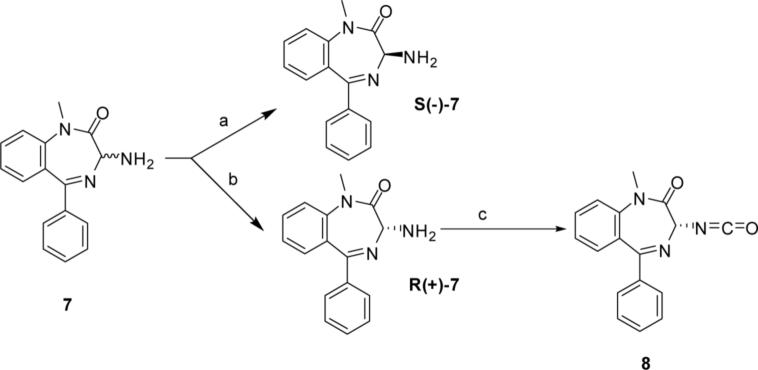
The precursor to the CCK2 antagonist pharmacophore 7 was synthesized using the method of Bock et al.31 Resolution of 7 was accomplished according to the procedure of Reider et al.32 Treatment of R(+)-7 with triphosgene gave the isocyanate 8 (scheme 1).
Scheme 1.
a) i. (1S)-(+)-10-camphorsulfonic acid, CH3CN/diethylether; ii. 10% NaOH, CH2Cl2. b) Mother liquor from first step taken. i. (1R)-(−)-10-camphorsulfonic acid, CH3CN/diethyl ether; ii. 10% NaOH, CH2Cl2. c) Triphosgen, aquas sat. Na2CO3/CH2Cl2, 0 °C.
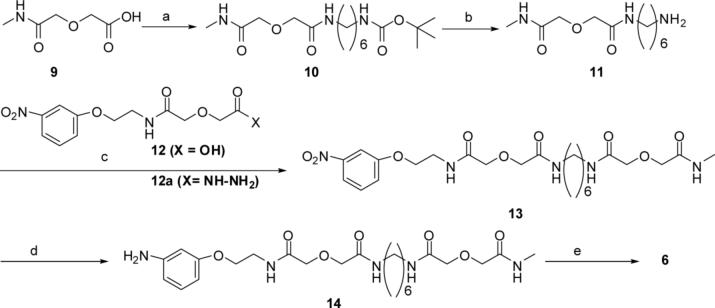
N-Methyldiglycolamide 9 was prepared by reaction of N-methylamine with diglycolic anhydride in anhydrous THF. Reaction of 9 with mono-Boc protected hexamethylene −1,6-diamine was mediated by HOBt/DCC in anhydrous DMF and gave the intermediate 10 which was converted in TFA/CH2Cl2 (1:1) at room temperature to the amine 11 (35%). Coupling of 11 to nitrophenyldiglycolamide 12 was mediated by HOBt/DCC in anhydrous DMF to give the intermediate nitrocompound 13 which was hydrogenated in methanol at 50 psi at room temperature to amine 14 using Pd/C (10%) catalyst. The reaction of amine 14 with isocyanate 8 in anhydrous DMF gave the monovalent ligand 6. This sequence is illustrated in scheme 2.
Scheme 2.
a) HOBt/DCC, DMF, Bochexadiamine1,6, r.t.; b) TFA/CH2Cl2, r.t.; c) only 12 used, HOBt/DCC, DMF, r.t.: d) Pd/C (10 %), methanol,50 psi.; e) 8, DMF, 5 days.
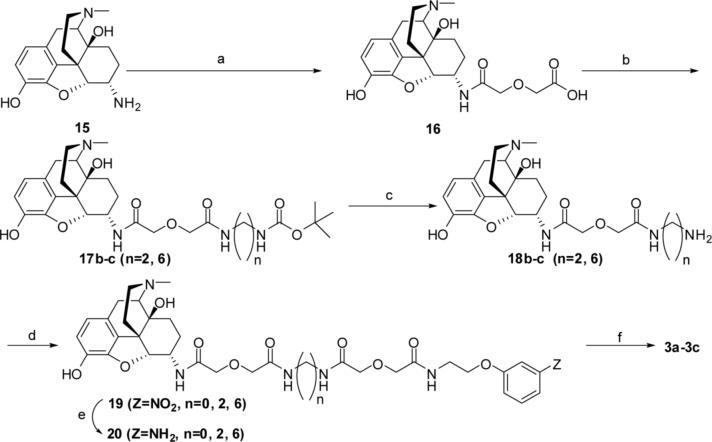
The intermediate 16 was obtained by condensation of 15 (α-oxmorphamine)26 with diglycolic anhydride in methylene chloride and coupled to mono-Boc protected diamines in anhydrous DMF using HOBt/DCC. These intermediates 17b-c were deprotected by TFA in methylene chloride and intermediate amines 18b-c were obtained in fair to good yields. The amines were coupled to 12 in DMF using HOBt/DCC. For the synthesis of 19a (n=0), the hydrazine derivative 12a (X=NHNH2) was first coupled to 16 in DMF mediated by HOBt/DCC. The intermediate nitro compounds 19a-c (n=0, 2, 6) were hydrogenated at 50 psi to amines 20a-c in the presence of Pd/C (10%) in methanol. The reaction of 20a-c with isocyanate 8 gave the bivalent ligands 3a-c (n=0, 2, 6). The reaction sequences of these reactions are illustrated in scheme 3.
Scheme 3.
a) Diglycolic anhydride, CH2Cl2, r.t.; b) HOBt/DCC, mono-Boc diamines (n=2; n=6), DMF; c) TAF/CH2Cl2; d) 12, HOBt/DCC; e) Pd/C (10%), methanol, 50 psi; f) 8, DMF, ∼24h, r.t.
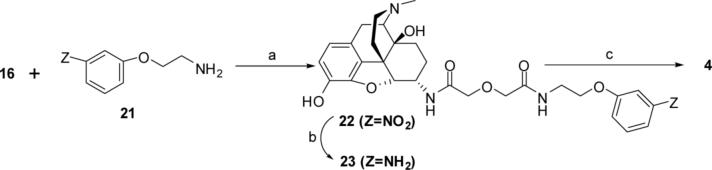
The synthetic sequence for the preparation of bivalent ligand 4 started with the HOBt/DCC-mediated coupling in DMF of 2-(3 nitrophenoxy)ethanolamine 21 33 with the glycolic acid derivative 16. The intermediate nitrocompound 22 was reduced to amine 23 via catalytic hydrogenolysis in methanol using Pd/C (10 %) at 50 psi. The reaction of 23 with 8 in DMSO gave bivalent ligand 4 (scheme 4).
Scheme 4.
a) HOBt/DCC, DMF; b) Pd/C (10%), methanol, 50 psi; c) 8, DMSO, 24h, r.t.
Biological Results
BRET studies
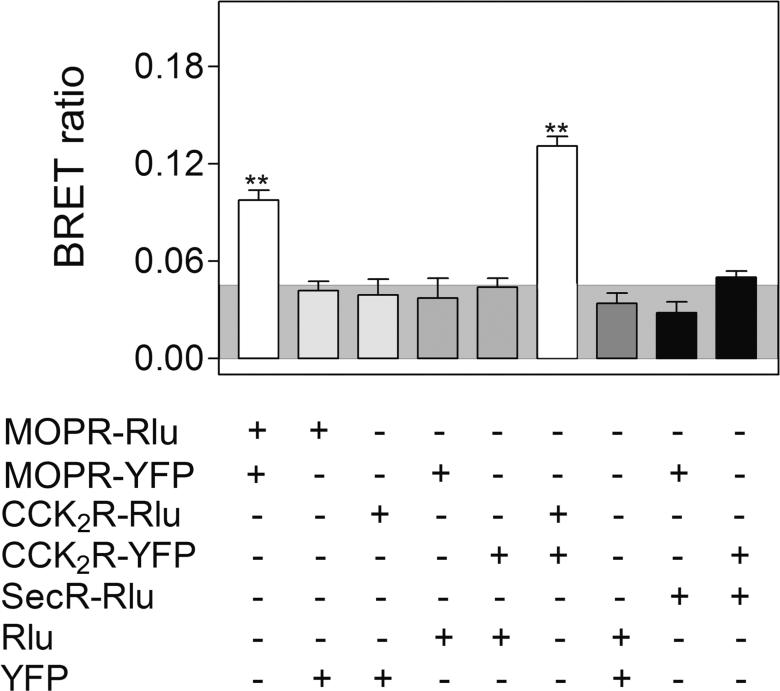
BRET technology has been utilized previously to demonstrate that CCK2 receptors exist as constitutive homodimers whose BRET signal is unaffected by the binding of endogenous agonist. 34 The literature supports similar constitutive homodimerization of MOP receptors.19, 35 This was confirmed in control studies with cells expressing only MOP or CCK2 receptors, in that a positive homologous receptor BRET signal was observed (Fig 2). Background non-specific BRET signals were determined by expressing the complementary fluorescent donor or acceptor in a soluble form or attached to a structurally unrelated Family B GPCR, the secretin receptor.
Figure 2. BRET controls.
Shown are the BRET ratios for COS cells expressing various constructs in the combinations noted. The shaded area represents the intensity of BRET signals felt to be non-specific, reflecting a signal that can be generated between Rlu-tagged receptor and soluble YFP protein or between YFP-tagged receptor and soluble Rlu protein. BRET signals above this are considered to be significant. Both CCK2 receptors and MOP receptors exist as constitutive homodimers with significant homologous receptor BRET signals when expressed in these cells. Values presented represent means±S.E.M. of data from five independent experiments. Values marked with ** represent BRET signals significantly above non-specific values at the p<0.001 level.
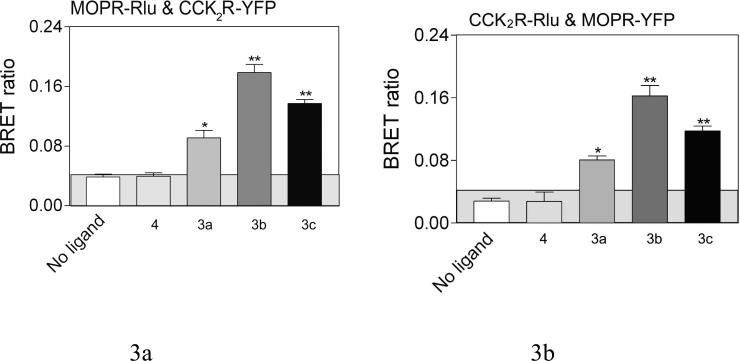
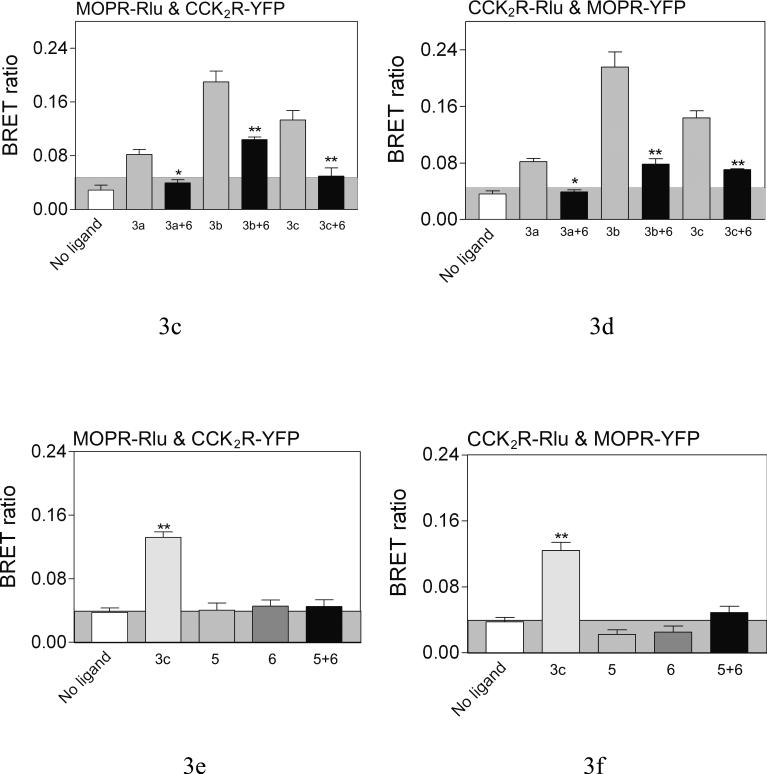
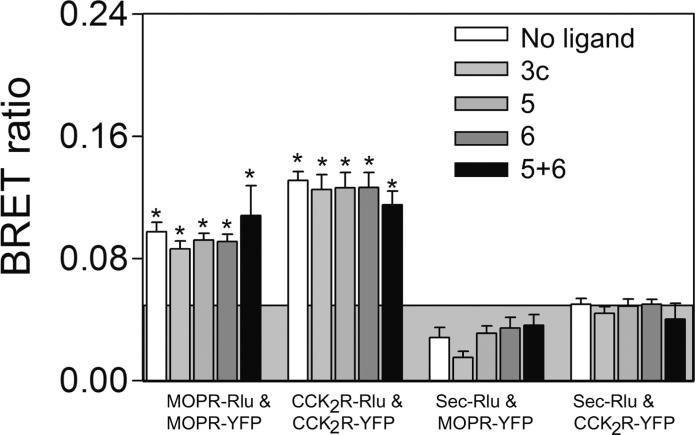
However, receptor association could be induced with bivalent ligands that were capable of bridging the CCK2 and MOP receptors, as reflected by a positive heterologous receptor BRET signal (Fig 3). The top panels show significant BRET signals were produced by 3a (16 atom spacer), 3b (18 atom spacer), and 3c (22 atom spacer), while the spacer in 4 (9 atoms) is apparently too short to generate such a signal. The highest BRET signal was generated with the bivalent ligand 3b having the 18 atom spacer. Competition between the monovalent CCK2 antagonist 6 and 3a-c, was reflected by a reduction in the BRET ratio, as shown in the middle panel. The bottom panel illustrates that no BRET signal was produced with monovalent ligands 5 and 6 individually or together. Thus, heterodimerization of these receptors was not induced by the occupation of the receptors with ligands that do not include a linking domain. It is noteworthy that neither the BRET ratios of MOP or CCK2 receptor homodimers were affected by either bivalent ligand 3c or monovalent ligands 5 and 6 utilized in this work (Fig 4).
Figure 3. Effects of bivalent ligands on heterodimerization of CCK2 and MOP receptors.
Shown are BRET ratios for COS cells expressing both tagged MOP and CCK2 receptor constructs with or without incubation of various bivalent ligands (10−6 M, 90 min at room temperature). The panels on the left reflect Rlu-tagged mu-opioid receptor expressed with YFP-tagged CCK2 receptor, while those on the right represent the opposite. The panels in the top row illustrate BRET ratios for these cells in the absence of ligand and in the presence of bivalent ligands of various spacer lengths. The panels in the second row reflect the saturability of the BRET ratios in the presence of the bivalent ligands that normally give a significant signal, that are reduced in the presence of competing monovalent CCK antagonist ligand 6. The panels in the third row demonstrate that monovalent ligands that recognize only the CCK2 receptor or the opioid receptor, even when mixed together, failed to produce the BRET signal observed with the bivalent ligand. The shaded area represents the non-specific BRET signal as described in Figure 2. Values represent means±S.E.M. of data from five independent experiments. * p<0.05, ** p<0.001.
Figure 4. Effects of ligands on receptor homodimerzation.
Shown are the BRET signals stimulated by bivalent or monovalent ligands in COS cells expressing Rlu- and YFP-tagged CCK2 receptor constructs or MOP receptor constructs or structurally-unrelated tagged receptor constructs. None of the ligands studied had any effect on the constitutive homodimers of the CCK2 or MOP receptors, and did not lead to heterodimerization of the unrelated receptors. Values represent means±S.E.M of data from six independent experiments. * p<0.05
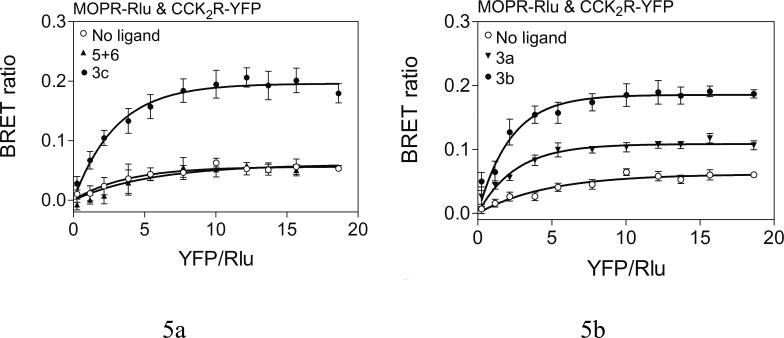
Saturation BRET assays were performed to distinguish specific molecular interactions from random interactions between donor and acceptor that can occur in a concentration-dependent manner. Figure 5 shows that COS cells expressing Rlu-tagged MOP receptor and YFP-tagged CCK2 receptor after transfection using a fixed amount of donor construct (1.0 μg DNA/dish) and increasing amounts of acceptor construct (0.3 μg to 6 μg DNA/dish). This produced the expected increase in BRET ratio that then reached a plateau for bivalent ligands 3b-c. These data support the saturable nature of these molecular interactions. The plateau of the BRET signal was typically reached with 1.5−2.0 μg acceptor DNA/dish, representing approximate molar ratios of acceptor:donor of 1.5−2.0:1. This supports the specific interactions between MOP and CCK2 receptors stimulated by the bivalent ligands that were suggested by the static BRET studies described above. The presence of the combination of monovalent ligands, 5 and 6, or the absence of ligand, yielded only a low level signal that was not saturable, with best fit of the data not different from a linear regression. These data are consistent with random interactions between these receptors taking place in the plasma membrane.
Figure 5. Effects of bivalent ligands on bystander BRET.
Shown are the saturation curves obtained by co-expressing pairs of Rlu-tagged MOP receptor and YFP-tagged CCK2 receptor constructs after incubation with bivalent ligands (10−6M), as indicated. Bivalent ligands 3a-c produced exponential curves that reached a plateau, supporting specific molecular interactions. Cells treated with either no ligand or with a combination of monovalent ligands for the CCK and mu-opioid receptors showed curves not significantly different from linear fits. Values represent means±S.E.M of data from five independent experiments.
Binding studies of the bivalent ligands were conducted in singly exspressed and coexpressed CHO cells. Only 3a and 3b shoved significantly higher affinity for CCK2 receptors in coexpressed cells relative to singly expressed cells, whereas little, if any affinity differences for MOP receptors were observed between singly and doubly expressed cells. The reason for these results is not clear, but one possibility is that the conditions employed in binding interfered with association of CCK2 and MOP receptors.
Given the results of the present study which demonstrate that MOP and CCK2 receptors do not constitutively form heterodimers, but can be induced to associate in the presence of appropriate bivalent ligands, we evaluated the functional properties of bivalent ligand 3b, and monovalent ligands 5, 6 in CHO cells coexpressed with MOP and CCK2 receptors using the calcium mobilization assay.36 The data (Table 2) revealed that 3b and the MOP receptor agonist 5 promoted Ca2+ release whereas the monovalent CCK antagonist 6 did not. Interestingly, both ligands were capable of activating opioid receptors, although 3b was 4.8-fold more potent than monovalent agonist 5 in this regard. These studies suggest that opioid receptor activation can occur in the induced, association state or as homomers.
Table 2.
Gαqi4myr-mediated Ca2+ release of CHO cells stably expressing MOP and CCK2 receptors.
| EC50 (nM) | ||||
|---|---|---|---|---|
| 3b | 5 | 6 | DAMGO | |
| MOPR-CCK2R CHO | 119 ± 75 (4) | 572 ± 71.1(3) | > 10000 (3) | 2.66 ± 0.70 (4) |
Experiments in mice were conducted to evaluate the tolerance of the bivalent ligands with 9-, 16-, 18- and 22-atom spacers 4, 3a-c relative to that of the control monovalent 5. These data are summarized in Table 3. With the exception of 5, none of the bivalents exhibited tolerance via the i.c.v. route. Given that bivalent ligand 4 with the shortest spacer did not show tolerance, and the quantitative BRET data suggesting the absence of bridging between MOP and CCK2 receptors for 4, there appears to be no relationship between ligand-induced association of receptors and blockage of tolerance.
Table 3.
Comparison of the ED50's (pmol/mouse) of bivalent ligands 3a-3c, 4, monovalent ligand 5 and simultaneous administration of monovalent ligands 5 and 6 ; a 24 hour tolerance study.
| 3a | 3b | 3c | 4 | 5 | 5+6 | |
|---|---|---|---|---|---|---|
| i.c.v. | 47.41 (37.19 − 60.44) |
390 (280 − 530) |
2020 (1420 − 2870) |
3036 (2390 − 3857) |
51.93 (39.00 − 69.14) |
96.29 (64.93 − 142.8) |
| Controla) | 83.86 ± 8.76 | 80.67 ± 9.79 | 83.93 ± 8.05 | 75.45 ± 9.84 | 70.61 ± 9.65 | 82.04 ± 12.08 |
| 24 hoursb),c) | 84.45 ± 7.81 | 71.29 ± 8.55 | 64.33 ± 13.10 | 57.31 ± 12.98 | 40.74 ± 11.86d) | 42.94 ± 13.40d) |
The control value represents the ED80 dose for each individual agonist on day one.
Twenty-four hours later the mice were first tested to see that the percent antinociception was back to baseline and then the ED80 dose was tested to see if there was any tolerance.
Peak time is between 10 and 20 minutes.
Tolerance formed.
DISCUSSION
Careful and systematic BRET analysis shows that MOP and CCK2 receptors coexpressed in COS cells are present as homodimers, with no evidence of spontaneous receptor heterodimerization. Evidently, the association of either MOP receptors or CCK2 receptors as homodimers is more favorable than their association to heterodimers. However, association of these receptors could be induced in the presence of bivalent ligands 3a-c that contain both mu-opioid agonist and CCK2 receptor antagonist pharmacophores connected through spacers whose length is in the range of 16 to 22 atoms. In this regard, the BRET signal for 3b-c plateaued close to a BRET ratio of ∼2, supporting specific saturable interaction between MOP and CCK2 receptors that was suggested by the BRET ratio enhancement. It is noteworthy that the BRET ratio plateau for 3a was approximately half that of 3b and 3c. This could reflect the low efficiency of induction of association by this ligand and different alignment of donor and acceptor fluorophores attached to their receptors as a consequence of this relatively short spacer. Significantly, the bivalent ligand 4 with a shortest spacer (9 atoms) as well as the simultaneous use of the monovalent ligands 5, 6 did not induce any recognizable association of MOP and CCK2 receptors. These data suggest that only bivalent ligands 3a-c were capable of inducing association because the spacers are of sufficient length to permit binding of each of the pharmacophores to their respective receptors through a bridging mechanism. The association of MOP and CCK2 receptors induced by 3a-c, as reflected by the heterologous receptor BRET ratios, appears to be most favorable with 3b which contains the 18-atom spacer. The finding that no significant difference of BRET ratio was observed with the monovalent ligands 5, 6 or bivalent ligand 4 in cells that contain either homodimerized MOP or CCK2 receptors indicates these ligands do not affect the degree of constitutive homodimerization.
In the presence of the CCK2 receptor monovalent antagonist ligand 6, the BRET ratios of bivalent ligands 3a-c were reduced. These data suggest that competition of 6 with the CCK2 receptor antagonist pharmacophore of the bivalent ligands involved in bridging associated MOP/CCK2 receptors, promotes a transition from the bridged binding mode to univalent binding. In the absence of constraints due to bridging, the heteromers dissociate and form the more stable homodimers.
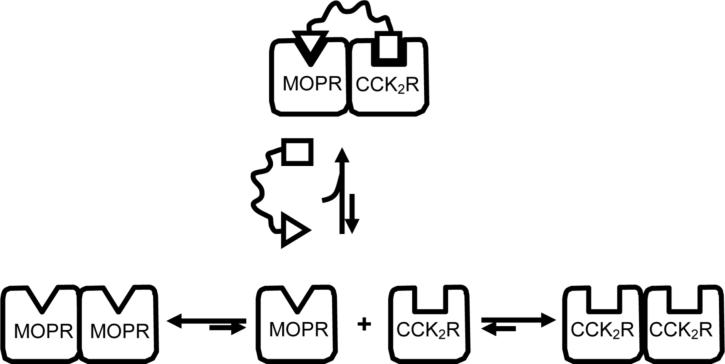
Based on the present work, a schematic for induction of association of MOP and CCK2 receptors is illustrated in Fig 6. Thus, GPCRs that do not easily associate to form heterodimers can be associated using an appropriately designed bivalent ligand. On the other hand, if coexpressed GPCRs exhibit greater ability to associate as heterodimers, as opposed to homodimers, it might be expected that they would exist as constitutive heterodimers. In this connection, the presence of constitutive heterodimers in cultured cells has been reported for a number of opioid 37, 38, 39 and CCK 40 receptors. Of significance is the finding that interaction of ligands that selectively target or activate putative heterodimers in vivo may have pharmacological profiles that differ from those that interact with homodimers. 30
Figure 6.
Schematic illustration of the effect of a MOP agonist/CCK2 antagonist bivalent ligand on the equilibrium between a mixture of MOP receptor (MOPR) and cholecystokinin receptors (CCK2R). Note that the bivalent ligand induces heterodimerization through constraint imposed by its spacer.
Because our results in cultured cells indicated the absence of constitutive association in MOP and CCK receptors (in contrast to literature41 which has reported numerous heterodimeric opioid receptors in cultured cells), thereby making it unlikely that this may be a mechanism for the modulation of morphine tolerance and potency, we decided to determine the i.c.v. ED50 values of the series in order to evaluate whether of not our ligands produce tolerance. The in vivo data (Table 3) revealed that only monovalent ligand 5 produced tolerance, either alone or in equimolar combination with monovalent CCK2 antagonist 6. On the other hand, none of the bivalent ligands 3a-c, 4 gave rise to significant tolerance, despite our finding that 4 does not induce association of MOP and CCK2 receptors in COS cells. Given our evidence for lack of constitutive association of MOP and CCK2 receptors in COS cells, it would be expected that 4 would produce tolerance in mice if a heterodimer were involved in the modulation process. When taken together with the results of the BRET data, the tolerance studies therefore suggest that the modulation of morphine's effects is possibly due to circuitry rather than to physical association of MOP and CCK2 receptors as heterodimers.
In conclusion, this study demonstrates proof of principle concerning the ability of bivalent ligands to induce association of receptors through a bridging mechanism. In this regard, it has not escaped our attention that other bivalent ligands21-25 whose pharmacologic profiles suggest bridging of dimeric opioid receptors has spacer lengths in the same range as the effective bivalent ligands in the present series. We believe this similarity may reflect a common structural organization of GPCR dimers. Thus, pharmacophores that bind within the helical bundle, and are linked by linear spacers greater than 16 atoms in length with optimal lengths in the range of 18 to 21 atoms, have been reported to most effectively bridge dimeric GPCRs.21-25
EXPERIMENTAL SECTION
Chemistry
General
Oxymorphone was obtained from Mallinckrodt & Co. All other chemicals and solvents were purchased from Aldrich or Fisher without further purification. Some dry solvents were made by MBRAUN MB-SPS Solvent Dispensing.
1H and 13C NMR spectroscopy were obtained on 300 MHz on an Oxford Varian VXR 300 MHz NMR Spectrometer. Polarities were obtained on AUTOPOL III Automatic Polarimeter. Mass spectroscopy was obtained on Bruker BioTOF II mass spectrometry.
Purities of the monovalent ligands 6 and bivalent ligands 3a-c and 4 were over 98% based on analysis on HPLC column (Phenomenex Luna SB-C18 (2) 5u 4.6×250mm) which was eluted with MeOH/Buffer (60:40) at a flow rate of 1 ml/min.
3(S)-(−)-Amino-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one (S(−)-7)
A solution of 7 (1.0 g, 3.9 mmol) in acetonitrile (4 mL) was treated with 1(S)-(+)-camphorsulfonic acid (0.91 g, 3.9 mmol) and diethyl ether (12 mL), and aged overnight at room temperature. The crystals were filtered and washed with acetonitrile/ether (1:3), yielding 0.92 g of solid as 3(S)-(−)-amine-1(S)-(+)-camphorsulfonate salt (98.1%). .
The salt (920 mg) was treated with 10% NaOH (6 mL) in CH2Cl2 (6 mL). The phases were separated, and the aqueous layer was extracted with CH2Cl2 (3 mL × 5). The organic layers were combined, dried over MgSO4 and concentrated by a rotary-evaporator to yield 459.4 mg (89.8%) of 3(S)-(−)-amine. , .31 1H NMR (CDCl3): δ 7.62−7.69 (m, ArH, 2H), 7.55−7.53 (m, ArH, 1H), 7.43−7.29 (m, ArH, 5H), 7.21−7.16 (m, ArH, 1H), 4.46 (s, CH, 1H), 3.45 (s, CH3, 3H), 2.75 (s, NH2, 2H). 13C NMR (CDCl3): δ 170.14, 165.85, 142.97, 138.03, 131.46, 130.29, 130.01, 129.50 (× 2), 129.04, 128.07 (× 2), 123.87, 121.14, 70.27, 35.27.
3(R)-(+)-Amino-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one (R(+)-7)
The Mother liquor from resolution of 7 with 1(S)-(+)-camphorsulfonic acid was concentrated by a rotary-evaporator. The residual was treated with 10% NaOH (30 mL) in CH2Cl2 (30 mL), and extracted with CH2Cl2 (10 mL × 5). The extracts were combined and concentrated by a rotary-evaporator. The residual was dissolved in acetonitrile (2.2 mL), treated with 1(R)-(−)-10-camphorsulfonic acid (0.49 g, 2.1 mmol) and diethyl ether (6.6 mL). The mixture was stored overnight at room temperature and the crystals were filtered and washed with cold acetonitrile/ether (1:3), yielding 0.81 g (86.4%) of solid as 3(R)-(+)-amine-1(R)-(−)-camphorsulfonate salt. .
The salt (810 mg) was treated with 10% NaOH (6 mL) in CH2Cl2 (6 mL). The phases were separated, and the aqueous layer was extracted with CH2Cl2 (3 mL × 5). The organic layers were combined, dried over MgSO4 and concentrated in a rotary-evaporator to yield 406 mg (97.3%) of 3(R)-(+)-amine. [α]22 = +262. 1H NMR (CDCl3): δ 7.62−7.60 (m, ArH, 2H), 7.56−7.53 (m, ArH, 1H), 7.44−7.30 (m, ArH, 5H), 7.21−7.17 (m, ArH, 1H), 4.46 (s, CH, 1H), 3.45 (s, CH3, 3H), 2.61 (s, NH2, 2H). 13C NMR (CDCl3): δ 170.20, 165.85, 143.01, 138.07, 131.49, 130.31, 130.03, 129.52 (× 2), 129.08, 128.10 (× 2), 123.89, 121.16, 70.32, 35.30.
1,3-Dihydro-3(R)-(+)-isocyanate-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one (8)
To the biphasic mixture of R(+)-7 (600 mg, 2.26 mmol) in CH2Cl2(10 mL)/NaHCO3 (saturated aqueous solution) cooled in an ice bath was added triphosgene (224 mg, 0.753 mmol). The reaction mixture was stirred in the ice bath for 20 min and then poured into a separator funnel. The organic layer was collected and the aqueous layer was extracted with CH2Cl2 (3 × 1.5 mL). The combined organic layers were dried over MgSO4 and concentrated in vacuum to give 553.9 mg (84.1%) of 8. 1H NMR (CDCl3): δ 7.67−7.4 (m, ArH, 9H), 4.94 (m, CH, 1H), 3.52 (s, CH3, 3H). MS 314.21 (M+ + Na).
N-Methyldiglycolamide (9)
To a cold (0 °C) 2M solution of methylamine in THF (10 mL, 20 mmol) was added commercially available diglycolic anhydride (2.32 g, 20 mmol). The reaction solution was stirred overnight at room temperature and concentrated by a rotary-evaporator. The residue was dried by a vacuum pump without further purification to give 2.82 g (95.8%) as an oil: 1H NMR (DMSO-d6): δ 12.76 (s, CO2H, 1H), 7.78 (s, NH, 1H), 4.07 (s, CH2, 2H), 3.93 (s, CH2, 2H), 2.61 (s, CH3, 3H). 13C NMR (DMSO-d6): δ 171.23, 168.95, 70.08, 67.75, 25.23.
N-(BOC-hexamethylamino)-N’-methyldiglycoldiamide (10)
To a solution (0 °C) of 9 (0.37 g, 2.5 mmol), DCC (0.57 g, 2.75 mmol) and HOBt (0.37 g, 2.75 mmol) in dry DMF (2 mL) was added a solution of mono-Boc protected hexamethylene amine (1,6) (0.54 g, 2.5 mmol) in DMF (1.5 mL). The reaction mixture was stirred for two days at room temperature and then the precipitate was filtered. The filtrate was concentrated and the residue was purified by flash chromatography (CH3OH/CH2Cl2/NH3H2O = 5:95:0.5) to give 0.42 g (61.0%) of 10: 1H NMR (CDCl3): δ 6.91 (s, CONH, 2H), 4.61 (s, CONH, 1H), 4.05 (s, diglCH2, 2H), 4.04 (s, diglCH2, 2H), 3.29 (m, hexCH2, 2H), 3.14 (m, hexCH2, 2H), 2.85 (m, CH3, 3H), 1.57−1.34 (m, hexCH2, 8H), 1.44 (s, CH3, 9H). MS 346.28 (M+ + H), 368.27 (M+ + Na).
N-(Aminomethyl)-2-(2-(methylamino)-2-oxoethoxy)acetamide (11)
N-(BOC-hexamethylamino)-N’-methyldiglycoldiamide 10 (0.4 g, 1.16 mmol) was dissolved in TFA/CH2Cl2 (1:1, 20 mL) and stirred at room temperature until no more starting materials could be detected on TLC. Solvent was evaporated and the residue was purified be flash chromatography (CH3OH/CH2Cl2/NH4OH) to give 100 mg (35.1%) of 11: 1H NMR (DMSO-d6): δ 8.03 (m, CONH, 2H), 7.71 (s br, CONH, 1H), 7.16 (t br, NH2, 2H), 3.89 (s, diglCH2, 4H), 3.09 (m, hexCH2, 2H), 2.75 (m, hexCH2, 2H), 2.63 (d, J = 7.8 Hz, CH3, 3H), 1.50 (m, hexCH2, 2H), 1.41 (m, hexCH2, 2H), 1.26 (m, hexCH2, 4H). MS 246.20 (M+ + H); 268.19 (M+ + Na).
2(-2-(3-Nitrophenoxyamino)-2-oxoethoxy)acetic acid (12)
To a suspension of diglycolic anhydride (5.8 g, 50 mmol) in dry CH2Cl2 (100 mL) was added a solution of commercially available 2-(3-nitrophenoxy)ethanamine (7.6 g, 55 mmol) in dry THF (100 mL) at 0 °C. The reaction mixture was stirred at room temperature overnight. Filtration of the precipitate gave 10.97 g (86.3%) of 12. 1H NMR (DMSO-d6): δ 12.87 (s br, CO2H, 1H), 10.37 (s, CONH, 1H), 8.67 (m, ArH, 1H), 8.01 (m, ArH, 1H), 7.92 (m, ArH, 1H), 7.61 (m, ArH, 1H), 4.22 (s, CH2, 4H), 4.21 (s, CH2, 4H). 13C NMR (DMSO-d3): δ 171.53, 168.56, 147.75, 139.40, 130.03, 125.51, 118.02, 113.55, 70.22, 67.97.
2-(2-Hydrazinyl-2-oxoethoxy)-N-(2(3nitrophenoxy)ethyl)acetamide (12a)
To a solution of 12 (149 mg, 0.5 mmol) in anhydrous DMF (1.5 mL) were added DCC (113.5 mg, 0.55 mmol), HOBt (148.6 mg, 1.1 mmol) and hydrazine hydrate (29 μL, 0.6 mmol) at room temperature. The reaction mixture was stirred 24 h at room temperature and the precipitate was removed by filtration. The filtrate was concentrated and the residual was purified by flash chromatography (CH2Cl2/MeOH/NH4OH = 85:14.5: 0.5) and gave 54 mg (34.6%) of 12a. 1H NMR (CDCl3): δ 7.86 (dd, J = 8.1, 2.1 Hz, 1H), 7.75 (dd, J = 2.1, 2.1 Hz, 1H), 7.68 (s, 1H), 7.46 (dd, J = 8.1, 8.1 Hz, 1H), 7.27 (dd, J = 8.1, 21 Hz, 1H), 6.95 (m, 1H), 4.17 (t, J = 5.4, 2H), 4.15 (s, 2H), 4.09 (s, 2H), 3.78 (dt, J = 5.4, 2H), 1.72 (s br, 2H). MS 335.32 (M+ + Na).
N-Methyl-2-(13-(3-nitrophenoxy)-2,6,10-trioxo-8-oxa-3,5,11-triazatridecyloxy)acetamide (13)
To a solution of 12 (0.714 g, 2.81 mmol), DCC (0.58 g, 2.81 mmol) and HOBt (0.38 g, 2.81 mmol) in dry DMF (2.5 mL) was added a 2.5 mL dry DMF solution of 11 (0.625 g, 2.55 mmol). The reaction mixture was stirred overnight at room temperature and the precipitate was filtered. The filtrate was concentrated in a rotary-evaporator and purified by flash chromatography (CH2Cl2/CH3OH/NH3H2O = 90:9.5:0.5) to give 0.48 g (39.1%) of 13: 1H NMR (DMSO-d6): δ 10.53 (s, CONH, 1H), 8.67 (m, CONH, 1H), 8.08 (m, ArH, 1H), 8.00 (m, ArH+CONH, 3H), 7.94 (m, ArH, 1H), 7.62 (m, ArH, 1H), 4.18 (s, CH2, 2H), 4.05 (s, CH2, 2H), 3.89 (s, CH2, 4H), 3.10 (m, CH2, 4H), 2.63 (d, J = 4.8 Hz, CH3, 3H), 1.42 (m, CH2, 4H), 1.25 (m, CH2, 4H). MS 482.4 (M+ + H); 503.3 (M+ + Na).
2-(13-(3-Aminophenoxy)-2,6,10-trioxo-8-oxa-3,5,11-triazatridecyloxy)-N-methylacetamide (14)
A mixture of 13 (90 mg, 0.171 mmol) and Pd/C of 10 wt % (30 mg) in MeOH (5 mL) was hydrogenated 50 psi for 4 h at room temperature. The catalyst was removed by filtration over a celite pad and the filtrate was concentrated by a rotary-evaporator to give 75 mg (88.5%) of product 14. 1H NMR (DMSO-d3): δ 8.21 (m, CONH, 1H), 8.01 (m, CONH, 3H), 6.86 (t, J = 8.1 Hz, ArH, 1H), 6.14−6.11 (m, ArH, 2H), 6.06 (m, ArH, 1H), 5.02 (m, NH2, 1H), 3.94 (s, CH2, 2H), 3.91 (s, CH2, 2H), 3.89 (m, CH2, 6H), 3.45 (m, CH2, 2H), 3.09 (m, CH2, 4H), 2.63 (d, J = 4.5 Hz, CH3, 3H), 1.41 (m, CH2, 4H), 1.24 (m, CH2, 4H). MS 496.61 (M+ + H), 518.59 (M+ + Na).
(R)-Z-N-Methyl-2-(13-(3-(3-(1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)ureido)phenoxy)-2,6,10-trioxo-8-oxa-3,5,11-triazatridecyloxy)acetamide (6)
To a solution of 14 (75 mg, 0.151 mmol) and isocyanate derivative 8 (71.7 mg, 0.166 mmol) in anhydrous DMF (4 mL) was added triethylamine (23.2 μL, 0.166 mmol). The reaction solution was stirred at room temperature for 5 days and concentrated. The residue was purified by flash chromatography to give 23 mg (19.4) of product 6. 1H NMR (CDCl3): δ 8.11 (m, CONH, 1H), 7.59 (m, CONH, 3H), 7.37 (m, ArH, 5H), 7.17−6.91 (m, ArH, 6H), 6.52 (m, CONH, 1H), 5.49 (d, J = 7.8 Hz, CH, 1H), 5.02 (m, CONH, 1H), 4.04 (s, CH2, 2H), 4.01 (m, CH2, 6H), 3.98 (s, CH2, 2H), 3.65 (m, CH2, 2H), 3.46 (s, CH3, 3H), 3.22 (m, CH2, 4H), 2.77 (d, J = 4.5 Hz, CH3, 3H), 1.42 (m, CH2, 4H), 1.23 (m, CH2, 4H). MS 787.61 (M+ + H), 809.65 (M+ + Na).
Oxymorphyldiglycolamide (16)
A suspension of diglycolic anhydride (0.645 g, 5.56 mmol) in anhydrous CH2Cl2 (20 mL) was added dropwise to oxymorphamine 15 (1.6 g, 5.29 mmol) in anhydrous CH2Cl2 (10 mL) under N2. The mixture was stirred at room temperature overnight. The precipitate was collected by suction filtration and washed with CH2Cl2 to give 2.20 g (99.4%) of product 16. 1H NMR (DMSO-d3): δ 8.75 (m, CONH, 1H), 6.59 (d, J = 7.8 Hz, ArH, 1H), 6.51 (d, J = 7.8 Hz, ArH, 1H), 4.45 (m, 1H), 4.32 (m, 1H), 3.92 (m, 4H), 3.16 (s, 1H), 2.72 (m, 1H), 2.55 (s, 2H), 2.42−2.25 (m, 5H), 1.69−1.61 (m, 1H), 1.49 (m, 1H), 1.33 (m, 2H), 0.88 (m, 1H). MS 417.14 (M− - H).
[N-(m-Nitrophenoxy)ethyldiglycolamidehydrazide-N’-oxymorphyldiglycolamide]dihydrazide (19a)
To a solution of 16 (69.7 mg, 0.167 mmol) in anhydrous DMSO (0.5 mL) were added DCC (37.8 mg, 0.183 mmol), HOBt (24.8 mg, 0.183 mmol) and a solution of 12a (52 mg, 0.167 mmol) in anhydrous DMSO (0.5 mL) at room temperature. The mixture was stirred for 24 h at room temperature and the precipitate was removed by filtration. The filtrate was concentrated and the residual was purified by flash chromatography (CH2Cl2/MeOH/NH4OH = 90:9.5: 0.5) to give 70 mg (58.8%) of 19a. 1H NMR (CDCl3): δ 7.80 (d, J = 8.1, 1H), 7.70 (m, 1H), 7.41 (t, J = 8.1 Hz, 2H), 7.21 (m, 2H), 6.69 (d, J = 8.1, 1H), 6.52 (d, J = 8.1 Hz, 1H), 4.58 (s, 2H), 4.23−4.10 (m, 8H), 3.75 (m, 2H), 3.10 (d, J = 18.6 Hz, 1H), 2.77 (m, 1H), 2.62−251 (m, 6H), 2.38 (m, 1H), 2.34 (s, 3H), 2.22 (m, 2H), 1.77−1.34 (m, 6H), 1.03 (m, 1H). MS 713.84 (M+ + H).
1-(N-Oxymorphyldiglycoldiamide)-2-[1-diglycoldiamide-2-(m-nitrophenoxy)ethane]ethane (19b)
To a solution of 12 (141 mg, 0.473 mmol) and 18b (198 mg, 0.43 mmol) in anhydrous DMSO (2.5 mL) were added DCC (97.6 mg, 0.473 mmol) and HOBt (64 mg, 0.473 mmol). The reaction solution was stirred overnight at room temperature and concentrated. The residue was purified by a flash chromatography (CH2Cl2/CH3OH/NH3H2O = 90:10:1) to give 220 mg (69.1%) of 19b. 1H NMR (CDCl3): δ 7.83 (d, J = 8.1 Hz, 1H), 7.74 (m, 1H), 7.54 (t, J = 6.0 Hz, 1H), 7.44 (m, 1H), 7.35−7.21 (m, 2H), 6.99 (d, J = 8.4 Hz, 1H), 6.70 (d, J = 8.4 Hz, 1H), 6.55 (d, J = 8.4 Hz, 1H), 4.58−4.53 (m, 2H), 4.19 (t, J = 5.7 Hz, 2H), 4.07 (m, 8H), 3.76 (q, J = 5.7 Hz, 2H), 3.61−3.49 (m, 4H), 3.13 (d, J = 18.0 Hz, 1H), 2.80 (m, 1H), 2.69−2.54 (m, 5H), 2.41 (m, 1H), 2.35 (s, 3H), 2.25 (m, 2H), 1.83−1.69 (m, 8H), 1.55 (d, J = 9.6 Hz, 1H), 1.44−1.36 (m, 1H), 1.25 (s, 1H), 1.03−0.88 (m, 1H). MS 741.24 (M+ + H).
1-(N-Oxymorphyldiglycoldiamide)-6-[1-diglycoldiamide-2-(m-nitrophenoxy)ethane]hexane (19c)
To an ice-cold solution of 12 (109 mg, 0.366 mmol), DCC (75.4 mg, 0.366 mmol) and HOBt (49.4 mg, 0.366 mmol) in dry DMF (1 mL) was added a solution of 18c (171.7 mg, 0.332 mmol) in dry DMF (1.5 mL). The reaction mixture was stirred overnight at room temperature. The precipitate was filtered and the filtrate was concentrated by a rotary-evaporator. The residue was purified by a flash chromatography (CH2Cl2/CH3OH/NH3H2O = 90:10:1) to give 255 mg (87.4%) of 19c. 1H NMR (CDCl3): δ 7.84 (d, J = 7.8 Hz, 1H), 7.72 (m, 1H), 7.46 (t, J = 7.8 Hz, 1H), 7.27 (m, 1H), 7.16 (m, 1H), 6.74(m, 2H), 6.54 (d, J = 7.2 Hz), 4.59 (m, 2H), 4.16 (m, 2H), 4.08 (m, 4H), 4.05 (m, 4H), 3.76 (m, 2H), 3.33 (m, 3H), 2.62 (s, 8H), 2.35 (s, 3H), 2.25 (m, 2H), 1.71 (m, 5H), 1.56 (m, 3H), 1.36 (m, 4H), 1.01 (m, 1H). MS 797.62 (M+ + H), 819.60 (M+ + Na).
N-(m-Aminophenoxy)ethyldiglycolamidehydrazide-N’-oxymorphyldiglycolamidedihydrazide (20a)
A mixture of 19a (66 mg, 0.093 mmol) and Pd/C of 10 wt % (15 mg) in MeOH (4 mL) was hydrogenated at 50 psi for 4 h at room temperature. The catalyst was removed by filtration over a celite pad and the filtrate was concentrated in a rotary-evaporator to give 60 mg (94.5%) of product 20a. 1H NMR (CDCl3): δ 8.03 (s br, 1H), 7.52 (s br, 1H), 6.99 (dd, J = 7.8 Hz, 1H), 6.69 (d, J = 7.8, Hz, 1H), 6.53 (d, J = 7.8 Hz, 1H), 6.26 (m, 2H), 4.57 (s, 2H), 4.18−4.01 (m, 8H), 3.63 (m, 2H), 3.12 (d, J = 18.3 Hz, 1H), 2.73−2.55 (m, 11H), 2.40 (m, 1H), 2.35 (s, 3H), 2.28−2.18 (m, 2H), 1.81−1.70 (m, 1H), 1.55 (m, 2H), 1.44−1.36 (m, 1H), 1.04 (m, 1H). MS 683.35 (M+ + H).
1-(N-Oxymorphyldiglycoldiamide)-2-[1-diglycoldiamide-2-(m-aminophenoxy)ethane]ethane (20b)
A mixture of 19b (120 mg, 0.162 mmol) and Pd/C of 10 wt % (30 mg) in MeOH (6.5 mL) was hydrogenated at 50 psi for 4 h at room temperature . The catalyst was removed by suction filtration and the filtrate was concentrated by a rotary-evaporator to give 100 mg (86.8%) of product 20b. 1H NMR (CDCl3): δ 7.58 (m, 1H), 7.48 (t, J = 5.7 Hz, 1H), 7.39 (m, 1H), 7.10−7.06 (m, 1H), 7.02 (d, J = 8.1 Hz, 1H), 6.70 (d, J = 8.1 Hz, 1H), 6.54 (d, J = 8.1 Hz, 1H), 6.31−6.23 (m, 3H), 4.59−4.52 (m, 2H), 4.08−3.97 (m, 8H), 3.88 (m, 2H), 3.68 (q, J = 5.4 Hz, 2H), 3.49 (s, 6H), 3.12 (d, J = 18.6 Hz, 1H), 2.78 (d, J = 6.0 Hz, 1H), 2.69−2.53 (m, 10H), 2.40 (m, 1H), 2.35 (s, 3H), 2.28−2.21 (m, 2H), 1.92−1.36 (m, 6H), 1.25 (m, 1H), 1.00 (m, 1H). MS 711.21 (M+ + H).
1-(N-Oxymorphyldiglycoldiamide)-6-[1-diglycoldiamide-2-(m-aminophenoxy)ethane]hexane (20c)
A mixture of 19c (250 mg, 0.314 mmol) and Pd/C of 10 wt % (80 mg) in MeOH (13 mL) was hydrogenated at 50 psi for 4 h at room temperature. The catalyst was removed by filtration over a celite pad and the filtrate was concentrated in a rotary-evaporator to give 110 mg (45.7%) of product 20c. 1H NMR (d6−DMSO): δ 8.21 (m, 1H), 8.03 (m, 2H), 7.57 (m, 1H), 6.86 (t, J = 7.5 Hz, 1H), 6.56 (d, J = 8.1 Hz, 1H), 6.46 (d, J = 8.1 Hz, 1H), 6.11−6.04 (m, 3H), 5.02 (m, 2H), 4.78 (s, 1H), 4.44 (m, 1H), 3.93−3.88 (m, 8H), 3.44 (q, J = 6.0 Hz, 2H), 3.32 (s, 2H), 3.06 (m, 4H), 2.71 (d, J = 6.0 Hz, 1H), 2.52 (m, 6H), 2.36 (m, 1H), 2.27 (s, 3H), 2.13 (m, 2H), 1.56 (m, 1H), 1.40 (m, 5H), 1.23 (m, 3H), 0.92 (m, 1H). MS 767.69 (M+ + H), 789.68 (M+ + Na).
N-{m-[R(+)-1]Ureaphenoxy}ethyldiglycolamidehydrazide-N’-oxymorphyldiglycolamidedihydrazide (3a)
To a solution of 20a (57 mg, 0.0835 mmol) in anhydrous DMSO (0.5 mL) was added a solution of 8 (26.8 mg, 0.0916 mmol) in anhydrous DMSO (0.5 mL). The reaction solution was stirred at room temperature for 24 h and concentrated. The residue was purified by flash chromatography (CH2Cl2/MeOH/NH4OH = 85/15/1.5) to give 10 mg (12.3%) of product 3a. 1H NMR (CDCl3): δ 7.65−7.33 (m, 10H), 7.30−7.22 (m, 2H), 6.97 (dd, J = 7.8 Hz, 1H), 6.89 (d, J = 7.8, Hz, 1H), 6.66 (d, J = 7.8 Hz, 1H), 6.31−6.23 (m, 2H), 5.29 (d, J = 6.0 Hz, 1H), 4.58 (m, 2H), 4.17−3.96 (m, 8H), 3.91−3.75 (m, 3H), 3.61 (m, 3H), 3.49 (s, 3H), 3.16 (d, J = 18.9 Hz, 1H), 2.81 (m, 1H), 2.41 (s, 3H), 2.22 (m, 2H), 1.97−1.67 (m, 4H), 1.59−1.48 (m, 2H), 1.41−1.38 (m, 2H), 1.00 (m, 1H). MS 974.36 (M+ + H).
1-{1-Diglycoldiamide-2-[m-(R(+)-1)ureaphenoxy]ethane}-2-(N-oxymorphyldiglycoldiamide)ethane (3b)
To a solution of 20b (95.7 mg, 0.135 mmol) in anhydrous DMSO (2 mL) was added a solution of 8 (39.2 mg, 0.135 mmol) in anhydrous THF (0.5 mL). The reaction solution was stirred at room temperature for 24 h and concentrated. The residue was purified by flash chromatography (CH2Cl2/MeOH/NH4OH = 90/10/1) to give 24.9 mg (18.4%) of product 3b. 1H NMR (CDCl3): δ 8.10 (s, 1H), 7.61−7.52 (m, 3H), 7.49−7.42 (m, 2H), 7.39−7.32 (m, 2H), 7.26−7.15 (m, 4H), 6.98 (d, J = 9.0 Hz, 1H), 6.92 (m, 1H), 6.71−6.61 (m, 2H), 6.53−6.50 (m, 2H), 5.50 (d, J = 7.8 Hz, 1H), 5.30 (s, 2H), 4.74−4.64 (m, 2H), 4.54−4.49 (m, 2H), 4.10−4.03 (m, 8H), 3.96 (m, 2H), 3.67 (m, 2H), 3.46 (m, 3H), 3.44 (s, 3H), 3.15−3.08 (m, 2H), 2.84−2.76 (m, 2H), 2.65−2.51 (m, 2H), 2.46−2.18 (m, 4H), 2.34 (s, 3H), 1.88−1.36 (m, 9H), 1.25 (s, 1H), 1.02−0.86 (m, 2H). MS 1002.20 (M+ + H).
1-{1-Diglycoldiamide-6-[m-(R(+)-1)ureaphenoxy]ethane}-2-(N-oxymorphyldiglycoldiamide)hexane (3c)
To a solution of 20c (194.8 mg, 0.254 mmol) and 8 (120 mg, 0.279 mmol) in anhydrous DMSO (5 mL) was added triethylamine (50 μL, 0.279 mmol). The reaction solution was stirred at room temperature for 6 h and concentrated. The residue was purified by flash chromatography (CH2Cl2/MeOH/NH4OH = 90/10/1) to give 21.1 mg (7.9%) of product 3c. 1H NMR (CDCl3): δ 7.65−7.58 (m, 2H), 7.50−7.32 (m, 6H), 7.24−7.13 (m, 3H), 7.02 (t, J = 7.8 Hz, 1H), 6.92 (d, J = 8.1 Hz, 1H), 6.81−6.72 (m, 2H), 6.67 (d, J = 8.1 Hz, 1H), 6.29−6.20 (m, 2H), 5.30 (d, J = 7.8 Hz, 1H), 4.58 (d, J = 4.2 Hz, 1H), 4.43 (m, 1H), 4.06−3.99 (m, 8H), 3.72−3.55 (m, 3H), 3.49 (s, 3H), 3.25−3.08 (m, 4H), 2.81 (m, 1H), 2.62 (s, 4H), 2.35 (s, 3H), 2.24 (m, 2H), 1.88−1.56 (m, 8H), 1.41 (m, 6H), 1.25 (m, 6H), 0.95 (m, 1H). MS 1058.44 (M+ + H), 1080.42 (M+ + Na).
N-(m-Nitrophenoxy)ethyl-N’-oxymorphyldiglycoldiamide (22)
To a solution of 16 (209 mg, 0.5 mmol) in anhydrous DMSO (1.5 mL) were added DCC (113 mg, 0.55 mmol) and HOBt (74.3 mg, 0.55 mmol) and the solution was stirred for 5 min. A solution of 1-amino-2-(3-nitro=phenoxy) ethane 21 (100 mg, 0.55 mmol) in anhydrous DMSO (1.0 mL) was added to the reaction solution and the reaction mixture was continued stirring overnight at room temperature. The precipitate was removed by filtration and the residue was purified by flash chromatography (CH2Cl2/MeOH/NH4OH=92:7.5:0.5) to give product 22 as oil (223.7 mg). 1H NMR (CDCl3): δ 7.84−7.80 (m, 1H), 7.70 (m, 1H), 7.42 (t, J = 8.4 Hz, 1H), 7.22−7.19 (m, 2H), 6.97 (m, 1H), 6.71 (d, J = 8.4 Hz, 1H), 6.56 (d, J = 8.4 Hz, 1H), 4.60−4.55 (m, 2H), 4.18−4.14 (m, 4H), 4.07 (d, J = 6.6 Hz, 1H), 3.84−3.77 (m, 2H), 3.12 (m, 1H), 2.84−2.68 (m, 2H), 2.40 (m, 1H), 2.34 (s, 3H), 2.27−2.21 (m, 1H), 1.79−1.52 (m, 6H), 1.37−1.25 (m, 2H), 0.92 (m, 1H). MS 583.14 (M+ + H).
N-(m-Aminophenoxy)ethyl-N’-oxymorphyldiglycoldiamide (23)
A mixture of 22 (220 mg, 0.338 mmol) and Pd/C (10 wt %, 72 mg) in MeOH (13 mL) was hydrogenated at room temperature and 50 psi for 4 h. The catalyst was removed by filtration over a celite pad and the filtrate was concentrated in a rotary-evaporator to give 196 mg of product 23. 1H NMR (DMSO-d3): δ 8.25 (s br, 1H), 7.57 (m, 1H), 6.85 (t, J = 8.4 Hz, 1H), 6.56 (d, J = 8.4 Hz, 1H), 6.45 (d, J = 8.4 Hz, 1H), 6.12−6.03 (m, 3H), 5.01 (s, 2H), 4.78 (s, 1H), 4.44 (m, 1H), 4.37−4.30 (m, 1H), 3.98 (m, 4H), 3.87 (t, J = 6.0 Hz, 2H), 3.43 (q, J = 6.0 Hz, 2H), 3.02 (d, J = 18.6 Hz, 1H), 2.53 (m, 3H), 2.37−2.32 (m, 1H), 2.27 (m, 3H), 2.17−2.10 (m, 2H), 1.58−1.51 (m, 1H), 1.33 (m, 3H), 0.92 (m, 1H). MS 553.20 (M+ + H).
N-{m-[R(+)-1]ureaphenoxy}ethyl-N’-oxymorphyldiglycoldiamide (4)
To a solution of 23 (193 mg, 0.349 mmol) in anhydrous DMSO (4.5 mL) was added a solution of 8 (96.6 mg, 0.332 mmol) in anhydrous DMSO (2 mL). The reaction solution was stirred at room temperature for 24 h and concentrated. The residue was purified by flash chromatography (CH2Cl2/MeOH/NH4OH=90:9:1) to give 21 mg (7.5%) of 4 as a solid. 1H NMR (CDCl3): δ 7.60 (m, 4H), 7.47−7.20 (m, 8H), 7,00 (m, 2H), 6.75 (m, 1H), 6.65 (d, J = 8.1 Hz, 1H), 6.23 (d, J = 8.1 Hz, 1H), 6.13 (m, 2H), 5.34 (d, J = 8.1 1H), 4.56 (m, 1H), 4.39 (m, 1H), 4.03 (m, 1H), 3.88 (t, J = 5.4 Hz, 1H), 3.77−3.63 (m, 2H), 3.58 (s, 1H), 3.48 (m, 3H), 3.44−3.39 (m, 2H), 3.14 (d, J = 18.9 Hz, 1H), 2.78 (m, 1H), 2.67−2.58 (m, 1H), 2.41 (m, 1H), 2.34 (s, 3H), 2.25−2.17 (m, 2H), 1.71−1.50 (m, 6H), 1.38−1.20 (m, 3H), 0.89 (m, 1H). MS 844.34 (M+ + H).
Biology
1. In Vitro Studies
1.1 Preparation of receptor constructs
Type 2 cholecystokinin (CCK2) and MOP receptor constructs tagged at their carboxyl-terminal ends with either Renilla luciferase (Rlu) or yellow fluorescent protein (YFP) were used as donor and acceptor, respectively, for BRET studies. We previously characterized the Rlu- and YFP-tagged CCK2 receptor constructs expressed in COS cells, demonstrating that their binding characteristics and biological activities are similar to those of wild type CCK2 receptor.34 The Rlu- and YFP-tagged MOP receptor constructs were prepared utilizing the Rlu and YFP sequences present in pBRET and pEYFP-N1 (Stratagene, La Jolla, CA), and cloning these in frame at the 3’- end of the receptor sequence into NheI/XbaI sites of pcDNA3.0 vector (Invitrogen, Carlsbad, CA). All sequences were verified by direct DNA sequencing.
1.2 Receptor-bearing cells
Chinese Hamster Ovary (CHO-K1) cells stably expressing the CCK2 receptors, the MOP receptors, and cells coexpressing both of these receptors were prepared and used as sources of receptor-enriched plasma membranes for receptor binding studies. CHO cells obtained from the American Type Culture Collection (Manassas, VA) and receptor-expressing CHO cell lines were grown in Ham's F-12 nutrient mixture containing 5% (v/v) Fetal Clone II supplement (Hyclone laboratories, Logan, UT) in a 5% CO2 environment. Cells were passaged approximately twice per week. Cell lines stably expressing receptor constructs were prepared by transfection with appropriate cDNA. Cells were selected with 1 mg/ml G-418 or 0.5 mg/mL zeocin, and then were subjected to a series of standard limiting dilutions, followed by screening using radioligand binding to assess receptor expression.
COS cells transiently expressing receptor constructs were used for receptor BRET studies. These cells were plated in 10 cm tissue culture dishes at a density of 0.5 million cells/dish and were propagated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5 % Fetal Clone II. Cells were transfected 48 h before use with approximately 3 μg of total DNA (either a single construct or a combination of two constructs (donor and acceptor)) per dish using the DEAE-dextran method. 42
1.3 CCK receptor binding
Receptor-enriched membranes were isolated from CHO cell lines using the previously-established density gradient centrifugation procedure. 43 The membranes were suspended in Krebs-Ringers-HEPES (KRH) medium (25 mM HEPES, pH 7.4, 104 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM KH2PO4, 1.2 mM MgSO4) containing 0.01 % soybean trypsin inhibitor and 1 mM phenylmethylsulfonyl fluoride and were stored at −80 °C until use. Radioligand binding studies were performed as described previously. 44 Receptor preparations were mixed with a constant amount of CCK-like radioligand, 125I-D-Tyr-Gly-[(Nle28,31)CCK-26−33] (1−5 pM) (specific radioactivity 2000 Ci/mmol), in the absence or presence of increasing concentrations of unlabeled ligand for 90 min at room temperature in KRH medium, pH 7.4, containing 0.01 % soybean trypsin inhibitor and 0.2 % bovine serum albumin. Receptor-bound and free radioligand were separated using a Skatron cell harvester (Molecular Devices, Sunnyvale, CA) with receptor-binding filtermats. Receptor-bound radioactivity was quantified using a γ spectrometer. Non-saturable binding determined in the presence of 1 μM CCK was consistently less than 10% of total binding. Saturable radioligand binding data were analyzed using the LIGAND program45 and were plotted using the nonlinear least-squares curve-fitting routine in Prism (GraphPad 3.0, San Diego, CA).
1.4 MOP receptor binding
Opioid receptor binding was performed with membranes isolated from CHO cells. Receptor-enriched membranes were mixed with a constant amount of specific MOP radioligand [3H]DAMGO (0.4nM) (specific radioactivity 56.8 Ci/mmol) (Perkin Elmer, Boston, MA ) in the absence or presence of increasing concentrations of unlabeled ligand for 2 h at room temperature in KRH medium, pH 7.4, containing 0.01 % soybean trypsin inhibitor and 0.2 % bovine serum albumin. The non-specific binding was determined in the presence of 1 μM DAMGO (Bachem, Torrace, CA), an agonist ligand for the MOP receptor. Receptor-bound and free radioligand were separated by rapid filtration using Whatman GF/C glass microfiber filters (Whatman Inc, Florham Park, NJ) that were presoaked in 0.3% polyethylenimine (Sigma, St Louis, MO). Receptor-bound radioactivity was quantified in a Beckman-Coulter LS6500 multipurpose scintillation counter. Saturable radioligand binding data were analyzed using the LIGAND program45 and were plotted using the nonlinear least-squares curve-fitting routine in Prism (GraphPad 3.0, San Diego, CA).
Values are expressed as means±S.E.M. of data from 3−5 independent experiments.
1.5 BRET studies
Bioluminescence and fluorescence measurements were performed with aliquots of approximately 25,000 receptor-bearing COS cells in suspension, as described previously.44 For this, the cells were lifted using non-enzymatic cell dissociation solution (Sigma) 48 h after transient transfection, and they were washed with KRH medium. The BRET assay was initiated by adding the cell-permeant Renilla luciferase-specific substrate, coelenterazine h (Biotium, Hayward, CA), to the cell suspension to yield a final concentration of 5 μM in a 96-well white Optiplate (PerkinElmer, Boston, MA). The BRET signal was collected by using the protocol designed for BRET studies in the 2103 Envision fluorescence plate reader (Perkin-Elmer, Boston, MA), and the BRET ratio was calculated based on emission ratios. For ligand effects, the transfected COS cells were incubated with specified ligands (1 μM) for 90 min at room temperature before performing the assay. Saturation BRET experiments were performed as described previously.44 COS cells were transiently transfected with a fixed amount of Rlu-tagged mu-opioid receptor construct (1.0 μg DNA/dish) and with increasing concentrations of YFP-tagged CCK2 receptor construct (0.3 μg to 6 μg DNA/dish). Curves were fit to these data and evaluated for quality-of-fit based on R2 values using Prism 3.0, and the BRET50 and BRETmax values were calculated.
1.6 Calcium mobilization36
Calcium mobilization in MOPR-CCK2R co-expressing CHO cells. Stably expressing CHO CCK2R cells were grown to 90% confluent in HAM F-12 nutrient media plus 10% bovine calf serum and 1 % penicillin/streptomycin in 75 mm2 tissue culture plates at 37 °C, were transiently transfected with MOPR construct and chimeric G-protein (Gqi4-myr) construct using lipofectamine 2000 transfection reagent and manufactor's protocol (GIBCO). Ratios were 20 μg MOPR construct, 24 μg Gqi4-myr construct to 80 mL lipofectamine reagent. Cells were incubated for 24 hrs after transfection, seeded into 96-well plates (Corning 96-well plates half area, black with clear bottom) and incubated overnight before experiments were conducted. Calcium mobilization was measured on a FlexStation3 using a FLIPR calcium kit (Molecular Devices) and analyzed using SoftMax Pro and PRISM software.
2. In Vivo studies
2.1 Materials and Methods
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Minnesota (Minneapolis, MN).
2.2 Subjects
Male ICR mice (17 − 25g; Harlan, Madison, WI), were housed in groups of 8 in a temperature- and humidity-controlled environment with unlimited access to food and water. They were maintained on a 12-h light/dark cycle.
2.3 Tail flick Assay
The tail flick assay was used to test for antinociception described by D'Amour and Smith 46 and modified by Dewey et. al. 47. For the measurement the latency of the tail-flick measurement, the mice were held gently in one hand with the tail positioned in the apparatus (Tail Flick Analgesia Meter, Columbus Instruments, Columbus, Ohio) for radiant heat stimulus. The tail-flick response was elicited by applying radiant heat to the dorsal side of the tail. The intensity of the heat was set at setting 8 so that the animal flicked its tail within 2 to 3 s. The test latency was measured once before drug treatment (control) and again after the drug treatment (test) at the peak time of the compound, a 10s maximum cut-off time was used to prevent damage to the tail. Antinociception was quantified according to the method of Harris and Pierson48 as the percent maximal possible effect (%MPE) which is calculated as:
At least three groups of eight to ten mice were used for each dose response curve, and each mouse was used only once. ED50 values with 95% confidence intervals (C.I.) were computed with GraphPad Prism 4 by using nonlinear regression methods.
2.4 Experimental Design
All the compounds were dissolved in 10% DMSO and then diluted to less than 1% DMSO in the test solutions. Controls given i.c.v. with 1% or less DMSO did not show any antinociception. All drugs were administered in a 5-μL volume in conscious mice according to the method of Haley and McCormick49 for i.c.v. injections. A time course study, including the times 10, 20, 30 and 60 minutes was used to determine the peak antinociception. List of compounds and peak times are summarized in the Table 3. A 24 h tolerance was measured with each compound using the ED80 − 90 doses measured on day1 and then repeated 24 h later on the same mouse.
Figure 1.
Opioid agonist 1 and CCK2 receptor antagonist 2 pharmacophores and related bivalent 3a-c, 4 and monovalent 5, 6 ligands.
Table 1.
Binding of DAMGO, 2 and bivalent ligands 3a-3c and monovalent ligands 4 and 6 to singly and co-expressed CCK2, MOP receptors in CHO cells.
| Ligands | 125-I-CCK Ki (nM) | Bmax pmoles/mg protein | 3H-DAMGO Ki (nM) | Bmax pmoles/mg protein |
|---|---|---|---|---|
| Cells expressing CCK2 receptor | ||||
| 2 | 18 ± 3 | 14.4 ± 3.4 | - | - |
| 3a | 356 ± 67 | 16.0 ± 3.7 | - | - |
| 3b | 71 ± 5 | 10.3 ± 1.8 | - | - |
| 3c | 260 ± 90 | 18.5 ± 1.7 | - | - |
| 4 | 223 ± 11 | 14.0 ± 3.9 | - | - |
| 6 | 48± 6 | 16.0 ± 1.1 | - | - |
| Cells expressing MOP receptor | ||||
| DAMGO | - | - | 6.7 ± 2.9 | 9.2 ± 2.7 |
| 3a | - | - | 8.3 ± 2.4 | 9.8 ± 3.5 |
| 3b | - | - | 5.7 ± 1.7 | 7.7 ± 1.9 |
| 3c | - | - | 29.4 ± 2.1 | 7.4 ± 0.5 |
| 4 | - | - | 24.1 ± 0.7 | 12.4 ± 1.9 |
| 5 | - | - | 29.8 ± 10.2 | 9.3 ± 1.4 |
| Cells expressing CCK2 receptor/MOP receptor | ||||
| DAMGO | - | - | 11.0 ± 6.2 | 9.5 ± 3.3 |
| 2 | 14 ± 2 | 9.1 ± 1.7 | ||
| 3a | 57 ± 10 | 9.5 ± 1.9 | 3.9 ± 2.1 | 8.3 ± 1.4 |
| 3b | 33 ± 3 | 12.4 ± 1.8 | 7.3 ± 1.5 | 9.7 ± 2.5 |
| 3c | 77 ± 11 | 10.9 ± 2.8 | 16.3 ± 0.5 | 9.3 ± 1.4 |
| 4 | 210 ± 44 | 9.3 ± 2.5 | 14.2 ± 2.5 | 10.5 ± 12.6 |
| 5 | - | - | 40.7 ± 9.5 | 7.4 ± 0.6 |
| 6 | 21 ± 3 | 9.5 ± 3.9 | - | - |
Values are expressed as means±S.E.M. of data from 3−5 independent experiments.
Acknowledgements
This work was supported by NIH grants DA 01533 (P.S.P.) and DK 32878 (L.J.M.). We thank Ajay Yekkirala for assistance with the graphics, and L.-A. Bruins and R. Happs for technical assistance.
Footnotes
Abbreviations: BRET, bioluminescence energy transfer; CNS, central nervous system; CCK, cholecystokinin; CCK1R, cholecystokinin-1 receptor; CCK2R, cholecystokinin-2 receptor; DCC, N,N’-dicyclohexylcarbodiimide; CDCl3, chloroform-d ; CHCl3, chloroform; CH2Cl2, dichloromethane; DAMGO, [D-Ala2, NMePHe4, Gly5-ol]enkephalin; DMEM, Dulbecco's modified Eagle's medium; DMF, N,N-dimethylformamide; DMSO, dimethyl sulfoxide; DOP, delta opioid peptide; ESI, electrospray ionization; EtOAc, ethyl acetate; FLIPR, Fluorometric Imaging Plate Reader; GPCR, G-protein-coupled receptor; HPLC, high performance liquid chromatography; HOBt, 1-hydroxybenzotriazole; KOP, kappa opioid peptide ; MeOH, methanol; MOP, mu opioid; MOPR, mu opioid receptor; NOP, nociceptin; Pd/C, palladium on carbon; Rlu, Renilla luciferase; RVM, rostral ventromedial medulla; THF, tetrahydrofuran; TFA, trifluoroacetic acid; YFP, yellow fluorescent protein;
References
- 1. IUPHAR RECEPTOR DATABASE, Opioid receptors, http://uphardb.org/PRODGPCR.
- 2.Gutstein HB, Akil H. The pharmacological Basis of Therapeutics(11th Ed. The McGraw-Hill Companies, Inc.; USA: 2006. Goodman and Gillman's. Chapter 21 opioid analgesics. [Google Scholar]
- 3.Saito A, Goldfine ID, Williams JA. Characterization of receptors for cholecystokinin and related peptides in mouse cerebral cortex. J. Neurochem. 1981;37:483–490. doi: 10.1111/j.1471-4159.1981.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 4.Wank A, Harkins R, Jensen RT, Shapira H, DeWeerth A, Slatery T. Purification, molecular cloning, and functional expression of the cholecystokinin receptor from ret pancreas. Proc. Natl. Acad. Sci. USA. 1992;89:3125–3129. doi: 10.1073/pnas.89.7.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopin AS, Lee Y-M, McBride EW, Miller LJ, Lu M, Lin HY, Kolakowski LF, Jr, Beinborn M. Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc. Natl. Acad. Sci. USA. 1992;89:3605–3609. doi: 10.1073/pnas.89.8.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulrich CD, Ferber I, Holicky E, Buell G, Miller LJ. Molecular cloning and functional expression of the human gallbladder cholecystokinin A receptor. Biochem. Biophy. Res. Commun. 1993;193:204–211. doi: 10.1006/bbrc.1993.1610. [DOI] [PubMed] [Google Scholar]
- 7.Pisegna JR, De Weerrth A, Huppi K, Wank SA. Molecular cloning of the human brain and gastric cholocystokinin receptor: structure, functional expression and chromosal loacalization. Biochem. Biophys. Res. Commun. 1992;189:296–303. doi: 10.1016/0006-291x(92)91557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holicky EL, Hadac EM, Ding X-Q, Miller LJ. Molecular characterization and organ distribution of type A and B cholocystokinin receptors in cynomolgus monkey. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G507–G514. doi: 10.1152/ajpgi.2001.281.2.G507. [DOI] [PubMed] [Google Scholar]
- 9.Noble F, Roques B. CCK-B receptor: Chemistry, molecular biology, biochemistry and pharmacology. Prog. Neurobiol. 1999;58:349–379. doi: 10.1016/s0301-0082(98)00090-2. [DOI] [PubMed] [Google Scholar]
- 10.Saito A, Sankaran H, Goldfine ID, Williams JA. Cholecytokinin receptors in the brain: characterization and distribution. Science. 1980;208:1155–1156. doi: 10.1126/science.6246582. [DOI] [PubMed] [Google Scholar]
- 11.Steengard-Pedersen K, Larson L-I. Localization and opiate receptor binding of enkephalin, CCK and ACTH/β-endorphin in the rat central nervous system. Peptides. 1981;2:3–19. doi: 10.1016/0196-9781(81)90050-4. [DOI] [PubMed] [Google Scholar]
- 12.Gall C, Lauterborn J, Burks D, Scroogy K. Co-localization of enkephalin and chlolecystokinin in discrete areas of rat brain. Brain Res. 1987;403:403–408. doi: 10.1016/0006-8993(87)90085-0. [DOI] [PubMed] [Google Scholar]
- 13.Kurrikoff K, Kõks S, Matsui T, Bourin M, Arend A, Aunapuu M, Vasar E. Deletion of CCK2 receptor gene reduces mechanical sensitivity and abolishes the development of hyperalgesia in mononeuropathic mice. Eur. J.Neurosci. 2004;20:1577–1586. doi: 10.1111/j.1460-9568.2004.03619.x. [DOI] [PubMed] [Google Scholar]
- 14.Pommier B, Beslot F, Simon A, Pophillat M, Matsui T, Dauge V, Roques BP, Noble F. Deletion of CCK2 receptor in mice results in an up regulation of the endogenous opioid system. J.Neurosci. 2002;22:2005–2011. doi: 10.1523/JNEUROSCI.22-05-02005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedrich AE, Gebhart GF. Modulation of visceral hyperalgesia by morphine and cholecystokinin from the rat rostroventral medial medulla. Pain. 2003;104:93–101. doi: 10.1016/s0304-3959(02)00469-4. [DOI] [PubMed] [Google Scholar]
- 16.Xie JY, Herman DS, Stiller C-O, Gardell LR, Ossipov MH, Lai J, Porreca F, Vanderah TW. Cholecystokinin in the rostral venromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J.Neurosci. 2005;25:409–416. doi: 10.1523/JNEUROSCI.4054-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalso E. Improving opioid effectiveness: from ideas to evidence. Eur. J. Pain. 2005;9:131–135. doi: 10.1016/j.ejpain.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 18.George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O'Dowd BF. Oligomerization of μ-and δ-opioid receptors. J. Biol. Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- 19.Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: a role in opiate synergy. J. Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Sun X, Bohn IM, Sadee W. Opioid receptor homo-and heterodimerization in living cells by quantative bioluminescence resonance energy transfer. Mol.Pharmacol. 2005;67:21713–2184. doi: 10.1124/mol.104.010272. [DOI] [PubMed] [Google Scholar]
- 21.Erez M, Takemori AE, Portoghese PS. Narcotic antagonist potency of bivalent ligands which contain β-naltrexamine. Evidence for bridging between proximal recognition sites. J.Med.Chem. 1982;25:847–849. doi: 10.1021/jm00349a016. [DOI] [PubMed] [Google Scholar]
- 22.Portoghese PS, Larson DL, Yim CB, Sayre LM, Ronsisvalle G, Lipkowski AW, Takemori AE, Rice KC, Tam SW. Stereostructure-activity relationship of opioid agonist and antagonists ligands. Evidence for bridging between vicinal opioid receptors. J.Med.Chem. 1985;28:1140–1141. doi: 10.1021/jm00147a002. [DOI] [PubMed] [Google Scholar]
- 23.Bhushan RG, Sharma SK, Xie Z, Daniels DJ, Portoghese PS. A bivalent ligand (KDN-21) reveals spinal delta and kappa opioid receptors are organized as heterodimers that give rise to delta1 and kappa2 phenotypes. Selective targeting af delta-kappa heterodimers. J.Med.Chem. 2004;47:2969–2972. doi: 10.1021/jm0342358. [DOI] [PubMed] [Google Scholar]
- 24.Daniels DJ, Kulkarni A, Xie Z, Bushan RG, Portoghese PS. A bivalent ligand (KDAN-18) containing delta-antagonist and kappa1-agonist pharmacophores bridges delta2 and kappa1 opioid receptor phenotypes. J.Med.Chem. 2005;48:1713–1716. doi: 10.1021/jm034234f. [DOI] [PubMed] [Google Scholar]
- 25.a Portoghese PS, Larson DL, Sayre LM, Yim CB, Ronsisvalle G, Tam SW, Takemori AE. Opioid agonist and antagonist bivalent ligands. The relationship between spacer length and selectivity at multiple opioid receptors. J.Med.Chem. 1986;29:1855–1861. doi: 10.1021/jm00160a010. [DOI] [PubMed] [Google Scholar]; b Daniels DJ, Lenard NR, Roerig SC, Portoghese PS. Tolerance and Dependence is Modulated by the Distance Between Opioid mu Agonist and delta Antagonist Pharmacophores in a Bivalent Ligand Series (MDAN) Evidence for Associated mu and delta Opioid Pharmacophores. Kyoto, Japan: Jul 18−22, 2004. 2004 INRC. [Google Scholar]; c Portoghese PS, Ronsisvalle G, Larson DL, Yim CB, Sayre LM, Takemori AE. Opioid agonist and antagonist bivalent ligands as receptors probes. Life Sci. 1982;31:1283–1286. doi: 10.1016/0024-3205(82)90362-9. [DOI] [PubMed] [Google Scholar]; d Xie Z, Bhushan RG, Daniels DJ, Portoghese PS. Interaction of biavalent ligand KDN21 with heterodimeric δ-κ opioid receptors in human embryonic kidney 293 cells. Mol Pharmacol. 2005;68:1079–1086. doi: 10.1124/mol.105.012070. [DOI] [PubMed] [Google Scholar]
- 26.Sayre LM, Portoghese PS. Stereospecific Synthesis of 6α- and 6β-Amino Derivatives of Naltrexone and Oxymorphone. J. Org. Chem. 1980;45:3366–3368. [Google Scholar]
- 27.Bock MG, DiPardo RM, Evans BE, Rittle KE, Whitter WL, Garsky VM, Gilbert KF, Leighton JL, Carson KL, Mellin EC, Veber DF, Chang RSL, Lotti VJ, Freedman SB, Smith AJ, Patel S, Anderson PS, Freidinger RM. Development of 1,4-Benzodiazepine Cholecystokinin Type B Antagonists. J. Med. Chem. 1993;36:4276–4292. doi: 10.1021/jm00078a018. [DOI] [PubMed] [Google Scholar]
- 28.Watkins LR, Kinscheck IB, Mayer DJ. Potentiation of opiate analgesia and apperant reversal of morphine tolerance by proglumide. Science. 1984;224:395–396. doi: 10.1126/science.6546809. [DOI] [PubMed] [Google Scholar]
- 29.Lu L, Huang M, Liu A, Ma L. Cholecystoinen-B receptor antagonists attenuate morphine dependence and withdrawal in rats. NeuroReport. 2000;11:829–832. doi: 10.1097/00001756-200003200-00034. [DOI] [PubMed] [Google Scholar]
- 30.Daniels DJ, Lenard NR, Etienne CL, Law P-Y, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice modulated by the bivalent ligand series. Proc. Natl. Acad. Sc.i USA. 2005;102:19208–13. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bock MG, Dipardo RM, Evans BE, Rittle KE, Veber DF, Freidinger RM, Hirshfield J, Sringer JP. Synthesis and Resolution of 3-Amine-1,3-dihydro-5-phenyl-2H-1,4-benzodiazepin-2-ones. J. Org. Chem. 1987;52:3232–3239. [Google Scholar]
- 32.Reider PJ, Davis P, Hughes DL, Grabowski EJJ. Crystallization-Induced Asymmetric Transformation: Stereospecific Synthesis of a Potent Peripheral CCK Antagonist. J. Org. Chem. 1987;52:955–957. [Google Scholar]
- 33.Wubbels GG, Halverson AM, Oxman JD, Bruyn VH. Regioselectivity of Photochemical and Thermal Smiles Rearrangements and Related Reaction of β-(Nitrophenoxy)ethylamines. J. Org. Chem. 1985;50:4499–4504. [Google Scholar]
- 34.Cheng ZJ, Harikumar KG, Holicky EL, Miller LJ. Heterodimerization of Type A and B Cholecystokinin Receptors Enhance Signaling and Promote Cell Growth. J. Biol. Chem. 2003;278:52972–52979. doi: 10.1074/jbc.M310090200. [DOI] [PubMed] [Google Scholar]
- 35.Gomes I, Filipovska J, Jordan BA, Devi LA. Oligomerization of opioid receptors. Methods. 2002;27:358–365. doi: 10.1016/s1046-2023(02)00094-4. [DOI] [PubMed] [Google Scholar]
- 36.Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, Portoghese PS, Whistler JL. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimmers. PNAS. 2005;102:9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling:β-arrestin2-mediated ERK activation by μ-δ opioid receptor heterodimer. FASEB J. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta A, Décaillot FM, Devi LA. Targeting opioid receptor heterodimers:Strategies for screening and drug development. AAPS j. 2006;8:E153–E159. doi: 10.1208/aapsj080118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milligan G. G protein-coupled receptor heterodimers: Pharmacology, function and relevance to drug discovery. DrugDiscoveryToday. 2006;11:541–549. doi: 10.1016/j.drudis.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Rashid AJ, O'Dowd BF, George SR. Minireview: Diversity and complexity of signaling through peptidergic G protein-coupled receptors. Endocrinoly. 2004;145:2645–2652. doi: 10.1210/en.2004-0052. [DOI] [PubMed] [Google Scholar]
- 41.a Lee PS, O'Dowd BF, George SR. Homo- and Hetero-oligomerization of G protein-coupled receptors. Life Sci. 2003;74:173–180. doi: 10.1016/j.lfs.2003.09.028. [DOI] [PubMed] [Google Scholar]; b Pan Y,-X, Bolan E, Pasternak GW. Dimerization of Morphine and Orphinan FQ/nociception Receptors: Generaton of Novel Receptor Subtype. Biochem. Biophys. Res. Comm. 2002;297:659–663. doi: 10.1016/s0006-291x(02)02258-1. [DOI] [PubMed] [Google Scholar]; c Wang HL, Hsu CY, Huang PC, Kuo YL, Li AH, Yeh TH, Tso AS, Chen YL. Heterodimerization of opioid receptor-like1 and mu opioid receptors impairs the potency of mu receptor agonist. J.Neurochem. 2005;92:1285–1294. doi: 10.1111/j.1471-4159.2004.02921.x. [DOI] [PubMed] [Google Scholar]; d Jordan BA, Trapaidza N, Gomes I, Nivarthi R, Devi LA. Oligomerization of opioid receptors with beta-2 adrenergic receptors: A role in trafficking and mitogen-activated protein kinase activation. Proc.Natl. Acad. Sci. U.S.A. 2001;98:343–348. doi: 10.1073/pnas.011384898. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Pfeiffer M, Koch T, Schroeder H, Laugusch M, Hollt V, Schulz S. Heterodimerization of somastatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J. Biol. Chem. 2002;277:19762–19772. doi: 10.1074/jbc.M110373200. [DOI] [PubMed] [Google Scholar]; f Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen L-Y. Heterodimerization and cross-desenitization between the μ-opioid receptor and the chemokine CCR5 receptor. Eur.J. Pharmcol. 2004;483:175–186. doi: 10.1016/j.ejphar.2003.10.033. [DOI] [PubMed] [Google Scholar]; g Suzuki S, Chuang LF, Yau P, Doi RH, Chuang RY. Interaction of Opioid and Chemokine Receptors: Oligomerization of mu, kappa, and delta with CCR5 on Immune Cells. Exp.Cell. Res. 2002;280:192–200. doi: 10.1006/excr.2002.5638. [DOI] [PubMed] [Google Scholar]; h Pfeiffer M, Kirsch S, Stumm R, Koch T, Wu D, Laugusch M, Schroeder H, Hollt V, Schulz S. Heterodimerization of Substance P and μ-Opioid Receptors Regulates Receptor Trafficking and Resensitization. J. Biol. Chem. 2003;51:51630–51637. doi: 10.1074/jbc.M307095200. [DOI] [PubMed] [Google Scholar]; i Jordan BA, Gomes I, Rios C, Filipovska J, Devi LA. Functional interactions between mu opioid and alpha 2A-adrenergic receptors. Mol.Pharmacol. 2003;64:1317–1324. doi: 10.1124/mol.64.6.1317. [DOI] [PubMed] [Google Scholar]; j Zhang YD, Limbird LE. Hetero-oligomers of alpha2A-adrenergic receptor and mu-opioid receptor do not lead to transactivation of G protein or altered endocytosis profiles. Biochem. Soc. Trans. 2004;32:856–860. doi: 10.1042/BST0320856. [DOI] [PubMed] [Google Scholar]; k Rios C, Gomes I, Devi LA. Interaction between delta opioid receptors and alpha2A-adrenoceptors. Clin. Exp. Pharmacol. Physiol. 2004;31:833–836. doi: 10.1111/j.1440-1681.2004.04076.x. [DOI] [PubMed] [Google Scholar]; l Rios C, Gomes I, Devi LA. Mu and CB1 cannabinoid receptor interaction: reciprocal inhibition of receptor signaling and neuritogenesis. Br. J. Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]; m Parenty G, Appelbe S, Milligan G. CXCR2 chemokine receptor antagonism enhances DOP opioid receptor function via allosteric regulation of CXCR2-DOP receptor heterodimer. Biochem. J. 2008;412:245–256. doi: 10.1042/BJ20071689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harikumar KG, Morfis MM, Lisenbee CS, Sexton PM, Miller LJ. Constitutive formation of oligomeric complexes between Family B G protein-coupled vasoactive intestinal polypeptide and secretin receptors. Mol. Pharmacol. 2006;69:363–373. doi: 10.1124/mol.105.015776. [DOI] [PubMed] [Google Scholar]
- 43.Hadac EM, Ghanekar DV, Holicky EL, Pinon DI, Dougherty RW, Miller LJ. Relationship Between Native and Recombinant Cholecystokinin Receptors: Role of Differential Glycosylation. Pancreas. 1996;13:130–139. doi: 10.1097/00006676-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Harikumar KG, Dong M, Cheng Z, Pinon DI, Lybrand TP, Miller LJ. Transmembrane segment peptides can disrupt cholecystokinin receptor oligomerization without effecting receptor function. Biochem. 2006;45:14706–14716. doi: 10.1021/bi061107n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munson PJ, Rodbard D. LIGAND: A versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 46.D'Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- 47.Dewey WL, Harris LS, Howes JF, Nuite JA. The effect of various neurochemical modulators on the activity of morphine and the narcotic antagonists in the tail-flick and phenylquinone tests. J Pharmacol Exp Ther. 1970;175:435–442. [PubMed] [Google Scholar]
- 48.Harris LS, Pierson AK. Some narcotic antagonists in the benzomorphan series. J Pharmacol Exp Ther. 1964;143:141–148. [PubMed] [Google Scholar]
- 49.Haley TJ, McCormick WG. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br J Pharmacol Chemother. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]